Summary
The purpose of this study was to evaluate the role of the endothelial cell reaction after endovascular coil embolization for the treatment of intracranial aneurysms. A scanning electron microscopic (SEM) study of the platinum coil, embolized into a middle cerebral aneurysm in a 35-year-old woman and subsequently removed surgically eight months later; revealed no endothelial coverage on the coil.
This finding prompted us to perform experimental studies. In the first in vitro study, endothelial cells from gerbil brain microvessels and canine carotid arteries were co-cultured with either bare-form platinum coils or type-1 collagen-coated coils for up to three weeks, and the endothelial cell population on the coils was ascertained. In the second in vivo study, platinum coils coated with type-1 collagen were delivered endovascularly into canine carotid arteries, while the contralateral side was treated with bare-form coils, and endothelialization over the coil was investigated.
SEM studies revealed that no endothelial cells, either from gerbil brain microvessels or from canine carotid artery, were found on the uncoated coils, whereas gerbil endothelial cells began to proliferate on the collagen-coated coils in three days, covering extensively in one week and reaching confluence in two weeks in vitro. The in vivo canine study demonstrated that bare-form platinum coils did not show endothelial coverage until two weeks, but endothelial cells proliferated directly on the collagen-coated coils in three days, and coils were completely covered in two weeks.
These results supported the SEM study of our case and several human histopathological reports in the literature in that endothelial cell coverage in the orifice of the intracranial aneurysm is exceptional after endovascular treatment. But if some extracellular matrix, like collagen in our study, is prepared, coverage could be possible, as is seen in a few human cases. Biological modification of the platinum coils, such as collagen coating, is awaited for the better long-term results of endovascular coil embolization without recanalization of the treated intracranial aneurysms.
Key words: aneurysms, collagen coating, endothelial cells, GDC, modification
Introduction
Intracranial aneurysms are increasingly treated by endovascular methods, mainly with Guglielmi detachable coils (GDCs). One drawback of endovascular coiling of aneurysms is the high incidence of recurrent lesions (recanalization of the aneurysm). Follow-up angiography has revealed recanalization of aneurysms with recurrences in 10 to 20% of patients Mechanisms involved in recurrence after coil embolization of human aneurysms have not been determined, and healing mechanisms or failure, particularly endothelial reactions, are poorly understood in human aneurysms treated by coil embolization.
In this paper, using cultured endothelial cells, in vitro and in vivo scanning electron microscopic (SEM) studies were performed to elucidate the role of neo-endotheliaization in the endovascular treatment of aneurysms. All described studies were inspired by SEM findings in a patient with unruptured left middle cerebral artery (MCA) aneurysm treated by endovascular coil embolization and the coils were subsequently removed surgically after partial migration of a coil.
Material and Methods
Clinical Material
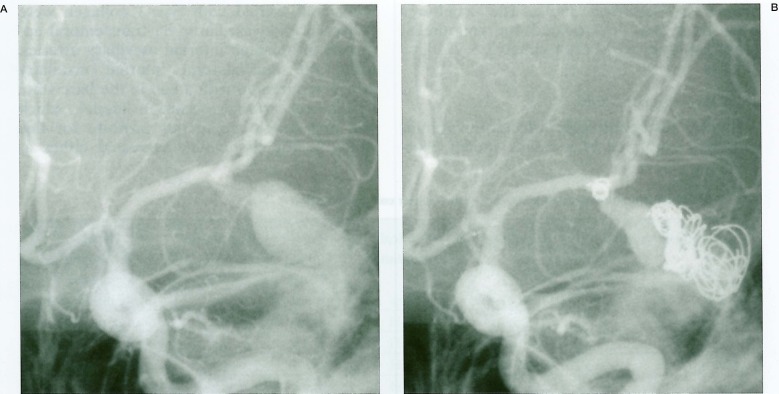
A 35-year-old woman presented for treatment of a giant cerebral aneurysm measuring 30 × 25 × 30 mm with a small neck at the left MCA trifurcation (figure 1A). The patient was treated by endovascular technique in two sessions using mechanical detachable coils (Target Therapeutics, CA).
The tail end of the last coil (2 mm × 4 cm) protruded into the parent artery. Immediate retrieval was not considered because of minimum protrusion of the coil. The patient was systemically heparinized during the proedure and for three days and oral anticoagulation was continued for three months. Clinically, the patient remained neurologically intact except for a transient right-hand numbness after five days. One-week follow-up angiography showed partial migration of the coil into the posterior branch of the MCA.
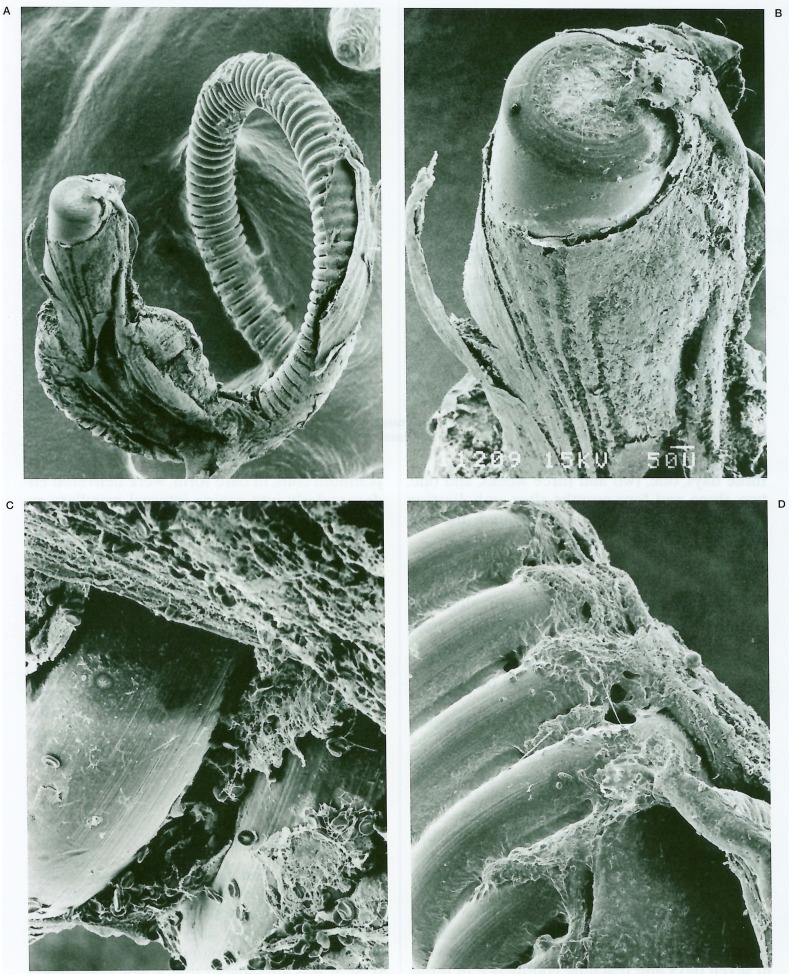
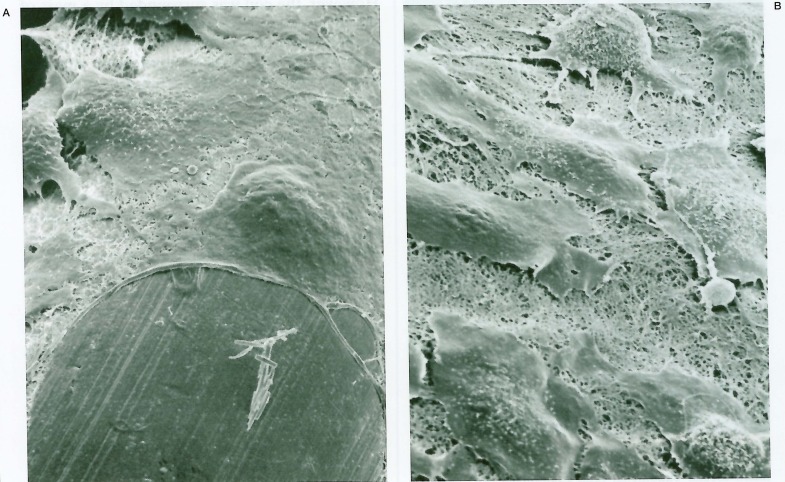
The eight month follow-up angiography showed unchanged coil position and enlargement of the residual aneurysm cavity (figure 1B); surgical exploration was conducted. Under temporary trapping, an aneurysmotomy was performed and the partially migrated coil was withdrawn gently through the aneurysm cavity. The aneurysm was excluded from the circulation by single neck clipping. The removed coil was submitted for SEM study. A mass of thrombus, composed primarily of red blood cells embedded in rich fibrinous tissue, was adherent around the end of the coil. The thrombus was laminated in some parts, presumably in the blood flow direction, and a lamina of thrombus projected like a wing (figures 2A,B). However, there was no evidence of endothelial coverage over the coil (figures 2C,D). A report of this case was published in 1994 3,4.
Figure 1.
Left internal carotid angiography of the case. A) Initial angiogram demonstrating left middle artery giant aneurysm. B) Angiogram 8 months after the two sessions of endovascular coil embolization. Partial migration of a small coil into the parent artery is seen.
Figure 2.
Scanning electron photomicrographs of the removed coil of the case. A) Low-power photomicrograph shows gross appearance of the detaching end of the coil with thrombus around the coil (original magnification × 40). B) Some thrombi around the coil are laminated, and a lamina projects like a wing (× 120). C) Thrombi are composed of red blood cells in fibrous tissue (× 700). D) There is no endothelial coverage even at the site of adhesion to the vascular wall (× 240).
These findings prompted us to investigate and perform in vitro and in vivo studies to assess the role and the amount, if present, of protective endotheliaization over the platinum coils against thrombus formation and recanalization.
Experimental Studies
1) In Vitro Gerbil Brain Microvascular Endothelial Cell Study
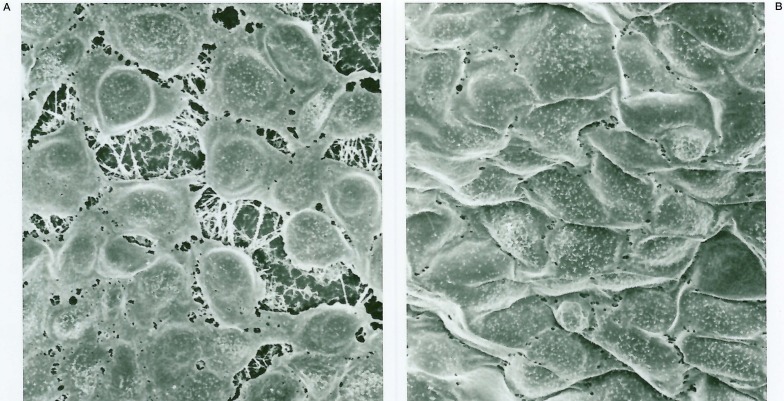
Histologic interaction of cultured endothelial cells and platinum coils were studied in vitro. Endothelial cells were isolated and cultured from gerbil brain microvessels as described elsewhere (figure 3)5. Strips of platinum coils, either in bare state or after coating with type-1 collagen, were placed on endothelial cells that had reached confluence in a culture chamber; then, coils and endothelial cells were cocultured in the CO2 incubator for either three days, one week, or two weeks.
Figure 3.
A) Scanning electron photomicrographs of the cultured gerbil brain microvascular endothelial cells on type-1 collagen fibers (× 1300). B) Endothelial cells that reached confluence with a cobblestone appearance (× 1300).
They were fixed with glutaraldehyde, dehydrated and dried, and then sputter coated with gold for SEM investigation. The endothelial cell population on the surface of the coil was ascertained. Coils that were coated with collagen in half of the length were also tested for endothelial coverage.
2) In Vitro Canine Carotid Artery Endothelial Cell Study
Since vascular healing varies from one species to another, this collagen-mediated endothelial proliferation on the surface of the platinum coils was further tested by another experimental animal study. Endothelial cells were isolated and cultured from a canine carotid artery as described elsewhere 6. Platinum coils, either in the bare state or after coating with type-1 collagen, were placed on endothelial cells and were cocultured in the same manner as described above. The cocultures were fixed and investigated for endothelial proliferation by SEM after either 2, 3, 7,14, or 21 days.
3) In Vivo Canine Study
In vivo histopathologic effect of platinum coils was evaluated in a canine model. Platinum coils coated with type-1 collagen were delivered endovascularly via transfemoral approach into canine internal maxillary arteries, while the contralateral internal maxillary artery was treated with a coil in the bare state (single-coil arterial occlusion model, which was first developed in our institute for this study7,8). The tissue was harvested either at three days, one, two and three weeks, and one, two, three and four months after embolization and was histologically investigated for endothelialization by SEM7.
Results
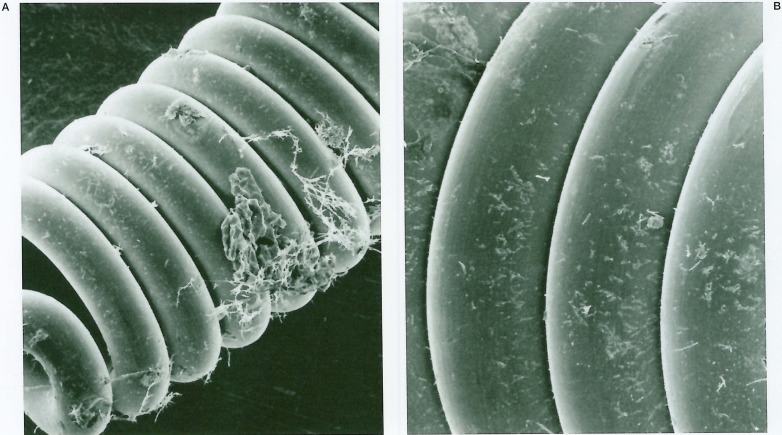
When the cocultures of the endothelial cells and platinum coils were investigated histologically in in vitro studies, no endothelial cells, either from gerbil brain microvessels or from canine carotid artery, were found on the uncoated bare platinum coils (figure 4).
Figure 4.
Scanning electron photomicrographs of the coil without collagen coating that was co-cultured with gerbil brain microvascular endothelial cells showing no endothelial coverage (A: × 290 and B: × 520).
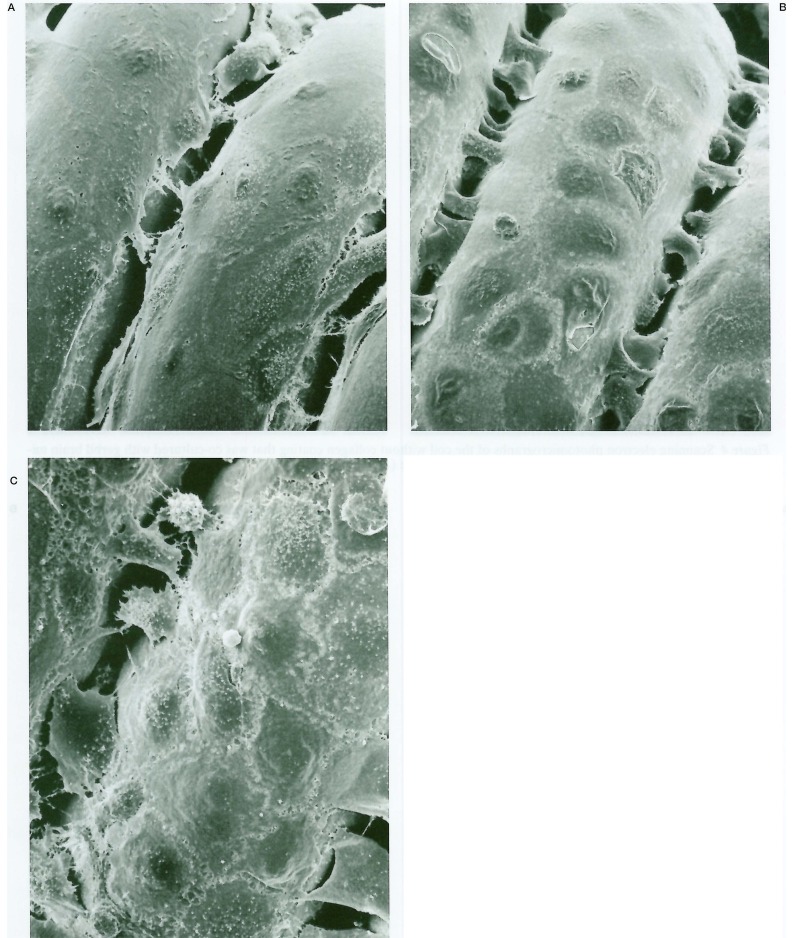
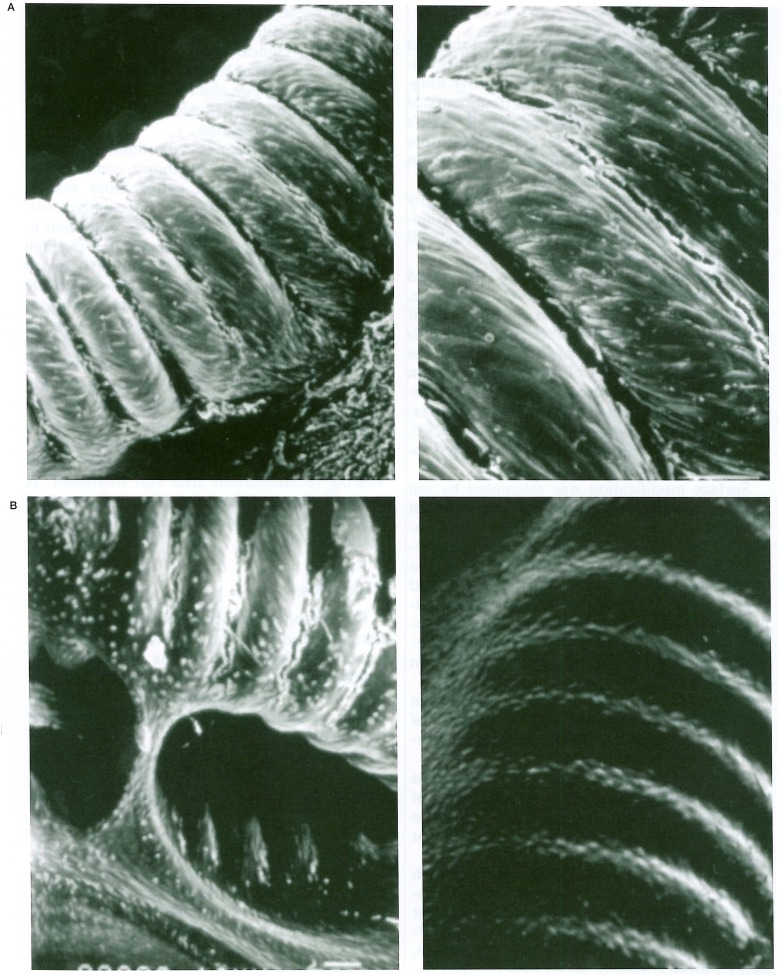
Endothelial cells were found on the collagen-coated coils, proliferating directly on the collagen fibers (figure 5). Gerbil brain endothelial cells began to proliferate on the collagen-coated platinum coils in three days, covering them extensively in one week and reaching confluence in two weeks (figure 6). Endothelial cells could proliferate only on the collagen-coated surface when coils were coated in half of the length (figure 7).
Figure 5.
Scanning electron photomicrographs of the coil with type-1 collagen coating that was co-cultured with gerbil brain microvascular endothelial cells showing endothelial cell proliferation on the coil surface. Note endothelial cells are directly proliferated on the collagen fibers (A: × 2300 and B: × 1400).
Figure 6.
Scanning electron photomicrographs of the coil with type-1 collagen coating co-cultured with gerbil brain microvascular endothelial cells. Endothelial cells begin to proliferate in 3 days (A: × 980), cover the coil extensively in one week (B: × 980), and reach confluence in 2 weeks (C: × 1000).
Figure 7.
Scanning electron photomicrographs of the coil coated with type-1 collagen in half of the length and was cocultured with gerbil brain microvascular endothelial cells. Note endothelial cells proliferate only on the collagen-coated surface (A, B: × 390).
Canine carotid endothelial cells also began to proliferate on the collagen-coated platinum coils in three days, covering them extensively in seven days and reaching confluence in three weeks6.
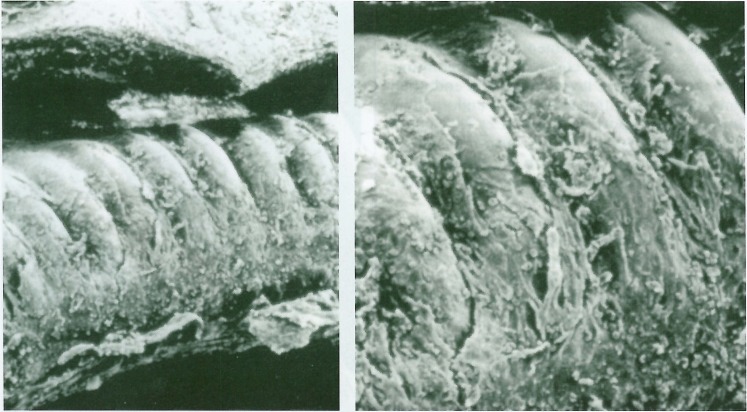
In vivo histopathologic effect of platinum coils was evaluated in a canine model. When platinum coils, in bare form, were delivered endovascularly into canine internal maxillary arteries, coils did not show endothelial coverage until two weeks after delivery (figure 8). By one month some endothelial coverage was seen on the uncoated coils, but endothelial cells proliferated only on the fibrous tissue formed on the coils.
Figure 8.
Scanning electron photomicrographs of the coil without collagen coating delivered endovascularly into canine carotid artery 2 weeks previously. Note no endothelial cell coverage and some unorganized clot on the coil (× 190 and × 350).
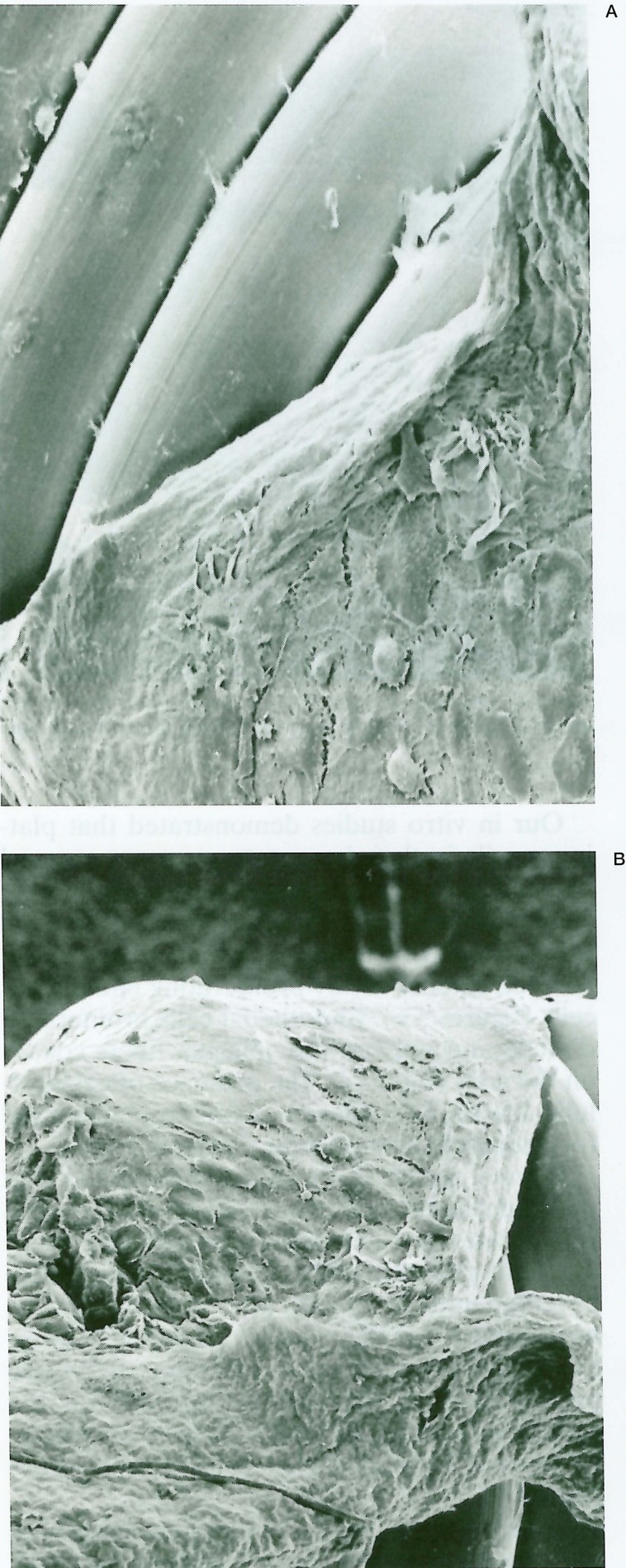
Instead, endothelial cells proliferated directly on the surface of the collagen-coated coils three days after delivery, and the coils were completely covered by endothelial cells in two weeks (figure 9A). After two months, endothelial cell showed a typical mosaic pattern of elongated spindle-shaped cells in flow direction7 (figure 9B).
Figure 9.
Scanning electron photomicrographs of the coil coated with type-1 collagen delivered endovascularly into canine carotid artery. A) The coil is completely covered by endothelial cells 2 weeks after delivery (left × 220 and right × 350). B) After 2 months, endothelial cells show a typical mosaic pattern of elongated spindle-shaped cells in flow direction (left × 250 and right × 280).
Discussion
Human histological studies
In 1994, we first reported a human clinico-pathological study of the platinum coil embolized into a cerebral aneurysm that was removed eight months after the embolization3. Before the histopathological investigation by SEM, it was anticipated that a new endothelial layer had developed over the coil and had played a protective role against thrombus formation, because most of the reported experimental animal studies had demonstrated neoendothelialization on the embolized coils 9. However, the coil was surrounded by a mass of thrombus and there was no evidence of endothelial coverage over the coil at all (figure 2). This case prompted us to investigate and perform in vitro and in vivo studies to assess endothelialization over the platinum coils after endovascular embolization.
In 1995, Molyneux et A110 reported two autopsy human cases who had been treated by GDCs two and six months previously. In both cases, coils were embedded in unorganized thrombus and no evidence of endothelialization was found at the aneurysm neck. Mizoi et A111 reported the histological findings of an anterior communicating artery aneurysm that had been treated by GDCs and was subsequently resected after six months due to aneurysm recanalization. In this case, the coils were directly exposed to the blood circulation without neointimal formation.
Following these reports, several human histopathological case reports revealed similar findings, namely fibrin membrane coverage of the coils and lack of re-endothelialization in the neck, indicating the potential risk of recanalization of the aneurysm 12-18.
Exceptionally, some neo-endothelialization over the coils were also reported. In the autopsy study reported by Castro et Al 19, one carotid-ophthalmic aneurysm was examined 33 months after GDC treatment: the ostium of the aneurysm was fully covered by a neointima, and the superficial layer comprised endothelium continuous with parent artery endothelium. In the other report by Koizumi et Al20, partial endothelial coverage on the surface of the coil was found in a carotid aneurysm, which was examined histologically four weeks after coil embolization.
In the largest histopathological series of GDC-treated aneurysms in humans (16 patients from whom 17 aneurysms were retrieved post mortem and one during surgical resection), Bavinzski et Al9 reported a sequential process of membranous reaction at the orifice of the aneurysms. During the first week after coiling, naked GDCs were embedded in fresh clot and coils were covered by fibrin; then, partial thin membrane, composed of fibrin, macrophages, and fibroblast, covered the coils in one to two weeks. Microscopically, no endothelial cell was found in the orifice of any of the cases in this period. Only one small narrow neck basilar tip aneurysm examined 40 days post GDC treatment showed endothelial lining on the granulation tissue membrane that covered the coils.
Table 1 summarizes the reported case in the literature; none of the 20 cases that were examined within one month after GDC treatment had any evidence of neo-endothelial coverage of the coils, whereas three of 11 cases examined one to 33 months after treatment showed some endothelial cell lining on the connective tissue. It seems that neo-endothelial coverage is exceptional in the present GDC technology, but may occur at least one month after embolization if conditions are prepared, although this remains unexplained so far.
Table 1.
Human histopathological studies after endovascular coil embolization
| Author, year (ref No.) | Aneurysm | Clinical Presentation |
Post- Coiling Period |
Endothelial Cell Coverage |
Other Histological Structures |
|---|---|---|---|---|---|
| Ozawa, 19943 | MCA giant | mass | 8 mo | no | unorganized clot |
| Molyneux, 199510 | IC giant | SAH | 2 mo | no | unorganized clot |
| BA trunk giant | SAH | 6 mo | no | unorganized clot | |
| Mizoi, 199611 | ACoA large | SAH | 18 mo | no | unorganized clot |
| Horowitz, 199712 | BA tip | SAH | 4 mo | no | thin film of fibrin |
| Otawara, 199713 | IC | SAH | 1 mo | no | unorganized clot |
| Koizumi, 199720 | IC | SAH | 1 mo | partial | no clot |
| Manabe, 199814 | IC large | SAH | 8 mo | no | unorganized clot |
| Stiver, 199815 | ACoA | SAH | 2 day | no | fibrin membrane |
| Takechi, 199816 | MCA | SAH | 12 day | no | fibrin membrane |
| BA tip | SAH | 1 day | no | fibrin membrane | |
| Shimizu, 199917 | BA tip | SAH | 6 mo | no | partially organized |
| Castro, 199919 | IC-oph | incident | 33 mo | yes | mature scar |
| Bavinzski, 19999 | PCoA | SAH | 3 day | no | fresh clot |
| PCoA | SAH | 3 day | no | fresh clot | |
| PCoA | SAH | 4 day | no | fresh clot | |
| SCA giant | mass | 5 day | no | fresh clot | |
| ACoA | SAH | 6 day | no | thin membrane | |
| PCoA large | SAH | 7 day | no | fresh clot | |
| PCoA giant | mass | 7 day | no | fresh clot | |
| PICA | SAH | 9 day | no | thin membrane | |
| BA tip | SAH | 11 day | no | partial membrane | |
| PCoA large | SAH | 11 day | no | partial membrane | |
| PCoA | SAH | 12 day | no | partial membrane | |
| ACoA | SAH | 14 day | no | thin membrane | |
| BA tip | SAH | 14 day | no | thin membrane | |
| BA tip large | SAH | 17 day | no | thin membrane | |
| PCoA large | SAH | 19 day | no | partial membrane | |
| IC-oph large | SAH | 22 day | no | partial membrane | |
| BA tip | SAH | 40 day | yes | organized clot | |
| Asai, 200018 | BA tip large | SAH | 25 days | no | fibrin membrane |
|
ACoA: anterior communicating artery, BA: basilar artery, IC: internal carotid artery, IC-oph: internal carotid-ophthalmic artery, MCA: middle cerebral artery, PCoA: posterior communicating artery, PICA: posterior inferior cerebellar artery, SCA: superior cerebellar artery, SAH: subarachnoid haemorrhage, mass: mass effect, incident: incidentally found. | |||||
Experimental Studies
In 1991, Guglielmi et Al reported endothelial covering of the aneurysm orifice after GDC treatment in pigs21. After this, several experimental reports supported the findings that if platinum coils are tightly packed in the aneurysm cavity, a new intimal layer is shown to cover the neck of aneurysm in swine, canine, and primate models 9,22. However, complete endothelialization was never seen in rabbit carotid aneurysm model23.
Our in vitro studies demonstrated that platinum coils, in their bare state, were not covered by endothelial cells in either gerbil and canine studies (figure 4). When the surface of the coil was coated with collagen, however, they were covered extensively by proliferating endothelial cells (figures 5,6). Endothelial cells proliferated directly and only on the collagen fibers coated on the coils (figure 7). The presence of extracellular matrix, i.e. collagen, seems to be essential for endothelialization over platinum coils.
In vivo canine study also demonstrated that the endothelial cell proliferation on the coils was accelerated extensively by collagen coating; bare platinum coils showed no endothelial coverage until two weeks after delivery into canine internal maxillary artery (figure 8), whereas collagen-coated coils exhibited extensive proliferation of the endothelial cells on the coils in three days and the coils were completely covered by endothelial cells in two weeks (figure 9).
These in vitro and in vivo results supported the results obtained in our clinical material. There was no endothelial coverage on the surface of bare-formed platinum coil removed from the aneurysm after eight months.
Our in vivo study, on the other hand, demonstrated that some endothelial cell coverage could be seen even on the bare coils by one month; the coverage, however, was limited to the fibrous tissue formed on the coil. This finding is compatible with the results of reported human materials; in some exceptional cases, endothelial lining over the fibrous membrane could be seen after one month of coil embolization as described above 9,19,20.
The degree and timing of extracellular matrix formation on the fibrous tissue as well as coil embolization density and size of aneurysm/ neck may contribute to this exceptional endothelial lining, but it could not be elucidated from our studies.
Our in vivo canine study had results very similar to human histopathological studies. Most reported experimental aneurysms are sidewall aneurysms or venous pouches, and the trauma caused by surgical procedures may produce thrombosis and fibrosis. We used a simple canine experimental study, in which coils were endovascularly delivered into the carotid artery lumens (single-coil arterial occlusion method7,8). We believe that this in vivo method is useful and essential to evaluate much less artificial histopathological reactions after embolization of the coils.
GDC Modification
GDCs are inert biologically and promote a limited inflammatory response and scar formation in aneurysms24. As a way to improve long-term results of endovascular treatment of aneurysms, biological modification of GDCs have been attempted and several investigators have reported GDC modifications in both in vitro and in vivo studies (table 2).
Table 2.
Biologically active platinum coils (experimental)
| Authors, year | Modification of the Coils | Description of the Effect |
|---|---|---|
| Ahuja and Hergenrother, 199325 | polyurethane coated platinum coils | increased thrombogenesity |
| Dawson and Krisht, 199526 | collagen-coated platinum coils | increased thrombogenesity and endothelialization |
| Dawson and Shengelaia, 199627 | collagen-core platinum coils | increased local fibroblast |
| Szikora and Wakhloo, 199728 | collagen-filled platinum coils | increased local fibroblast |
| Tamatani and Ozawa, 19976 | collagen-coated platinum coils | increased thrombogenesity and endothelialization |
| Murayama and Viñuela, 199729,199924 | surface modified by ion implantation and protein coated platinum coils |
fastened re-endothelial coverage |
| Murayama and Viñuela, 200131 | bioabsorbable copolymer-coated platinum coils |
accelerated fibrosis and neointima formation |
| Shimozuru and Kamezawa, 200130 | hydroxyapatite-coated platinum coils |
increased fibroblast response and endothelialization |
| Abrahams and Forman, 200132 | biodegradable polyglycolide suture coils |
increased cellular response and intimal hyperplasia |
| Marx and Cloft, 200134 | fibroblast coated platinum coils |
fibroblast progresses cellular proliferation |
| Abrahams and Forman, 200133 | collagen and recombinant human VEGF coated platinum coil |
enhanced fibrosis and wall thickening |
| Raymond and Leblanc, 20028 | radioactive (32P ion implantation) platinum coils |
inhibited recanalization of intima |
In our studies, we proposed a modification by collagen coating and demonstrated that collagen coating induced extensive endothelial cell coverage over platinum coils.
Other investigators have also attempted GDC modification by coating technique. Polyurethane-coated platinum coils in a rabbit study increased coil thrombogenicity but also changed its physical characteristics such as shape and softness25. Collagen-coated coils in swine experimental model demonstrated increased thrombogenicity and re-endothelialization across the venous-pouch aneurysm neck26. Collagen-filled coils in swine model showed local increase in fibroblast27,28.
Surface modification was attempted by ion implantation technique coupled with collagen or other protein coating: more intense inflammatory response in the aneurysm and faster re-endothelial coverage of the neck of the aneurysm were indicated in in vitro bovine and in vivo swine studies24,29. Hydroxyapatite coating of platinum coils, which mimics extracellular matrix surface, had increased fibroblast response and endothelial coverage of the neck in swine model30.
Bioabsorbable copolymer-coated platinum coils accelerated and intensified the degree of aneurysm scarring and neck neointimal formation in swine model 26.
Biodegradable coils, in which platinum was replaced with biodegradable sutures made of polyglycolide, exhibited a marked increase in cellular response and intimal hyperplasia in rat ligated carotid arteries32.
Fibroblast or vascular endothelial growth factor coated on platinum coils enhanced cellular proliferation and wall thickening in rat carotid arteries 33,34. Radioactive coils (32P-coils), produced by ion implantation technique, showed more complete neointimal (fibrous tissue) coverage and inhibited recanalization in the canine, porcine, and rabbit single-coil arterial occlusion models, and in the canine experimental aneurysm8. It is not known which of these coil modification techniques is being considered for clinical application, but we speculate that the earlier and more intense transformation of aneurysm clot into fibrous scar tissue without re-endothelialization cannot decrease aneurysm recanalization.
The organized scar or neointima, a non-specific vascular response to injury, composed of mesenchymal cells and extracellular matrix, would be re-vasculalized by neocapillary formation, which, in a long process, could lead to tissue degeneration and recanalization through the channels that restore circulation between the occluded cavity and the parent artery. Only neo-endothelialization across the aneurysm neck, or at least on the surface of coils facing the lumen of the parent artery, ensures a living nonthrombogenic surface that completely excludes the aneurysm cavity from circulation and protects from recanalization of the aneurysm.
At this point, the extracellular matrix, especially collagen, seems to play a key role in preventing recanalization after coil treatment, as most of the modifications using collagen had evidence of neo-endothelialization over the platinum coils.
Conclusions
Our histopathological and other human histological studies in the literature indicate that neo-endothelialization in the orifice of the aneurysm can occur after one month, but it is very limited and exceptional in the present GDC technology for intracranial aneurysm treatment.
The reason and mechanism of the failure of neo-endothelialization are unclear. Our gerbil and canine in vitro studies demonstrated that bare-formed platinum coils would not be covered by endothelial cells when coils were cocultured with endothelial cells, whereas collagen-coated platinum coils were rapidly and extensively covered by endothelial cells. An in vivo single-coil arterial occlusion canine model confirmed acceleration of endothelial cell proliferation over the collagen-coated coils. Collagen, as an extracellular matrix, seems to play a key role in endothelial proliferation.
Modifications of GDCs to enhance the biological activity and to induce endothelialization, such as collagen-coating, are under experimental tests.
We hope some modification will promise effective aneurysmal treatment with prevention of recanalization in the endovascular GDC technology for intracranial aneurysms.
Acknowledgements
The authors express their thanks to Kiyoshi Uesugi and Kazuo Adachi for their assistance in scanning electron microscopic work.
This work was supported by the Grant-in-Aid for Exploratory Research from the Japan Society for the Promotion of Science (No. 13877221)
References
- 1.Byrne JV, Sohn MJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: Outcome and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa M, Murayama Y, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa T, Koike T, et al. Scanning electron microscopical study of a partially migrated coil after giant middle cerebral artery aneurysm embolization. Proc Jpn Society for Intravascular Neurosurgery (suppl) 1994;10:107–110. [Google Scholar]
- 4.Ozawa T, Koike T, et al. Scanning electron microscopic study of the migrated platinum coil after endovascular embolization of a giant cerebral aneurysm. Am J Neuroradiol. 1998;19:594–595. [PMC free article] [PubMed] [Google Scholar]
- 5.Minakawa T. Long-term culture of microvascular endothelial cells derived from Mongolian gerbil brain. Stroke. 1989;20:947–951. doi: 10.1161/01.str.20.7.947. [DOI] [PubMed] [Google Scholar]
- 6.Tamatani S, Ozawa T, et al. Histological interaction of cultured endothelial cells and endovascular embolic materials coated with extracellular matrix. J Neurosurg. 1997;86:109–412. doi: 10.3171/jns.1997.86.1.0109. [DOI] [PubMed] [Google Scholar]
- 7.Tamatani S, Ozawa T, et al. Radiologic and histopathologic evaluation of canine artery occlusion after collagen-coated platinum microcoil delivery. Am J Neuroradiol. 1999;20:541–545. [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond J, Leblanc P, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke. 2002;33:421–427. doi: 10.1161/hs0202.104474. [DOI] [PubMed] [Google Scholar]
- 9.Bavinzki G, Talazoglu V, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux A, Ellison D, et al. Histological findings in giant aneurysm treated with Guglielmi detachable coils. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 11.Mizoi K, Yoshimoto T, et al. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery. 1996;39:165–169. doi: 10.1097/00006123-199607000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz MB, Purdy PD, et al. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi Detachable coils. Am J Neuroradiol. 1997;18:688–690. [PMC free article] [PubMed] [Google Scholar]
- 13.Otawara Y, Sugawara T, et al. An autopsy case of a ruptured cerebral aneurysm treated with interlocking detachable coils. No Shinkei Geka. 1997;25:829–833. (in Jpn with Eng Abst) [PubMed] [Google Scholar]
- 14.Manabe H, Fujita S, et al. Rerupture of coil-embolized aneurysm during long-term observation: ease report. J Neurosurg. 1998;88:1096–1098. doi: 10.3171/jns.1998.88.6.1096. [DOI] [PubMed] [Google Scholar]
- 15.Stiver S, Porter P, et al. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of he literature. Neurosurgery. 1998;43:1203–1208. doi: 10.1097/00006123-199811000-00106. [DOI] [PubMed] [Google Scholar]
- 16.Takechi A, Inagawa T, et al. Two autopsy cases with ruptured cerebral aneurysm treated by embolization. Jpn J Neurosurg (Tokyo) 1998;7:678–682. (in Jpn with Eng Abst) [Google Scholar]
- 17.Shimizu S, Kurata A, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. Am J Neuroradiol. 1999;20:546–548. [PMC free article] [PubMed] [Google Scholar]
- 18.Asai J, Suzuki R, et al. Correlation of magnetic resonance imaging and histological findings in a large basilar tip aneurysm after coil embolization: case report. Neurol Med Chir (Tokyo) 2000;40:519–523. doi: 10.2176/nmc.40.519. [DOI] [PubMed] [Google Scholar]
- 19.Castro E, Fortea F, et al. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1999;20:549–552. [PMC free article] [PubMed] [Google Scholar]
- 20.Koizumi T, Kawano T, et al. Histological findings in aneurysm treated with IDC: scanning electron microscopic study. No Shinkei Geka. 1997;25:1027–1031. (in Jpn with Eng Abst) [PubMed] [Google Scholar]
- 21.Guglielmi, Viñuela F, et al. Electrothrombosis of saccular aneurysms via the endovascular approach part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 22.Teijin H, Fushiki S, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysm in primates. Stroke. 1995;26:2075–2080. doi: 10.1161/01.str.26.11.2075. [DOI] [PubMed] [Google Scholar]
- 23.Reul J, Weis J, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. Am J Neuroradiol. 1997;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Murayama Y, Viñuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysm part II: an experimental study in a swine aneurysm model. Am J Neuroradiol. 1999;20:1992–1999. [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja AA, Hergenrother RW, et al. Platinum coil coatings to increase thrombogenicity: a preliminary study in rabbits. Am J Neuroragiol. 1993;14:794–798. [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson RC, Krisht AF, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery. 1995;36:133–140. doi: 10.1227/00006123-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Dawson III, RC, Shengelaia GG, et al. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. Am J Neuroragiol. 1996;17:853–858. [PMC free article] [PubMed] [Google Scholar]
- 28.Szikora I, Wakhloo AK, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. Am J Neuroradiol. 1997;18:667–670. [PMC free article] [PubMed] [Google Scholar]
- 29.Murayama Y, Viñuela F, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery. 1997;40:1233–1244. doi: 10.1097/00006123-199706000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Shimozuru T, Kanezawa T, et al. Hydroxyapatite coating of detachable coils for endovascular occlusion of experimental aneurysm. Intervent Neuroradiol. 2001;7(suppl 1):105–110. doi: 10.1177/15910199010070S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murayama Y, Vinuela F, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg. 2001;94:454–463. doi: 10.3171/jns.2001.94.3.0454. [DOI] [PubMed] [Google Scholar]
- 32.Abrahams JM, Forman MS, et al. Biodegradable polyglycolide endovascular coils promote wall thickening and drug delivery in a rat aneurysm model. Neurosurgery. 2001;49:1187–1195. [PubMed] [Google Scholar]
- 33.Abrahams JM, Forman MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. Am J Neuroradiol. 2001;22:1410–1417. [PMC free article] [PubMed] [Google Scholar]
- 34.Marx WF, Cloft HJ, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. Am J Neuroradiol. 2001;22:323–333. [PMC free article] [PubMed] [Google Scholar]