Summary
The object of this study is to evaluate the radiological and pathological changes in the sinus of an experimental arteriovenous fistula of the rat.
Twenty-five male Sprague-Dawley rats, including two control rats, were used for this study. A venous hypertension model in the transverse sinus was induced by means of anastomosis of a common carotid artery (CCA) to the ipsilateral external jugular vein (EJV). Rats were sacrificed 11 to 42 weeks after the procedure, then histopathological and immunohistopathological examinations were performed for the resected transverse sinus. Follow-up angiography was performed two to three weeks after the anastomosis in every case, and five months later in two rats.
Patency of the anastomosed portion was confirmed in 12 of the 23 anastomosed rats. An ipsilateral carotid angiogram demonstrated a high-flow arteriovenous (AV) shunt from the CCA to the sigmoid-to-transverse sinus and draining into the contralateral juglar vein. A contralateral angiogram displayed a steal phenomenon via the communicating artery.
Histopathologically, the vein of the anastomosed portion and the transverse sinus were markedly dilated in with cases. There was a thickening the connecting tissue and a proliferation of fibroblast in four (50%) of the eight cases. Thrombus formation in the transverse sinus was found in one case. VEGF stained strongly in the endothelial hypertrophied area and in fibrous connective tissue around the transverse sinus compared to the control sinuses.
Our results from this long-term observation of the radiological and pathological changes in the sinus exposed to hypertension resembled the clinical findings of a dural AV fistula.
Key words: dural arteriovenous fistula, experimental model, pathology
Introduction
There have been many reports on the pathogenesis of dural arteriovenous fistulas (AVFs) in clinical cases and experimental models. Recent reports indicate that the dural AVFs are acquired rather than congenital lesions5,8. Some investigators have claimed that a physiological arteriovenous shunt exists in the sinus wall4 and that it may open due to various causes resulting in dural AVF2. In recent clinical and experimental studies, the most important factor related to the pathogenesis of a dural AVF is thought to be sinus thrombosis and venous or intrasinus hypertension 1-3,5-7. Terada et A1 suggested from their experimental study that only venous hypertension without thrombosis could cause the development of newly acquired AVFs7. Thus venous or intrasinus hypertension may be a more important factor than venous or sinus thrombosis2,7.
Vascular endothelial growth factor (VEGF) is one of the main cytokines secreted during wound healing, tissue hypoxia, or embryonic vasculogenesis. VEGF stained in the surgically resected sinuses with dural AVFs at the transverse-sigmoid and superior sagittal sinuses8.
In this study, to clarify the relationship between angiogenic growth factor and the development of dural AVFs, we investigated the expression of VEGF at the sinus wall with an experimental high-flow AVF of rats and compared this expression to that in the dural sinuses of control rats.
Material and Methods
Twenty-five male Sprague-Dawley rats, each weighing 300-350 gm, were used for this study, which was performed according to a protocol approved by the Nagoya University Animal Care Committee.
Surgical Protocol
Venous hypertension in the transverse sinus was induced in 23 rats by means of the following procedure. Each rat was anesthetized with halothane (induction 3.5%, maintenance 0.5%, in a mixture of 70% N20 and 30% 02) using a face mask.
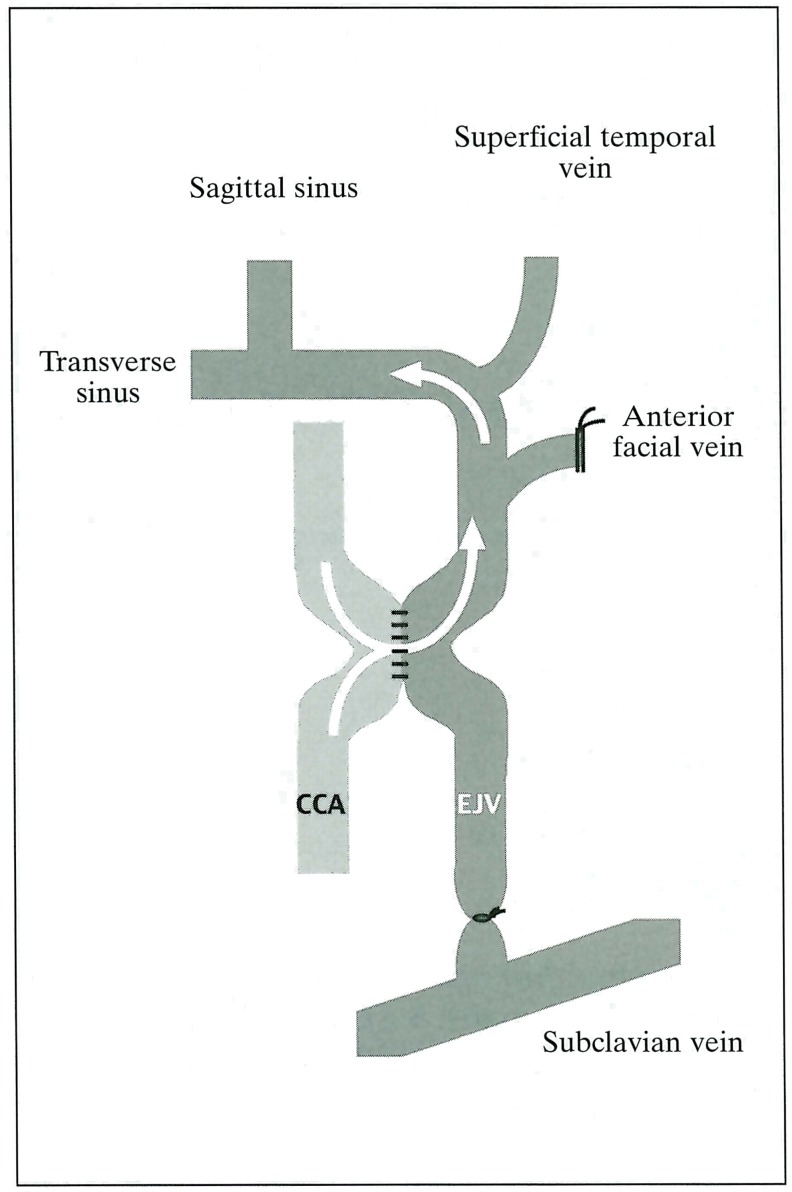
A ventral midline neck incision was made, then a left common carotid artery (CCA) and the ipsilateral external jugular vein (EJV) were exposed. All branches from the external jugular vein, such as the anterior facial vein and the proximal portion of the EJV, were ligated or obliterated by a bipolar coagulator except for the largest draining vein from the dural sinus7. The CCA and the EJV were anastomosed in a side-to-side fashion, using 10-0 microsutures. The EJV was then ligated on the side of the heart (figure 1). Two rats that did not undergo any surgical procedure were used as the control.
Figure 1.
Diagram of the surgery for making AV shunt.
Angiographic Protocol
Angiography was performed two to three weeks after the anastomosis, and was then repeated over three months later; a 0.018 system microcatheter was used. Each rat was anesthetized as described above. The right femoral artery was exposed by the cutdown method, and a microcatheter (0.018 inch) was introduced. An intraperitoneal injection of sodium pentobarbital (50 mg/kg) was given, followed by transfer to the angiography room where the catheter was navigated into the bilateral common carotid and vertebral arteries under fluoroscopic guidance. Those two sites were subjected to rapid-sequence digital subtraction angiography at 8 frames/sec, using 0.1 to 0.15 ml of contrast material for each angiogram.
Pathological Examination
Rats were sacrificed 11,17, 22 and 42 weeks after the procedure and histopathological and immunohistopathological examinations were performed. ĩìiey were perfused transcardially with 4% buffered formalin after 50 mg/kg of sodium pentobarbital was administered by intraperitoneal injection. The rat brains, including the transverse sinus, were removed and sections stained by hematoxylin/eosin and VEGF were examined by light microscopy.
Results
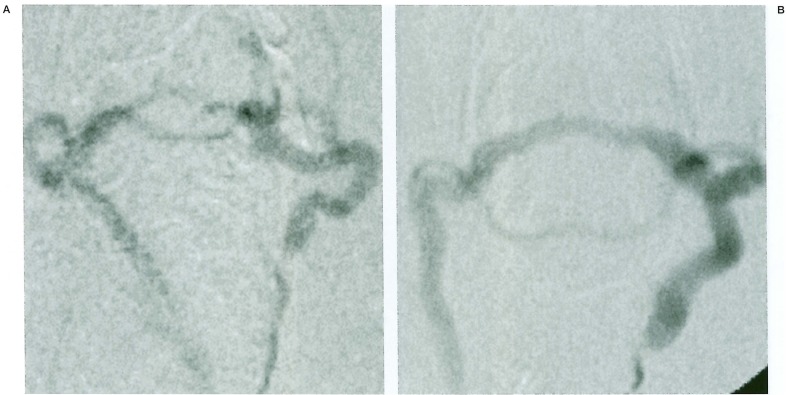
Patency of the anastomosed portion was confirmed in 12 of 23 anastomosed rats by means of angiograms and direct exposure. Initial angiography could be performed in seven of the 12 cases. Ipsilateral common carotid angiograms demonstrated a high-flow arteriovenous (AV) shunt from the CCA to the sigmoid-to-transverse sinus draining into the contralateral jugular vein. Contralateral common carotid angiograms displayed a steal phenomenon via a communicating artery. Long-term follow-up angiography was performed in two cases which had survived for more than five months (22 weeks and 42 weeks after the anastomosis), showing increases in both shunt flow and the size of the anastomosed vein (figure 2).
Figure 2.
A) Ipsilateral common carotid angiogram taken 2 weeks after surgery demonstrated a high-flow arteriovenous (AV) shunt draining into the contralateral jugular vein. B) Follow-up angiogram 22 weeks later. Note the dilatation of sinus and draining veins.
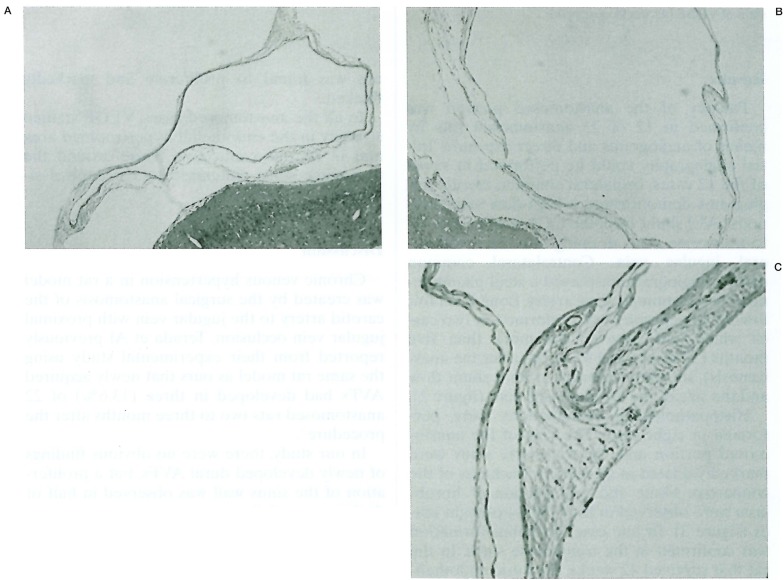
Histopathological examinations were performed in eight cases. The vein of the anastomosed portion and the transverse sinus were markedly dilated in all cases. A thickness of the connective tissue and proliferation of fibroblasts were observed in four (50%) of eight cases (figure 3). In one case, thrombus formation was confirmed in the transeverse sinus. In the rat that survived 42 weeks, the dural endothelium was found to proliferate and markedly thicken.
Figure 3.
A) Normal transverse sinus of control rat. B) Dilated sinus (17 weeks post op.). C) Thickness of sinus wall (11 weeks post op.).
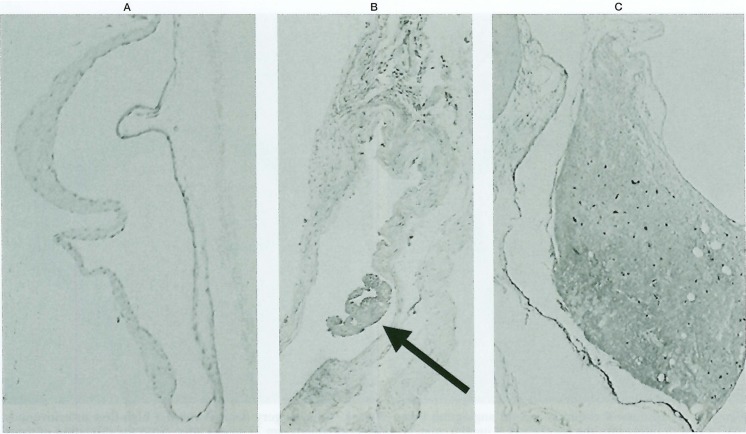
Figure 4.
A) No obvious expression of VEGF in a normal sinus (control). B) Stain of VEGF with subintimal proliferation of fibroblasts (22 weeks post op.). Note the intrasinus thrombus formation (arrow). C) Marked intimal proliferation with strong stain of VEGF (42 weeks post op.).
In all the anastomosed cases, VEGF stained strongly in the endothelial hypertrophied area and in fibrous connective tissue around the transverse sinus compared to the control sinuses.
Discussion
Chronic venous hypertension in a rat model was created by the surgical anastomosis of the carotid artery to the jugular vein with proximal jugular vein occlusion. Terada et A1 previously reported from their experimental study using the same rat model as ours that newly acquired AVFs had developed in three (13.6%) of 22 anastomosed rats two to three months after the procedure 7.
In our study, there were no obvious findings of newly developed dural AVFs, but a proliferation of the sinus wall was observed in half of the long-term follow-up rats with the successful formation of an AV shunt. This change may suggest hypertrophic vasculopathy due to long-term venous hypertension.
Furthermore, one case showed intrasinus thrombogenesis, which may also have resulted from an intimal change in the sinus wall under hemodynamic stress.
Uranishi et A1 reported from their clinical study that VEGF had stained positively in the endothelium of surgically resected sinuses with dural AVF, but that it was not expressed in any of the normal specimens. VEGF is expressed in tissue requiring rapid vasculogenesis to proliferate or perfuse the ischemic area. From this point of view, the expression of VEGF in the sinus wall in our series may be compatible with sinus wall proliferation, and this may be the cause to sprout the regional AV shunt in the sinus wall or the sinus leaf.
Although this study is limited by the small sample size, long-term observation revealed radiological and pathological changes in the sinus exposed to sinus hypertension. Further study using the various conditions and designs will be needed.
References
- 1.Bederson JB, Wiestler OD, et al. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29:341–350. doi: 10.1097/00006123-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hamada Y, Goto K, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452–458. doi: 10.1097/00006123-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Herman JM, Spetsler RE, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539–545. doi: 10.3171/jns.1995.83.3.0539. [DOI] [PubMed] [Google Scholar]
- 4.Kerber CW, Newton TH. The macro and microvasculature of the dura mater. Neuroradiology. 1973;6:175–179. doi: 10.1007/BF00335317. [DOI] [PubMed] [Google Scholar]
- 5.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267–274. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- 6.Nishijima M, Takaku A, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76:600–606. doi: 10.3171/jns.1992.76.4.0600. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Higashida RT, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 8.Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781–786. doi: 10.3171/jns.1999.91.5.0781. [DOI] [PubMed] [Google Scholar]