Summary
We report the histological findings in two patients treated using Guglielmi detachable coils with almost complete occlusion of the aneurysms. Autopsies of these patients were performed one week and one year after GDC embolization respectively. In one aneurysm that was obtained at autopsy one week after embolization, the histological findings revealed coils and an unorganized thrombus-filled aneurysm sac; an incomplete cell-lining on the luminal side of fibrin thrombi in the region of the neck of the aneurysm was recognized. In the other aneurysm in which autopsy was performed one year after embolization, an organized fibrous tissue at the margin of the aneurysmal wall and vascular granulation tissue at the center of the aneurysm were observed. There is a single layer of endothelium covering fibrous tissue in the neck of the aneurysm. We discuss the healing process after GDC treatment.
Key words: cerebral aneurysm, Guglielmi detachable coil, embolization, pathological findings
Introduction
Since aneurysmal embolization by means of Guglielmi detachable coil (GDC) was first introduced by Guglielmi and colleagues in 1991, this treatment has become a popular alternative to conventional surgery for cerebral aneurysms. Although many investigators reported the clinical effectiveness of this treatment to prevent rebleeding2-4, there is a paucity of data regarding histologic changes after embolization in humans. This study is important for understanding the mechanism of the healing process after intraaneurysmal embolization using GDC. We report two cases with cerebral aneurysms successfully embolized with GDCs, and studied pathologically one week and one year after treatment respectively.
Case Reports
Case 1
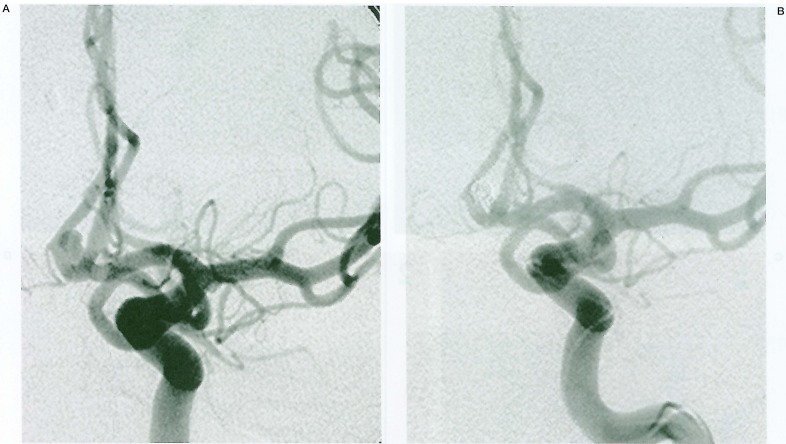
A 52-year-old man presented to an emergency room with a Hunt & Hess grade IV subarachnoid haemorrhage (SAH). Computed tomography (CT) revealed a Fisher group 3 SAH. Emergency cerebral angiography demonstrated a small anterior communicating artery aneurysm (figure 1A). The aneurysm was 4×5×4 mm in size, and size of aneurysmal neck was 2 mm. The patient was believed to be a poor surgical candidate because of associated medical comorbidities.
Figure 1.
Serial angiograms in Case 1. A) A left carotid angiography shows a small aneurysm in the anterior communication artery. B) Almost complete obliteration of the aneurysm is achieved.
Endovascular Treatment: After informed consent was obtained, we proceeded with GDC treatment following a diagnostic angiography under general anesthesia. Three coils of GDC 10 were deployed without any complications. There was good packing of the aneurysm with a 3-mm × 6-cm, a 3-mm × 2-cm and a 2mm × 6-cm GDC 10. An angiogram immediately after the procedure demonstrated a small neck remnant and patency of the parent vessels (figure 1B).
Posttreatment Course: There was no evidence of subsequent rebleeding. The patient, however, died due to brain swelling seven days after the procedure.
Neuropathological Findings: The formalin-fixed aneurysmal specimen was embedded in methyl methacrylate, and the complete specimen was sectioned with a diamond saw. The sections were then stained with hematoxylineosin (HE) and phosphotungstic acid hematoxylin (PATH). On microscopic examination, coils and an unorganized thrombus, which composed of fibrin and red blood cell, filled aneurysm sac (figure 2A). Near the aneurysmal neck the fibrin net was densely infiltrated (figure 2C). An incomplete cell-lining on the luminal side of fibrin thrombi in the region of the neck of the aneurysm was recognized. This celllining was composed of endothelial cell or fibroblast (figures 2B,D).
Figure 2.
Light microscopic examination of case 1. The coils within the aneurysm cavity are coated by fibrin and red cells (Hematoxylin and eosin stain: magnification × 2.5) (A). Near the aneurysmal neck the fibrin net is densely infiltrated (Phosphotungstic acid hematoxylin: magnification × 16) (C). An incomplete cell-lining on the luminal side of fibrin thrombi in the region of the neck of the aneurysm was recognized (Hematoxylin and eosin stain: magnification × 50) (B). This cell-lining is composed of endothelial cells or fibroblasts (Hematoxylin and eosin stain: magnification × 80) (D).
Case 2
A 60-year-old man was referred to our hospital with a one-month history of headaches. Magnetic resonance images revealed two brain tumors situated at the right occipital lobe and left temporal lobe. Magnetic resonance angiography showed an unruptured aneurysm at the origin of the right posterior communicating artery. We diagnosed metastatic brain tumor from lung cancer by results of lung CT and serum examination. Cerebral angiography demonstrated a small aneurysm in the right posterior communicating artery. The aneurysm measured 9×4×4 mm with a small neck (3 mm).
Endovascular Treatment: The aneurysm was treated with an endovascular technique one week before removal of the metastatic brain tumor. Eight GDCs were deposited within the aneurysm cavity. Total length of GDC was 54 cm. Almost complete obliteration of the aneurysm was obtained without any complications.
Posttreatment Course: Follow-up angiography was performed at approximately seven months after the procedure (figure 3A). Follow-up angiograms showed almost complete obliteration of the aneurysm and the rate of aneurysmal embolization did not change. There were no episodes of aneurysmal bleeding or cerebral ischemia during the follow-up period. The patient died due to recurrence of lung cancer one year after the procedure.
Figure 3.
A) In case 2, a right carotid angiogram 7 months after embolization demonstrates almost complete occlusion of the aneurysm. B)Gross pathological examination of case 2. The aneurysmal neck was covered completely by thin membrane.
Neuropathological Findings: On gross examination, the aneurysmal neck was covered completely by a thin membrane (figure 3B). Microscopic examination revealed that an organized fibrous tissue existed at the margin of the aneurysmal wall (figure 4A). The center of the aneurysm contained vascular granulation tissue. These findings suggested that maturation proceeded from the periphery (figure 4B). There was a single layer of endothelium covering fibrous tissue in the neck of the aneurysm (figure 4C)
Figure 4.
Histological findings of case 2 (Hematoxylin and eosin stain). Coils and an organized fibrous tissue filled aneurysm sac (magnification × 2.5) (A). The center of the aneurysm contained vascular granulation tissue (magnification × 10) (B). There is a single layer of endothelium covering fibrous tissue in the neck of the aneurysm (magnification × 25) (C).
Discussion
The goal of GDC treatment for a cerebral aneurysm is its complete isolation from the parent artery. Pathologically complete obliteration of an aneurysm after GDC treatment could be obtained by proof of endothelialization at the orifice and organized thrombi filling the fundus. In order to follow patients after GDC treatment, it is very important to understand the histological healing process after this treatment. Therefore, pathological analyses of embolized aneurysm are needed. In previous experimental studies using animals, complete isolation of aneurysms from the parent artery in angiographic and pathological examinations has been reported after GDC treatment 5-8. Limiting factors to apply experimental studies using animals to human aneurysms are the following: requirement of surgical intervention, usage of cervical artery and vein pouch, differences in coagulation system and differences in haemodynamics. There is a lack of histological data in human subjects regarding the changes found in aneurysms treated with GDC embolization. To date, histological findings of 25 patients with 28 embolized aneurysms at autopsy, including our two cases, have been reported (table 1) 9-18. The healing process after the coiling suspected from the data of previous reports is the following. By a deployment of the coils within the aneurysm, the aneurysm is isolated from the parent artery transiently by an unorganized thrombus within the aneurysm and an intraaneurysmal blood flow was stagnant. This stagnation of intraaneurysmal blood flow led to the infiltration of fibrin surrounding the deposited coils. Near the aneurysmal neck the fibrin net is densely infiltrated in the early period after coiling as in our Case 1. Early membrane formation covering fibrin mesh-work was occurred from the luminal sides of parent artery. Histologically, this membrane was composed of fibroblasts or endothelial cells 11. This membrane made isolation of theembolized aneurysm from parent artery firmer, and this phenomenon promoted the appearance of endothelialization across the neck and an organized thrombus within the aneurysm. In the largest series with histological data at the autopsy after coiling, Bavinzski et Al9 reported that unorganized thrombi were found in aneurysms within one week earlier, and replacement of the intraluminal blood clot by fibrous tissue and partial membrane covering at the neck were observed two or three weeks after the treatment. Furthermore, they reported that in the aneurysm six weeks after treatment, a complete endothelialization across the neck was recognized. Wound healing normally completes within one week after tissue injury. Based on previous studies in humans with embolized aneurysm, it takes a longer time than expected when compared with normal wound healing. This delayed healing might contribute to not only intrinsically poor vascularity of the wall of the aneurysm but also the difference in the degree of aneurysmal embolization, especially at the neck side.
Table 1.
Summary of autopsy cases in humans
| size | No. | histological findings | angiographical results (%) |
time after embolization |
||
| sac | neck | neck | sac | |||
| small | small | 4 | endothelialization | organized thrombus | 95 (3), 100 | 40d, 12m, 33m (2) |
| 18 ans | 6 | membrane | unorganized thrombus | 90, 100 (5) | 36h, 6d, 9d, 14d (2), 4w | |
| 4 | incomplete membrane | unorganized thrombus | 90 (3), 100 | 7d, 11d, 12d, 4w | ||
| 4 | no membrane | unorganized thrombus | 90, 95, 100 (2) | 3d, 4d, 26d | ||
| large | wide | 1 | membrane | unorganized thrombus | 100 | 17d |
| 6 ans | 3 | incomplete membrane | unorganized thrombus | 90, 100 (2) | 11d, 19d, 22d | |
| 2 | no membrane | unorganized thrombus | 95,100 | 7d, 8m | ||
| giant | wide | 4 | no membrane | unorganized thrombus | 40, 90 95 100 | 5d, 7d, 2m, 6m |
| 4 ans | ||||||
| * ans: aneurysms, d: days, m: month. | ||||||
In the healing process after the GDC treatment, the appearance of a membrane across the neck during the early phase after the treatment could be a very important step in determining whether the aneurysm goes on to obliteration or recanalization 17. The histological findings of our Case 1 indicated that the fibrin meshwork near the neck might function as a scaffold for promoting early membrane formation across the neck. We thought the early membrane formation allowed the early migration of endothelial cells, which led to endothelializaton covering the aneurysmal orifice. When early membrane formation could not be obtained, immature thrombus within the aneurysm was lysed by continuous exposure to blood flow and this fibrinolysis caused the recanalization during follow-up period. From clinical data, it is well known that large or giant aneurysms with wide necks were likely to cause recanalization during the follow-up period 19-20. The pathological findings in many large or giant aneurysms with wide necks demonstrated tiny open spaces between the coils at the neck, even if angiographically complete occlusion was obtained. This tiny open spaces might prevent complete membrane formation covering in the region of the neck. To date, only four reports including our Case 2 are available on complete occlusion after coiling in humans was proved pathologically 9,10. All four aneurysms were small aneurysms with small necks. Requisites of pathologically complete occlusion of the aneurysm treated with GDCs might be an giographically complete obliteration in small aneurysms with small necks. Therefore, this may be critical to the achievement of complete obliteration by endovascular means.
Conclusions
We reported histological findings of two patients, who performed autopsies one week and one year after the procedure respectively. The formation of a membrane covering fibrin thrombi in the region of the neck is thought to play an important role in achieving complete aneurysmal obliteration. Biological response after endovascular embolization regarding mechanism of occlusion, coil compaction and regrowth should be evaluated by gathering a large number of postmortem cases.
References
- 1.Guglielmi G, Viñuela F, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77:515–524. doi: 10.3171/jns.1992.77.4.0515. [DOI] [PubMed] [Google Scholar]
- 2.Byrne J, Molybeux A, et al. Embolization of recently ruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1995;59:616–620. doi: 10.1136/jnnp.59.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malisch T, Guglielmi G, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm results in a consecutive series of 100 patients. J Neurosurg. 1997;87:176–183. doi: 10.3171/jns.1997.87.2.0176. [DOI] [PubMed] [Google Scholar]
- 4.Graves V, Strother C, et al. Early Treatment of Ruptured Aneurysms with Guglielmi Detachable Ciols: Effect on Subsequent Bleeding. Neurosurgery. 1995;37:640–648. doi: 10.1227/00006123-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Byrne J, Hope J, et al. The Nature of Thrombosis Induced by Platinum and Tungsten Coils in Saccular Aneurysms. Am J Neuroradiol. 1997;18:29–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Pile-Spellman J, Wu J. Coil Embolization of Aneurysms: Angiographic and Histologic Changes. Am J Neuroradiol. 1997;18:43–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Mawad M, Mawad J, et al. Long-term Histopathologic Changes in Canine Aneurysms Embolized with Guglielmi Detachable Coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Tenjin H, Fushiki S, et al. Effect of Guglielmi Detachable Coils on Experimental Carotid Artery Aneurysms in Primates. Stroke. 1995;26:2075–2080. doi: 10.1161/01.str.26.11.2075. [DOI] [PubMed] [Google Scholar]
- 9.Bavinzski G, Talazoglu V, et al. Gross and microscopic histological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 2001;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 10.Castro E, Fortea F, et al. Long-term Histopathologic Findings in Two Cerebral Aneurysms Embolized with Guglielmi Detachable Coils. Am J Neuroradiol. 1999;20:549–552. [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz M, Purdy P, et al. Scanning Electron Microscopic Findings in a Basilar Tip Aneurysm Embolized with Guglielmi Detachable Coils. Am J Neuroradiol. 1997;18:688–690. [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi T, Kawano T, et al. Histological findings in aneurysm treated interlocking detachable coils: scanning electron microscopical study (in Japanese) Neurol Surg. 1997;25:1027–1031. [PubMed] [Google Scholar]
- 13.Manabe H, Fujita S, et al. Rerupture of coil-embolized aneurysm during long-term observation. J Neurosurg. 1998;88:1096–1098. doi: 10.3171/jns.1998.88.6.1096. [DOI] [PubMed] [Google Scholar]
- 14.Molyneux A, Ellison D, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 15.Otawara Y, Sugawara T, et al. An autopsy case of a ruptured cerebral aneurysm treated with interlocking detachable coils (in Japanese) Neurol Surg. 1997;25:829–933. [PubMed] [Google Scholar]
- 16.Shimizu S, Kurata A, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. Am J Neuroradiol. 1999;20:546–548. [PMC free article] [PubMed] [Google Scholar]
- 17.Stiver S, Porter PI, et al. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: Case report and review of the literature. Neurosurgery. 1998;43:1203–1208. doi: 10.1097/00006123-199811000-00106. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi T, Kawano T, et al. Histological findings in aneurysm treated interlocking detachable coils: scanning electron microscopical study (in japanese) Neurol Surg. 1997;25:1027–1031. [PubMed] [Google Scholar]
- 19.Hayakawa M, Murayama Y, et al. Natural history of neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 20.Hope J, Byrne J, et al. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. Am J Neuroradiol. 1999;20:391–399. [PMC free article] [PubMed] [Google Scholar]