Abstract
Requirements for the physical presence of the cell's nucleus for the establishment and maintenance of the interferon-induced antiviral state were investigated. Enucleated chicken embryo fibroblasts were obtained by cytochalasin B treatment during centrifugation. The inhibition of vaccinia virus cytoplasmic DNA synthesis, monitored by autoradiography, was used to measure the antiviral activity resulting from interferon treatment. The antiviral state is not established in cells treated with interferon after removal of their nuclei. On the other hand, cells first treated with interferon for 6 or 12 h and then enucleated express the antiviral state. Furthermore, the antiviral state is maintained in enucleated cells for 16 h after enucleation. The antiviral state appears to be more stable in enucleates than in the residual nucleated cells found in the same cultures. Single cells of antiviral populations are found to be either fully permissive or fully restrictive to vaccinia DNA synthesis. The effect of an increasing intracellular multiplicity of infectious virus is to overcome the antiviral cell's block against viral DNA synthesis.
Full text
PDF


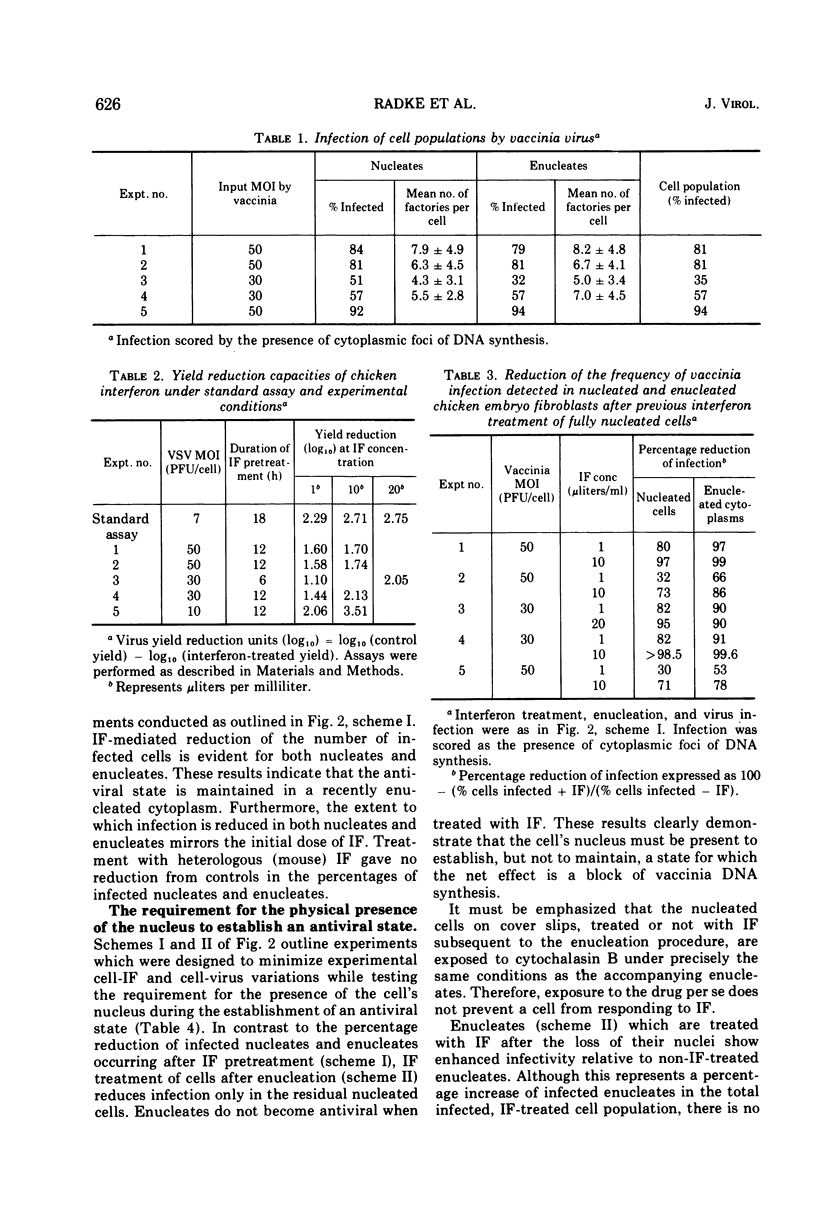
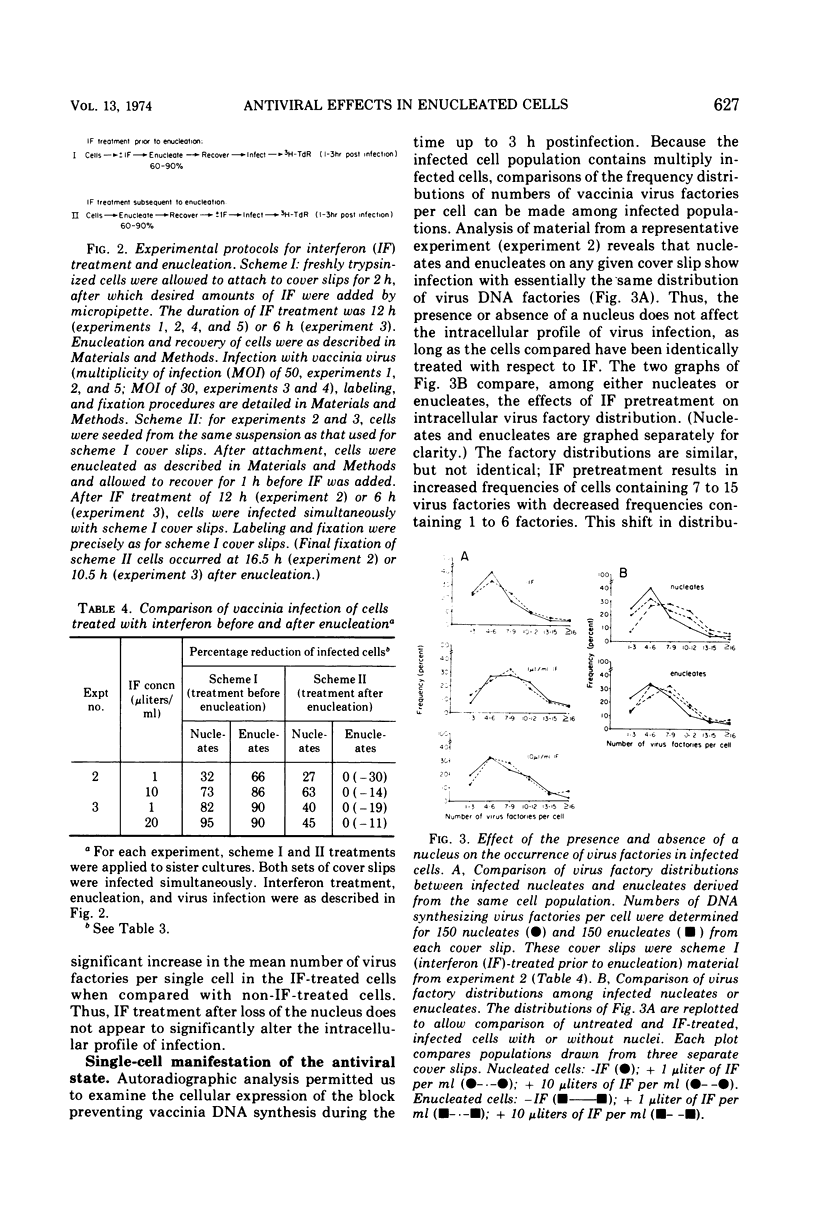



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A., MCDEVITT H. INTERFERON INHIBITION OF CYTOPLASMIC DNA ACCUMULATION IN VACCINIA VIRUS INFECTION. A RADIOAUTOGRAPHIC STUDY. Proc Soc Exp Biol Med. 1965 Jun;119:551–553. doi: 10.3181/00379727-119-30235. [DOI] [PubMed] [Google Scholar]
- Fleischmann W. R., Simon E. H. Effect of interferon on virus production from isolated single cells. J Gen Virol. 1973 Aug;20(2):127–137. doi: 10.1099/0022-1317-20-2-127. [DOI] [PubMed] [Google Scholar]
- Ghosh S. N., Gifford G. E. Effect of interferon on the dynamics of H3-thymidine incorporation and thymidine kinase induction in chick fibroblast cultures infected with vaccinia virus. Virology. 1965 Oct;27(2):186–192. doi: 10.1016/0042-6822(65)90158-3. [DOI] [PubMed] [Google Scholar]
- Hiller G., Jungwirth C., Bodo G., Schultze B. Biological activity of poly rI:poly rC: effect on poxvirus-specific functions. Virology. 1973 Mar;52(1):22–29. doi: 10.1016/0042-6822(73)90394-2. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan G. W. Quantitative aspects of interferon-induced plaque reduction: kinetics of interferon action. Virology. 1972 May;48(2):425–432. doi: 10.1016/0042-6822(72)90053-0. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPSON G. P., TYTELL A. A., NEMES M. M., HILLEMAN M. R. Purification and characterization of chick embryo interferon. Proc Soc Exp Biol Med. 1963 Feb;112:468–478. doi: 10.3181/00379727-112-28080. [DOI] [PubMed] [Google Scholar]
- Levine S., Magee W. E., Hamilton R. D., Miller O. V. Effect of interferon on early enzyme and viral DNA synthesis in vaccinia virus infections. Virology. 1967 May;32(1):33–40. doi: 10.1016/0042-6822(67)90249-8. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Levine S., Miller O. V., Hamilton R. D. Inhibition by interferon of the uncoating of vaccinia virus. Virology. 1968 Aug;35(4):505–511. doi: 10.1016/0042-6822(68)90280-8. [DOI] [PubMed] [Google Scholar]
- Pollack R., Goldman R. Synthesis of infective poliovirus in BSC-1 monkey cells enucleated with cytochalasin B. Science. 1973 Mar 2;179(4076):915–916. doi: 10.1126/science.179.4076.915. [DOI] [PubMed] [Google Scholar]
- Poste G. Enucleation of mammalian cells by cytochalasin B. I. Characterization of anucleate cells. Exp Cell Res. 1972 Aug;73(2):273–286. doi: 10.1016/0014-4827(72)90049-3. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]



