Summary
We developed a new type of coil with a polyvinyl alcohol core (PVA-core coil) to absorb and release various types of biologically active materials, for the endovascular treatment of intracranial aneurysms. A 10 mm segment of the PVA-core coil was used in this study. PVA-core coils were immersed in basic fibroblast growth factor (b-FGF) solution. The PVA-core coil, which absorbed b-FGF in the PVA core, was named FGF-core coil. This coil gradually released b-FGF in the solution without b-FGF. In vitro study, FGF-core coils, PVA-core coils and unmodified coils were cultured with fibroblasts (NIH3T3) respectively and their surface was observed with scanning electron microscopy (SEM). In vivo study, each coils were inserted into the rat common carotid artery. Rats were sacrificed and the arterial lumen were histologically examined 14 days and 28 days after coil implantation. Electron microscopy findings demonstrated remarkable cellular adhesion to the surface of the FGF-core coils, while no adhesion to the surface of the PVA-core coils and unmodified coils was found. Histologically, remarkable cellular proliferation and wall thickness like neointimal hyperplasia was demonstrated in the implanted common carotid artery of the FGF-core coil group at 14 days and 28 days. On the other hand, these changes did not occur in PVA-core coil group and unmodified coil group. We suggest that FGF-core coils may be effective to induce fibrotic changes inside cerebral aneurysms.
Key words: polyvinyl alcohol, b-FGF, drug delivery, fibrosis
Introduction
The endovascular treatment of intracranial aneurysms using Guglielmi detachable coils (GDCs) is widely accepted as a safe and less invasive procedure alternative to standard surgical therapy. On the other hand, some limitations of GDC treatment have been pointed out, that is to say, difficulty in treating the large or giant aneurysms with or without thrombus. Furthermore, histopathological findings of experimental aneurysms or human autopsy cases have recently shown the lack of organization in the aneurysm cavity after GDC embolization1. Over the past few years, various modifications have been made to the coil in order to enhance organization in aneurysmal cavity. In this study, we designed a new coil with polyvinyl alcohol rod, which can deliver biologically active substance inside of the aneurysms. We evaluated its effect as drug delivery system in vitro and in vivo study by using basic fibroblast growth factor (b-FGF) as a candidate to induce fibrosis inside of aneurysms.
Material and Methods
Coil Preparation
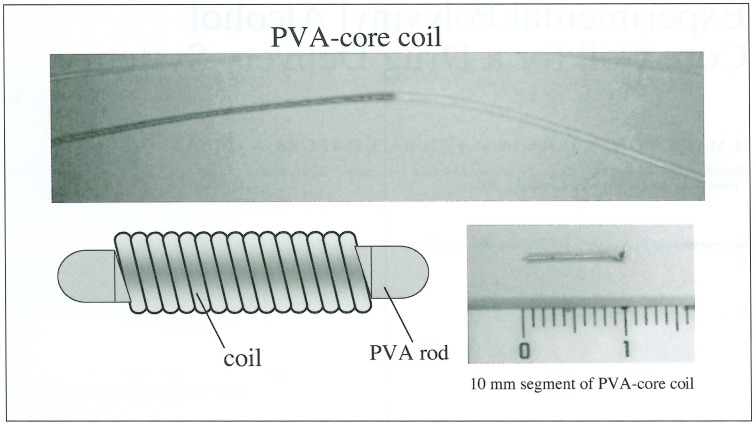
Polyvinyl alcohol (PVA) was processed to small thread (polymerization; 2000, diameter; 0.018 mm) and inserted into the central core space of the coil. The coil is constructed from platinum filament (filament diameter; 0.07 mm, primary coil diameter; 0.35 mm, length 10 cm). The schema of the coil, named PVA-core coil, is shown in figure 1. These were sectioned into 10 mm segments and used in vitro and in vivo experiment.
Figure 1.
PVA-core coil (produced by Kaneka Medix). Small PVA rod (polymerization; 2000, diameter; 0.018 mm) is inserted into the central core space of the coil (filament diameter; 0.07 mm, primary coil diameter; 0.35 mm, length 10 cm).
In Vitro Study
Under sterile conditions, 10 mm-long segments of PVA-core coil were immersed in the b-FGF solution (25µg/ml) for 1 hour to absorb b-FGF into the PVA. We call this coil, which absorbed b-FGF solution, FGF-core coil. After one coil segment was placed in the plates, cell suspensions containing mouse fibroblast (NIH 3T3) and DMEM (Dulbecco’s modified Eagle medium with 3% calf serum, b-FGF free) were added to the plates. These plates were incubated at 37° C until the cells reached the confluent state over the plates. Unmodified coils and PVA-core coils were treated in the same way for control samples. After incubation, each coils were fixed to prepare SEM samples according to standard protocols. The surface of each coil was examined with SEM.
In Vivo Study
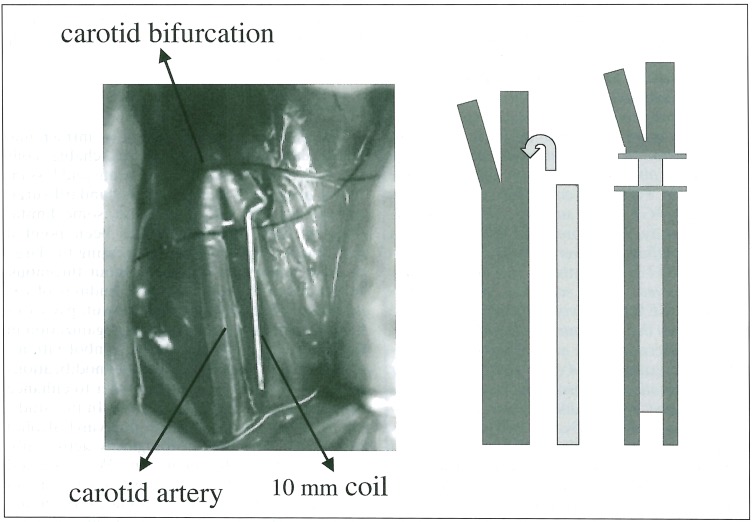
Sprague-Dawley rats (400-450 g) were anesthetized and the right common carotid artery was carefully dissected. 10 mm segments of unmodified coils, PVA-core coils and FGF-core coils were prepared. Each of the coil was inserted into the right CCA (figure 2). The coil segment of unmodified coil was implanted in the CCA of 16 rats and that of PVA-core coil in the CCA of 16 rats, and that of FGF-core coil in the CCA of 16 rats respectively. Half of the rats were sacrificed at 14 days and the rest of them at 28 days. They were anesthetized and the right CCA containing 10 mm segments of the coil was carefully harvested in a similar fashion. The removed artery with the coil was immediately fixed by 10% formaldehyde for at least 48 hours. Only the coil segments were then carefully removed from the vessel seg-ment. The vascular tissue samples without the coil were mounted in paraffin and sectioned and each specimen was stained with hematoxylin-eosin (H&E) and Masson trichrome according to standard protocols. Specimens were examined histopathologically under light microscope. H&E stain was used primarily to assess cellular component, whereas Masson trichrome stain was used to evaluate collagen component.
Figure 2.
Simple aneurysmal model of rat carotid artery A 10 mm segment of the coil was inserted into the right CCA of the rat.
Results
Scanning Electron Microscopy Observation of the Coil Surface
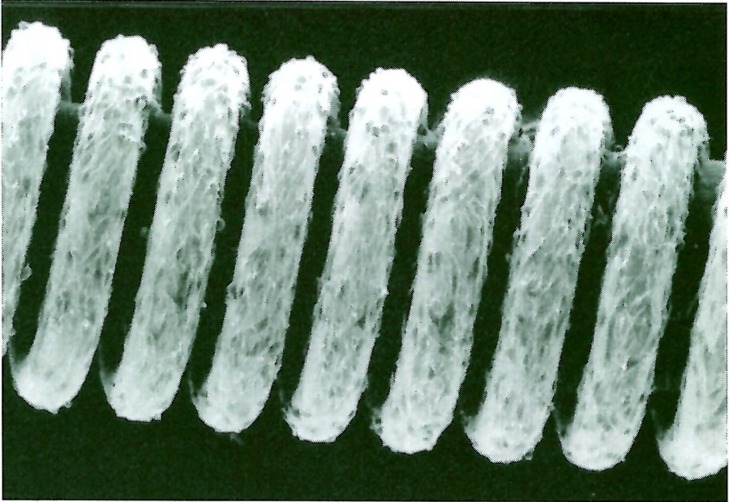
Both unmodified coils and PVA-core coils had no cell on their surface. On the other hand, surface of FGF-core coils was completely covered with NIH 3T3 cells. These cells showed spindle formation and extended to the primary coil space(figure 3).
Figure 3.
Scanning electron microscopy findings of the surface of FGF-core coil. Electron microscopy image demonstrated remarkable cellular adhesion on the surface of FGF-core coils. These cells showed spindle formation and extended into the primary coil space.
Histopathological Findings
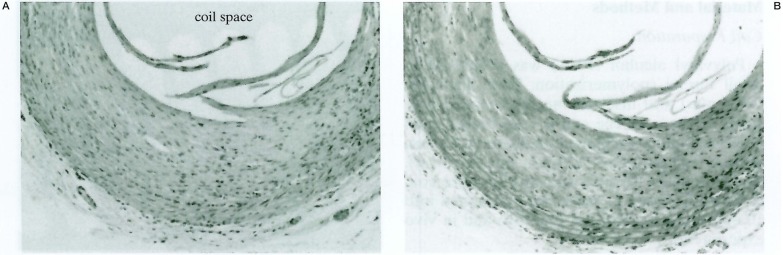
The CCA lumen implanted unmodified coils was filled with blood element and displayed thin cellular lumen at 14 days and 28 days’ specimen. The CCA lumen implanted PVA-core coils showed almost same appearance as the unmodified coils. On the other hand, the CCA lumen implanted FGF-core coil was filled with cellular proliferation like thick intimal hyperplasia. The vessel wall of FGF-core coil was much thicker than that of unmodified coil and PVA-core coil. The wall like intimal hyperplasia was mainly stained blue with Masson trichrome, which suggested that these were composed of collagen component (figure 4).
Figure 4.
Histopathologic findings in the CCA segments implanted FGF-core coil after 28 days. The implanted coil was surrounded by thick cellular proliferation. The wall-like intimal hyperplasia was mainly stained blue with Masson trichrome stain. A) H & E, original magnification × 50, B) masson trichrom stain, original magnification × 50).
Discussion
Modification of coils aim to increase intraaneurysmal organization and to make GDC therapy more effective. Previously many studies were performed in vitro and in vivo models in order to modify GDCs with various factors, such as exracellular matrix proteins which promote wound healing, vasoactive growth factors, polymers, and immortalized fibroblasts that secrete growth factors 2-8.
Modifications have been made to the surface of the coil. However, the problem was that simple coating process increases the diameter of the coil and coated materials may be easily stripped away during coil delivery through the microcatheter and results in a potential source of distal thromboembolism. To overcome this problem, Murayama et A19-11 coated GDC with alubmin, fibronectin, and collagen by using high-energy ion plantation technology. These coils improved the strength of surface adhesion against flow shear stress and showed an intense cellular response in the aneurysm and faster reendothelial coverage of the neck of the aneurysm in swine model. On the other hand, another modification was done to the space of the coil or new materials were used as the coil. Dawson et Al12 filled central space of the interlocking detachable coils with collagen core. Abrahams et Al13 designed a new type of coil, which was constructed from monofilament, synthetic, absorbable suture, as drug delivery system. Myrayama et A114 developed bioabsorbable polymeric coils, which decreased aneurysm recanalization by organized fibrous tissue in swine model.
In this study, we developed a new type of coil which enabled local drug delivery by using PVA as the core of the platinum coil. Originally PVA was often used for feeding artery embolization of tumors or arteriovenous malformations as particle embolic materials in endovascular therapy. It has good absorbability of liquid and expands when it absorbs liquid. It also has good biocompatibility and it is used for other biomaterials. Especially characteristics of good absorbability is used for drug delivery systems in some studies15,17. We applied these characteristics to the coil system, that is, the PVA rod was used as the central core of the coil. In this study we chose b-FGF as a delivered substance, because it affects on NIH3T3 fibroblast in vitro study and it also has some influence on intravascular reaction such as vascular smooth muscle cell in vivo study.
In vitro study, b-FGF absorbed in PVA-core was released in the medium and affected on fibroblasts. The released b-FGF led the fibroblasts to the surface of the coil and helped them to scaffold on the surface of it. In vivo study, b-FGF released from the PVA-core influenced on the arterial wall and also enhanced cellular proliferation in the* vascular lumen and wall thickness like intimal hyperplasia. These components were mainly composed of collagen fibers according to the Masson trichrome stain.Our study indicated that b-FGF released from PVA-core effectively worked in vitro and in vivo to induce fibrosis. FGF-core coils had a potential to be used as an effective embolic material for cerebral aneurysm. These coils has some advantages as follow: At first it is easy to prepare the coils before treatment. We only have to immense them in solution for about one hour. Secondly these coils may deliver various factors other than b-FGF to implanted portion respectively. On the other hand, they have a disadvantage that they may become slightly hard when PVA expands in the central lumen of the coils.
Conclusions
We have developed a PVA-core coil which has a potential to deliver bioactive materials around the implanted coil. A FGF-core coil may be effective to induce fibrosis inside the aneurysm.
References
- 1.Bavinzski G, Talazoglu V, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams JM, Diamond SL, et al. Topic review: surface modifications enhancing biological activity of Guglielmi detachable coils in treating intracranial aneurysms. Surg Neurol. 2000;54:34–41. doi: 10.1016/s0090-3019(00)00269-x. [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF, Borland MK, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblast on platinum coils. Radiology. 1998;206:237–243. doi: 10.1148/radiology.206.1.9423678. [DOI] [PubMed] [Google Scholar]
- 4.Kallmes DF, Williams AD, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology. 1998;207:519–523. doi: 10.1148/radiology.207.2.9577504. [DOI] [PubMed] [Google Scholar]
- 5.Tamatani S, Ozawa T, et al. Histological interaction of cultured endothelial cells and endovascular embolic materials coated with exracellular matrix. J Neurosurg. 1997;86:109–112. doi: 10.3171/jns.1997.86.1.0109. [DOI] [PubMed] [Google Scholar]
- 6.Abrahams JM, Forman MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickness and coil impregnation in rat aneurysmal model. Am J Neuroradiol. 2001;22:1410–1417. [PMC free article] [PubMed] [Google Scholar]
- 7.Marx WF, Cloft HJ, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissure allografts. Am J Neuroradiol. 2001;22:323–333. [PMC free article] [PubMed] [Google Scholar]
- 8.Gast AN, Altes TA, et al. Transforming growth factor β-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery. 2001;49:690–769. doi: 10.1097/00006123-200109000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Murayama Y, Vineuela F, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery. 1997;40:1233–1244. doi: 10.1097/00006123-199706000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Murayama Y, Suzuki Y, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part 1: in vitro study. Am J Neuroradiol. 1999;20:1986–1991. [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama Y, Vineuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part 2: an experimental study in a swine models. Am J Neuroradiol. 1999;20:1992–1999. [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson RC, Shengelaia GG, et al. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. Am J Neuroradiol. 1996;17:853–858. [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahams JM, Forman MS, et al. Biogredable polyglycolide endovascular coils promote wall thickness and drug delivery in a rat aneurysmal model. Neurosurgery. 2001;49:1187–1195. [PubMed] [Google Scholar]
- 14.Murayama Y, Vinuera F, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms; a preliminary experimental study. J Neurosurg. 2001;94:454–463. doi: 10.3171/jns.2001.94.3.0454. [DOI] [PubMed] [Google Scholar]
- 15.Buruczak K, Gamian E, et al. Long-term in vivo performance and biocompatibility of polyvinyl alcohol hydrogel macrocapsules for hybrid type artificial pancrreas. Biomaterials. 1996;17:2351–2356. doi: 10.1016/s0142-9612(96)00076-2. [DOI] [PubMed] [Google Scholar]
- 16.Chuang WY, Young TH, et al. Properties of the polyvinyl alcohol/chitosan blend and its effect on the culture of fibroblast in vitro. Biomaterials. 1999;20:1479–1487. doi: 10.1016/s0142-9612(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 17.Sano K, Tokoro T, et al. A new drug delivery system utilizing piggyback contact lenses. Acta Ophthalmol Scand. 1996;74:243–248. doi: 10.1111/j.1600-0420.1996.tb00085.x. [DOI] [PubMed] [Google Scholar]