Summary
Modulation of NF-κB-dependent responses is critical to the success of attaching/effacing (A/E) human pathogenic E. coli (EPEC and EHEC) and the natural mouse pathogen Citrobacter rodentium. NleB, a highly conserved type III secretion system effector of A/E pathogens, suppresses NF-κB activation, but the underlying mechanisms are unknown. We identified the mammalian glycolysis enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an NleB interacting protein. Further, we discovered that GAPDH interacts with the TNF receptor associated factor 2 (TRAF2), a protein required for TNF-α-mediated NF-κB activation, and regulates TRAF2 polyubiquitination. During infection, NleB functions as a translocated N-acetyl-D-glucosamine (O-GlcNAc) transferase that modifies GAPDH. NleB-mediated GAPDH O-GlcNAcylation disrupts the TRAF2-GAPDH interaction to suppress TRAF2 polyubiquitination and NF-κB activation. Eliminating NleB O-GlcNAcylation activity attenuates C. rodentium colonization of mice. These data identify GAPDH as a TRAF2 signaling cofactor and reveal a virulence strategy employed by A/E pathogens to inhibit NF-κB dependent host innate immune responses.
Introduction
Enterohemorrhagic E. coli (EHEC) are attaching/effacing (A/E) pathogens that are especially detrimental to human health because they cause hemorrhagic colitis and a type of renal failure (hemolytic uremic syndrome; HUS) for which therapy is limited. A related E. coli virotype, enteropathogenic E. coli (EPEC), is an important cause of infantile diarrhea. These human pathogens, as well as Citrobacter rodentium, a mouse pathogen that shares virulence strategies with E. coli (Deng et al., 2003), translocate virulence proteins (effectors) into intestinal epithelial cells using a type III secretion system (T3SS) to subvert the activity of various cell functions (Dean and Kenny, 2009).
E. coli and C. rodentium T3SS effectors modulate the innate immune system, including host responses regulated by the transcription factor NF-κB, via several different mechanisms (Rahman and McFadden, 2011). The NleB effector is highly conserved among the A/E pathogens (Kelly et al., 2006). NleB-deficient C. rodentium do not cause mortality (Wickham et al., 2007) or significant colonic hyperplasia (Kelly et al., 2006) in mice. These bacteria show markedly reduced colonization, as compared with wild-type (WT) C. rodentium, indicating the importance of NleB to C. rodentium virulence. NleB is associated with human EHEC outbreaks and the subsequent development of HUS (Wickham et al., 2006). The presence of NleB in atypical EPEC strains is associated with diarrheal disease (Bugarel et al., 2010). Thus, NleB plays an important role in the virulence of A/E pathogens. Two recent studies determined that NleB suppresses NF-κB activation (Nadler et al., 2010, Newton et al., 2010).
Here, we characterize a mechanism by which NleB inhibits NF-κB signaling. NleB functions as a translocated N-acetyl-D-glucosamine (O-GlcNAc)-transferase that modifies the mammalian glycolysis enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH). We provide evidence that GAPDH is a co-activator of the TNF receptor associated factor 2 (TRAF2) and that NleB-mediated GAPDH O-GlcNAcylation inhibits both TRAF2 activation and downstream NF-κB signaling.
Results
NleB inhibits NF-κ B activation by inhibiting TRAF2 polyubiquitination
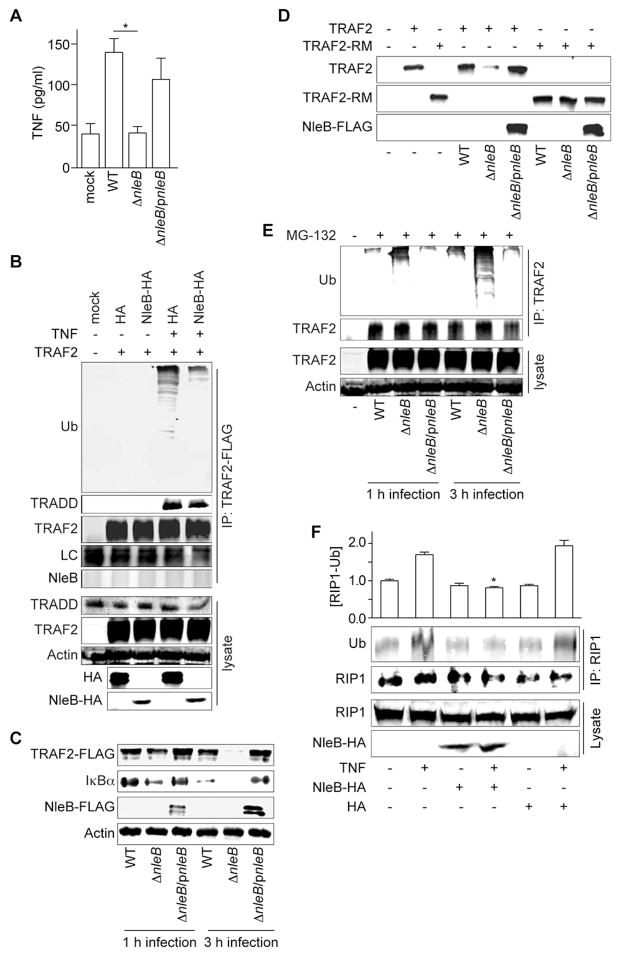
WT C. rodentium colonized C57BL/6J mice to a greater magnitude than a strain bearing an isogenic deletion of NleB (ΔnleB; Fig. S1A). Complementing ΔnleB C. rodentium with a FLAG-tagged NleB expression plasmid (ΔnleB/pnleB) restored bacterial colonization to nearly WT levels. WT C. rodentium was shed in mouse feces in greater numbers over the duration of infection, as compared with ΔnleB C. rodentium (Fig. S1B). TNF serum concentrations were significantly higher in mice infected with WT C. rodentium, than in mice infected with ΔnleB (Fig. 1A), indicating that NleB contributes to bacterial-induced host inflammation.
Figure 1. Figure 1, see also Figure S1. NleB inhibits NF- B activation by preventing TRAF2 polyubiquitination.
A. TNF concentrations (pg/ml; mean ± SEM, n = 8) in serum (7 d post-infection) from uninfected mice or mice infected with indicated C. rodentium strains. Asterisks indicate significantly different [TNF] as compared with WT infection (one-way ANOVA with Bonferonni’s multiple comparison test). B. TRAF2-FLAG was co-transfected with NleB-HA and immunoprecipitated using α-FLAG antibody. Immunoprecipitates were immunoblotted for FLAG, TRADD, ubiquitin, and HA. LC, antibody light chain. C. HeLa cells were transfected with TRAF2-FLAG and then infected with C. rodentium for 1 or 3 h. TRAF2 and IκBα abundance were analyzed using immunoblotting. D. HeLa cells were transfected with either TRAF2-FLAG or ΔRING TRAF2 (TRAF2-RM) plasmids and then infected with C. rodentium for 3 h. E. HeLa cells were transfected with TRAF2-FLAG, pre-treated with 10 μM MG-132 for 2 h, and then infected with C. rodentium. TRAF2 was immunoprecipitated and polyubiquitination was assessed using immunoblotting. F. RIP1 was co-transfected with NleB-HA and immunoprecipitated using α-RIP1 antibody. Immunoprecipitates were immunoblotted for ubiquitin and RIP1. Data are represented as mean ± SEM, n = 3. Asterisks indicate significantly different [RIP1-Ub] as compared with TNF treatment (Bonferonni’s).
NleB inhibits tumor necrosis factor (TNF)-induced NF-κB activation (Newton et al., 2010, Nadler et al., 2010), but its mechanism of action is not clear. We transfected HeLa cells with NleB-HA, stimulated these cells with TNF, and subsequently examined the extent of degradation of the NF-κB inhibitor, IκBα. NleB prevented TNF-induced IκBα degradation, as well as the subsequent translocation of the NF-κB p65 subunit to the nucleus (Fig. S1C).
Deleting nleB from C. rodentium did not significantly alter nleE expression, another effector that inhibits NF-κB activation (Fig. S1D; (Nadler et al., 2010)]. Both NleB and NleE, when transfected into HeLa cells, inhibited TNF-induced IκBα degradation (Fig. S1E). Neither ΔnleB nor ΔnleE C. rodentium prevented IκBα degradation during infection, by contrast to WT C. rodentium (Fig. S1F), indicating a potential coordinated activity between the effectors during C. rodentium infection.
After TNF stimulation, the cytosolic death domain of the TNF receptor-1 (TNFR1) recruits proteins such as the TNFR-associated death domain protein (TRADD), the TNFR associated factor 2 (TRAF2), and the receptor-interacting protein 1 (RIP1) to form complexes that activate the NF-κB pathway (Chen and Goeddel, 2002). During this process, TRAF2, an E3 ubiquitin ligase, becomes polyubiquitinated and activates RIP1 kinase activity. TRAF2 polyubiquitination was induced by TNF in control cells (Fig. 1B), whereas cells transfected with NleB-HA exhibited significantly reduced TRAF2 polyubiquitination. The TRAF2 interaction partner TRADD was still recruited to TRAF2 in NleB-transfected cells, suggesting how NleB inhibits TRAF2 polyubiquitination is independent of TRADD.
Bacterial infection induces production of additional TNFR ligands, including RANKL and CD40L (Zheng et al., 2008). These molecules can cause not only TRAF2 polyubiquitination, but also can promote its degradation. We transfected HeLa cells with TRAF2-FLAG and then infected these cells with C. rodentium strains that either express or lack NleB. Infecting cells with C. rodentium ΔnleB induced TRAF2 degradation (Fig. 1C), with almost complete degradation of TRAF2 observed after 3 h. By contrast, TRAF2 was stable in cells infected with either WT or ΔnleB/pnleB C. rodentium (Fig. 1C). TRAF2 degradation was mediated by ubiquitination, as ΔnleB C. rodentium failed to induce the degradation of a TRAF2 ΔRING mutant (Fig. 1D). Treating cells with the 26S proteasome inhibitor MG-132 also stabilized TRAF2 during infection (Fig. 1E).
We hypothesized that NleB might target the TRAF2 polyubiquitination normally induced upon TNFR stimulation. TRAF2 polyubiquitination was prevented in cells infected with either WT or ΔnleB/pnleB C. rodentium, but not ΔnleB C. rodentium (Fig. 1E). NleB also inhibited the ubiquitination of RIP1, a substrate of TRAF2 Ub ligase activity [(Alvarez et al., 2010); Fig. 1F]. However, NleB did not directly inhibit TRAF2 self-ubiquitination in vitro (Fig. S1G). Overall, our data instead suggested that NleB might target an unidentified co-activator(s) of TRAF2 to prevent its polyubiquitination upon either TNFR stimulation or after bacterial infection, subsequently attenuating NF-κB activation.
NleB interacts with GAPDH
We did not obtain evidence that NleB interacts with TRAF2 from immunoprecipitation experiments (Fig. 1B). We conducted a proteomic screen to identify NleB interaction partner(s). By purified NleB-FLAG (Fig. 2A) and used it in affinity column experiments to identify a human protein of ~37 kDa that interacted with NleB (Fig. 2B). By using mass spectrometry and immunoblotting affinity-purified cell lysates, we identified this protein as human glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Fig. 2C–D), a component of the glycolysis pathway.
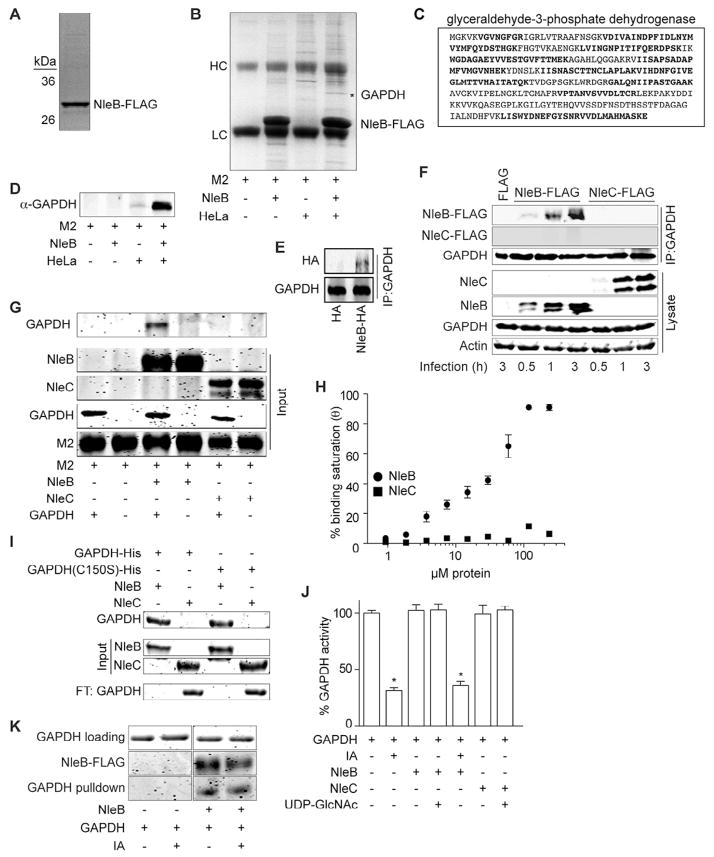
Figure 2. NleB binds GAPDH.
A. NleB-FLAG protein purity assessed by Coomassie Blue staining after 12 % SDS-PAGE. B. HeLa cell lysates were incubated with purified NleB-FLAG pre-bound to FLAG M2 beads and analyzed by SDS-PAGE. The band indicated with an asterisk was excised and analyzed using mass spectrometry. C. GAPDH amino acid sequence. Tryptic peptides identified by mass spectrometry are indicated in bold. D. Affinity column samples were analyzed using immunoblotting with α-GAPDH antibody. E. NleB-HA was transfected. GAPDH was immunoprecipitated and immunoblotted for GAPDH and HA. F. Immunoprecipitation of GAPDH from HeLa cells by translocated NleB- or NleC-FLAG after C. rodentium infection for 0.5, 1 or 3 h. Samples were immunoprecipitated with α-GAPDH antibody and immunoblotted for FLAG and GAPDH. G. FLAG-tagged NleB or NleC were coupled to M2 beads and incubated with GAPDH. Samples were analyzed using IRDye Blue protein staining after electrophoresis through 12 % SDS-PAGE. H. ELISA plates were coated with 2 μg GAPDH. Coated plates were overlaid with increasing amounts of NleB- or NleC-FLAG. Protein binding was detected using α-FLAG antibody and 1-Step Ultra TMB-ELISA solution. Absorbance at 450 nm was measured (mean ± SEM, n = 4). I. In vitro pulldown assay for the interaction between GAPDH C150S and NleB. Purified GAPDH- and GAPDH C150S-His proteins were incubated with NleB-FLAG or NleC-FLAG proteins prebound to M2 beads. J. GAPDH enzyme inhibition by iodoacetate (IA; mean ± SEM, n = 3). Asterisks indicate significantly different GAPDH activity (Bonferroni’s). K. Impact of IA on GAPDH-NleB binding was measured using in vitro pulldown assay. GAPDH was treated with IA and then incubated with NleB-FLAG prebound to M2 beads.
GAPDH co-immunoprecipitated ectopically expressed NleB-HA, but not an HA-epitope control (Fig. 2E). Infecting HeLa cells with C. rodentium strains expressing either NleB or NleC [an effector that also targets the NF-κB pathway; (Pearson et al., 2011)], showed that NleB, but not NleC, immunoprecipitated with GAPDH during C. rodentium infection (Fig. 2F). We then developed an in vitro pulldown assay with purified recombinant proteins. NleB-FLAG, but not NleC-FLAG, binds directly to GAPDH (Fig. 2G), as NleB-FLAG was pulled down in vitro when mixed with GAPDH.
We quantified the affinity of the NleB-GAPDH interaction in vitro by performing ELISAs. NleB-FLAG bound to immobilized GAPDH with an apparent dissociation constant (Kd) of 32 ± 6 μM (Fig. 2H). By contrast, NleC-FLAG did not bind GAPDH with significant affinity. We did not detect significant changes in the abundance or in the cytoplasmic localization of GAPDH as a function of expressing NleB (data not shown).
Some apoptotic stimuli generate nitric oxide, which subsequently causes GAPDH to be S-nitrosylated on its catalytic cysteine (C150) residue (Hara et al., 2005). We found that both WT and a C150S GAPDH mutant protein bound directly to NleB in vitro (Fig. 2I). Likewise, treating GAPDH with iodoacetate (IA), a small molecule inhibitor that modifies the GAPDH C150 sulfhydryl moiety and prevents disulfide bond formation (Harrison et al., 2003), at a concentration sufficient to inhibit GAPDH enzyme activity (10 μM; Fig. 2J), did not disrupt the GAPDH-NleB interaction in vitro (Fig. 2K). NleB binding to GAPDH did not inhibit GAPDH enzyme activity (Fig. 2J), either alone, or in concert with IA, indicating the NleB-GAPDH interaction is unlikely to affect host glycolysis directly. Overall, our data suggest that GAPDH is a eukaryotic interaction partner for NleB and this interaction is independent of GAPDH C150.
GAPDH is required for TNF-induced NF-κ B activation
The role of GAPDH in stress response pathways has been studied previously (Hara et al., 2005). GAPDH participates in NF-κB signaling (Bouwmeester et al., 2004, Mookherjee et al., 2009), but the precise mechanism has not been elucidated. Transfecting siRNAs targeting the GAPDH-3′ UTR GAPDH depleted GAPDH abundance by approximately 50 % (Fig. S2A), activated multiple stress-induced signaling pathways (Fig. S2B), possibly due to the generation of reactive oxygen species (ROS; Fig. S2C) and the release of cytochrome c from mitochondria (Fig. S2D). Complementing GAPDH knockdown cells with either WT or C150S GAPDH-Myc prevented cytochrome c release (Fig. S2D) and reduced NF-κB pathway activation (Fig. S2E–F).
Complementing GAPDH knockdown cells with a GAPDH K160R plasmid, which encodes a form of GAPDH that cannot be acetylated by the p300/CREB binding protein [CBP; (Sen et al., 2008)], did not inhibit TNF-induced IκBα degradation (Fig. S2E). The K160 residue is essential for GAPDH binding to p300/CBP in the nucleus, an interaction that subsequently activates downstream apoptotic events. Because initiation of apoptosis typically leads to NF-κB pathway activation (Van Antwerp et al., 1996), we conclude that K160 is not likely to be involved in TNF/NF-κB signaling under the conditions of our assays.
By contrast to WT and K160R GAPDH, complementing GAPDH knockdown cells with GAPDH C150S resulted in a ~30 % reduction in cellular ATP levels (Fig. S2G). This ~30 % reduction in [ATP] is not likely to contribute to the attenuation of NF-κB activation, as a larger reduction in [ATP] was observed by inhibiting the rate-limiting glycolytic enzyme, pyruvate kinase, with potassium oxalate (PO; Fig. S2H). However, pyruvate kinase inhibition only caused a mild attenuation of TNF-induced NF-κB activation (Fig. S2I).
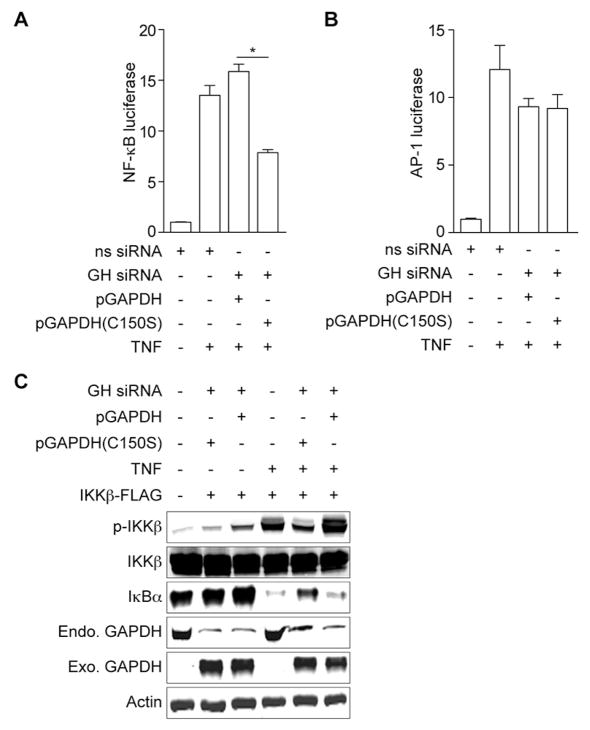
We hypothesized that GAPDH enzyme activity might be required for NF-κB activation after TNF stimulation. Whereas complementation with WT GAPDH only slightly enhanced NF-κB activation, complementation with GAPDH C150S instead significantly attenuated NF-κB activation after TNF treatment (Fig. 3A). AP-1 luciferase activity was independent of GAPDH enzyme activity (Fig. 3B), suggesting a degree of specificity of GAPDH to the NF-κB pathway. Expressing GAPDH C150S failed to restore TNF-induced IKKβ phosphorylation in GAPDH knockdown cells (Fig. 3C). We concluded that WT GAPDH is required for TNF-induced NF-κB activation.
Figure 3. Figure 3, see also Figure S2. GAPDH is essential for TNF-induced NF-κB activation.
A. NF-κB luciferase activity as a function of GAPDH complementation (mean ± SEM, n = 3). Asterisks indicate significantly different luciferase activity between GAPDH WT and C150S complementation (t-test). B. AP-1 luciferase activity as a function of GAPDH complementation (mean ± SEM, n = 3). C. Analysis of IKKβ phosphorylation and IκBα degradation in GAPDH knockdown cells complemented with WT GAPDH or GAPDH C150S. HeLa cells were co-transfected with GAPDH siRNA, as well as with IKKβ-FLAG and either WT GAPDH-Myc or GAPDH-C150S-Myc plasmids, and then stimulated with TNF.
We used a pharmacological approach to evaluate the role of glycolysis on TNF-induced NF-κB activation. We first pre-treated HeLa cells with IA to inhibit GAPDH and then treated these cells with TNF. While IA targets many other proteins containing reactive cysteines, including deubiquitinating peptidases, a number of other studies have used this reagent to inhibit both GAPDH and glycolysis (Fujiki et al., 2011, Wentzel et al., 2003). We also pre-treated cells with two other drugs that target glycolytic enzymes, either a pyruvate kinase inhibitor, potassium oxalate (PO), or a hexokinase inhibitor, 2-deoxy-D-glucose (2DG). Treating cells with either PO or with 2DG depleted [ATP] to ~20 % of that detected in untreated cells (Fig. S2H). By contrast, IA treatment only reduced [ATP] s ~2-fold. However, IA significantly inhibited GAPDH enzyme activity, whereas both 2DG and PO had less impact (Fig. S2J).
Following TNF treatment, we conducted gene expression analyses. Our microarray (data not shown) and RT-PCR assays (Fig. S2K) showed that IA significantly inhibited TNF-induced increases in the expression of numerous NF-κB-regulated genes. By contrast, neither 2DG nor PO significantly reduced the NF-κB-dependent gene expression normally induced by TNF. We reached similar conclusions by studying NF-κB luciferase reporter activation after inhibitor treatment (Fig. S2I). By contrast to the NF-κB-regulated genes, the expression of NF-κB-independent genes was not impaired by drug treatment (Fig. S2K), again suggesting a degree of specificity for GAPDH activity in the NF-κB pathway.
Likewise, TNF failed to induce IκBα degradation and IKKβ phosphorylation in IA-treated cells, but had normal activity in cells treated with either PO or with bromopyruvate (BP), a hexokinase inhibitor (Fig. S2L). Both p38 and JNK phosphorylation were unaffected by IA (Fig. S2L). These data suggest that GAPDH regulates the NF-κB signaling pathway using a mechanism distinct from its enzymatic role in glycolysis.
GAPDH binds and activates TRAF2
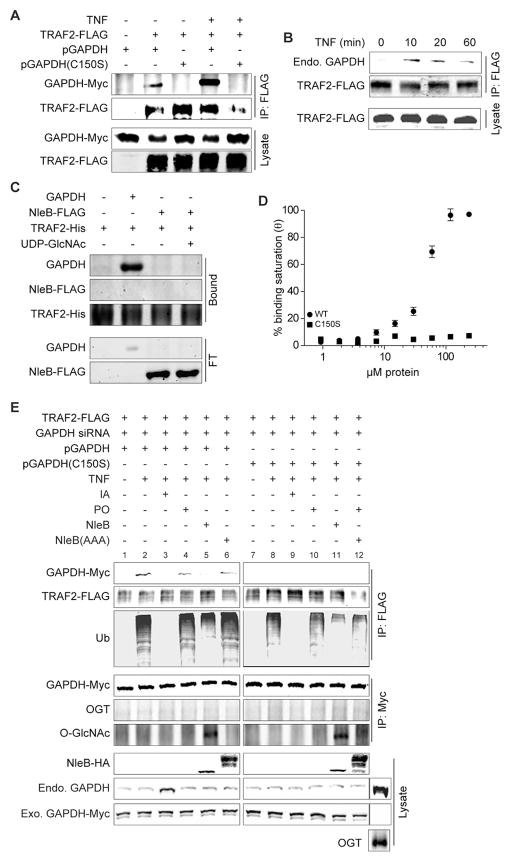
TRAF2 is structurally similar to the E3 ligase Siah1, which interacts with GAPDH under stress (Hara et al., 2005). To determine whether GAPDH interacts with TRAF2, we co-transfected TRAF2-FLAG with GAPDH-Myc and then immunoprecipitated TRAF2-FLAG. WT GAPDH was associated with TRAF2 and this interaction was enhanced by TNF (Fig. 4A). The association of endogenous GAPDH with TRAF2 was also promoted by TNF (Fig. 4B). Using in vitro pulldown assays with TRAF2-His, NleB-FLAG, and recombinant GAPDH, we determined that TRAF2 interacts directly with GAPDH, but not with NleB (Fig. 4C). GAPDH C150S did not immunoprecipitate with TRAF2 (Fig. 4A), suggesting the importance of the C150 residue for the interaction between GAPDH and TRAF2 in vivo. TRAF2 bound to WT GAPDH with an apparent Kd of 96 ± 31 μM (Fig. 4D). TRAF2 did not bind to GAPDH C150S with significant affinity.
Figure 4. GAPDH interacts with TRAF2.
A. HeLa cells were transfected with TRAF2-FLAG ± GAPDH-Myc plasmids, immunoprecipitated using α-FLAG antibody, and then immunoblotted using α-Myc antibody. B. Experiment was performed as described in panel A, except that endogenous GAPDH association with TRAF2 was monitored using α-GAPDH antibody. TNF treatment time is indicated. C. TRAF2-His protein was coupled to Ni-NTA agarose and incubated with GAPDH and/or NleB-FLAG ± 1.0 mM UDP-GlcNAc. The bound and flow-through (FT) samples are indicated. D. ELISA plates were coated with 2 μg of either WT GAPDH or C150S GAPDH. Coated plates were overlaid with increasing amounts of TRAF2. Protein binding was detected using α-TRAF2 antibody (mean ± SEM, n = 4). E. HeLa cells were transfected and treated 60 h later with indicated glycolysis enzyme inhibitors. Cell lysates were immunoprecipitated using α-FLAG antibody to capture TRAF2-FLAG and immunoblotted for ubiquitin and GAPDH-Myc. Additional immunoprecipitations were conducted using α-Myc antibody to capture GAPDH-Myc and immunoblotted for O-GlcNAc and OGT.
We investigated whether the GAPDH-TRAF2 interaction is important for TRAF2 activity in response to TNF. After transfecting GAPDH siRNA and GAPDH-Myc plasmids, we treated cells with TNF and immunoprecipitated TRAF2. TRAF2 polyubiquitination was enhanced in cells expressing WT GAPDH (Fig. 4E), whereas TRAF2 polyubiquitination was attenuated in cells either complemented with GAPDH C150S or in cells treated with IA. This phenotype appears to be unrelated to cellular ATP levels, as treating cells with PO (Fig. 4E, lane 4), which significantly reduced ATP stores (Fig. S2H) did not impair TRAF2 polyubiquitination. Thus, GAPDH binds TRAF2, its recruitment is dependent upon the C150 residue, and the interaction is enhanced by TNF.
NleB is a glycosyltransferase that O-GlcNAcylates GAPDH
Analysis of the NleB amino acid sequence using the HHPred/HHSearch package (Soding et al., 2005), revealed that the central region of NleB is significantly similar (p-value 10−7) to GT-8 family glycosyltransferases. These glycosyltransferases have a conserved sequence and three-dimensional structure, represented by either a canonical or by a modified Rossman fold. These proteins are also characterized by a DxD catalytic signature, which chelates the divalent cation implicated in catalysis (Liu and Mushegian, 2003). The DxD signature, in the form of DAD tripeptide, is present in all NleB homologs we examined, as well as in the most closely related glycosyltransferases within the GT-8 family, namely the eukaryotic glycogenins (Fig. S3A).
Glycogenins are glycosyltransferases that attach glucose to the growing glycogen chain (Lazarus et al., 2011). Multiple sequence alignments indicated the conservation of several beta-strands and connecting α-helices that form the modified Rossmann fold in the glycogenin structure (Fig. S3A). Particularly well conserved are two beta-strands connected by the loop in which the conserved catalytic DxD motif is located, as well as the upstream motif between two alpha-helices that makes direct contacts to the uridine diphosphate (UDP), the dinucleotide substrate of human glycogenin 1.
In eukaryotic cells, O-linked-N-acetylglucosamine (O-GlcNAc) regulates numerous cellular signaling networks, including the NF-κB pathway (Yang et al., 2008), often in opposition with protein phosphorylation. O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) regulate protein O-GlcNAcylation (Hart et al., 2011). OGT catalyzes the transfer of N-acetylglucosamine from UDP-GlcNAc to the hydroxyl oxygen of serine or threonine, whereas OGA removes O-GlcNAc from targeted proteins.
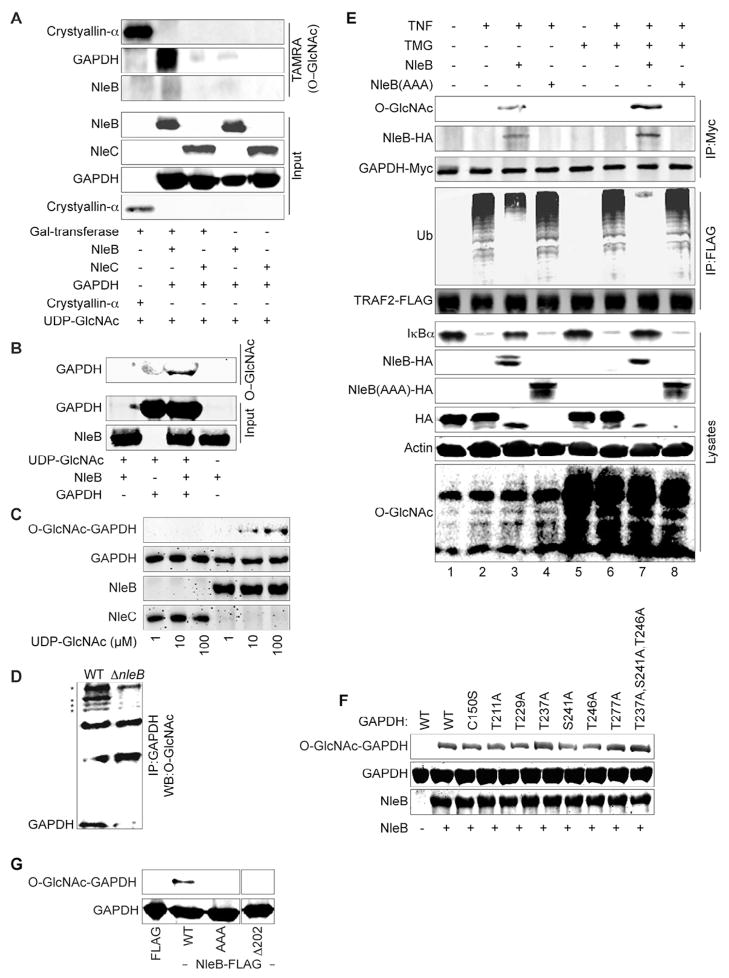
We used an O-GlcNAc in vitro labeling assay to determine whether NleB possess glycosyltransferase activity. Alpha-crystallin, which contains a number of amino acids that can be O-GlcNAcylated, was used as a positive control. We incubated purified NleB-FLAG with GAPDH in the presence of UDP-GlcNAc and discovered that NleB-FLAG is capable of O-GlcNAcylating GAPDH (Fig. 5A). We confirmed these data by using an antibody (110.6) that specifically recognizes O-GlcNAcylation sites on proteins (Fig. 5B). The intensity of NleB-mediated GAPDH O-GlcNAcylation was proportional to the concentration of the sugar nucleotide donor UDP-GlcNAc (Fig. 5C).
Figure 5. Figure 5, see also Figure S3. NleB O-GlcNAcylates GAPDH.
A. Detection of GAPDH O-GlcNAcylation by NleB using 5-carboxytetramethylrhodamine (TAMRA). B. Detection of GAPDH O-GlcNAcylation by NleB using α-O-GlcNAc antibody (110.6). C. GAPDH O-GlcNAcylation as a function of [UDP-GlcNAc]. NleB- or NleC-FLAG was incubated with GAPDH and UDP-GlcNAc. Proteins were analyzed by SDS-PAGE and immunoblotted for the O-GlcNAcylation using α-GlcNAc antibody. D. GAPDH O-GlcNAcylation during bacterial infection. HeLa cells were infected with C. rodentium WT or ⊗nleB for 1 or 3 h. Cell lysates were immunoprecipitated with α-GAPDH antibody and analyzed for O-GlcNAcylation. Asterisks indicate other proteins detected in GAPDH immunoprecipitates more heavily O-GlcNAcylated in the presence NleB. E. NleB(AAA) does not O-GlcNAcylate GAPDH. HeLa cells were co-transfected with NleB, TRAF2, and GAPDH plasmids for 60 h and treated with 20 μM TMG for 8 h ± TNF (20′). Cell lysates were immunoprecipitated for TRAF2-FLAG or GAPDH-Myc. Cell lysates were also immunoblotted for total protein O-GlcNAcylation. F. Purified GAPDH mutant proteins were incubated with NleB-FLAG and UDP-GlcNAc. GAPDH O-GlcNAcylation was analyzed using α-GlcNAc antibody. G. FLAG-NleB WT and mutants were purified and incubated with WT GAPDH. Samples were analyzed using SDS-PAGE and immunoblotted with α-GlcNAc and α-GAPDH antibodies.
To investigate whether NleB O-GlcNAcylates GAPDH during bacterial infection, we infected HeLa cells for 3 h with C. rodentium. GAPDH O-GlcNAcylation was induced in cells infected with WT C. rodentium, but not in cells infected with ΔnleB C. rodentium (Fig. 5D). GAPDH was not O-GlcNAcylated in control cells or in cells treated only with TNF (Fig. 5E). However, after transfecting NleB-HA, GAPDH was modified with O-GlcNAc (Figs. 4E & 5E). NleB also O-GlcNAcylated GAPDH C150S in vivo (Fig. 4E) and in vitro (Fig. 5F), consistent with results suggesting GAPDH C150 is not required for the GAPDH-NleB interaction.
To begin to identify GAPDH O-GlcNAcylation sites, we first considered several GAPDH residues that are modified by cellular enzymes. Although we did not detect OGT in TRAF2-GAPDH complexes (Fig. 4E), OGT has been suggested to O-GlcNAcylate GAPDH on T229 (Park et al., 2009). We mutated T229 (T229A) and a neighboring threonine residue, T211 (T211A), to determine if NleB targets them for modification. However, neither mutant differed in NleB-mediated GAPDH O-GlcNAcylation (Fig. 5F). GAPDH is phosphorylated by protein kinase B (AKT) on T237, which prevents GAPDH nuclear translocation and suppresses GAPDH-mediated apoptosis (Huang et al., 2011). We also mutated T237 (T237A), as well as the neighboring residues S241 (S241A), T246 (T246A), and T277 (T277A). However, we still did not detect significant changes to NleB-mediated GAPDH O-GlcNAcylation, indicating there may be other or multiple GAPDH residues targeted by NleB (Fig. 5F).
To confirm further that NleB is an O-GlcNAc transferase, we mutated the predicted catalytic site 221DAD223 of NleB to 221AAA223. The NleB 221DAD-AAA223 mutant [hereafter designated NleB(AAA)] failed to O- GlcNAcylate GAPDH proteins either in vivo (Fig. 4E & Fig. 5E) or in vitro (Fig. 5G). Expressing an NleB mutant in which the modified Rossmann fold was deleted (Δ202) also abolished the O-GlcNAcylation of GAPDH by NleB (Fig. 5G). However, GAPDH, in the presence or absence of NleB and UDP-GlcNAc did not directly inhibit TRAF2 self-ubiquitination in vitro (Fig. S3B).
O-GlcNAcase catalyzes the cleavage of O-GlcNAc from glycosylated proteins. Treating cells with an O-GlcNAcase inhibitor, thiamet G (TMG), increased total cellular protein O-GlcNAcylation (Fig. 5E). We tested whether O-GlcNAcylation of GAPDH by NleB is enhanced by TMG treatment. Although we did not detect any basal GAPDH O-GlcNAcylation in our experimental system, we found that GAPDH O-GlcNAcylation was significantly enhanced by NleB (Fig. 5E), and further enhanced after TMG treatment. By contrast, the NleB(AAA) mutant failed to induce GAPDH O-GlcNAcylation, irrespective of TMG. Thus, NleB activity appears to be linked to cellular O-GlcNAc concentrations. These experiments were conducted using ectopic expression of NleB and NleB(AAA) to allow specific analysis of the TNF/TRAF2 pathway, in the absence of other potential inducers generated during bacterial infection (e.g. IL-1β, LPS).
Most proteins that are O-GlcNAcylated by OGT are sensitive to glucose concentrations because this sugar affects the activity of the hexosamine biosynthetic pathway (Hart et al., 2011). In our system, total protein O-GlcNAcylation was decreased in cells cultured in hypoglycemic conditions, as compared with hyperglycemic conditions (Fig. S3C). However, the level of GAPDH O-GlcNAcylation mediated by NleB did not change significantly in the different glucose conditions (Fig. S3D), indicating that the activity of NleB may be largely insensitive to changes in UDP-GlcNAc concentrations. Taken together, our data suggest that NleB is a translocated glycosyltransferase that modifies host GAPDH with O-GlcNAc.
NleB O-GlcNAc transferase activity disrupts the GAPDH-TRAF2 interaction and inhibits NF-κ B activity
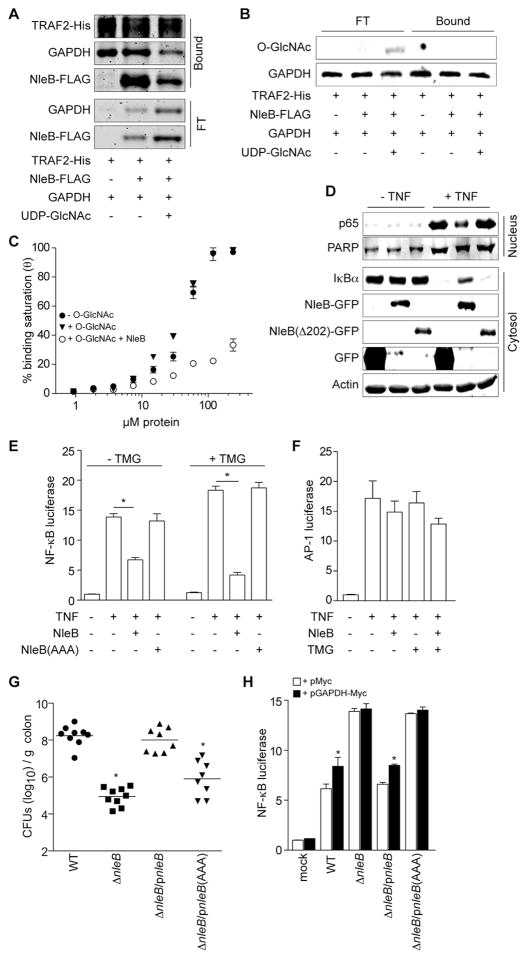
We immobilized TRAF2-His on Ni-NTA agarose and then applied either purified GAPDH or NleB-FLAG ± UDP-GlcNAc to assess the binding interactions among these three proteins. More NleB-FLAG was pulled down by GAPDH/TRAF2 in the absence of UDP-GlcNAc than when UDP-GlcNAc was present (Fig. 6A). When incubated with NleB and UDP-GlcNAc, only the GAPDH that had dissociated from TRAF2 possesses a significant O-GlcNAcylation signal, as compared with GAPDH still bound to TRAF2 (Fig. 6B). We therefore hypothesized that NleB might interact with GAPDH to disrupt the formation of TRAF2-GAPDH complex through its O-GlcNAc transferase activity. Indeed, while WT NleB blocked the association of GAPDH with TRAF2 after TNF treatment, the glycosyltransferase-deficient mutant NleB(AAA) failed to do so (Fig. 4E).
Figure 6. NleB O-GlcNAc transferase activity is essential for inhibiting the NF-κB pathway.
A. TRAF2-His was coupled to Ni-NTA beads and then incubated with NleB-FLAG and GAPDH. UDP-GlcNAc (1 mM) was added where indicated. Bound and flow-through (FT) proteins were stained with IRDye Blue. B. O-GlcNAcylation of free (FT) and TRAF2-associated GAPDH. Samples were immunoblotted using α-GlcNAc antibody. C. ELISA plates were coated with 2 μg of either unmodified GAPDH or GAPDH that had been labeled in vitro by O-GlcNAc after incubation with NleB and UDP-GlcNAc. Coated plates were overlaid with increasing amounts of TRAF2 ± 100 μM UDP-GlcNAc. Protein binding was detected using α-TRAF2 antibody (mean ± SEM, n = 4). D. Immunoblotting of IκBα and p65 nuclear translocation in the presence of NleB(Δ202). E. NF-κB luciferase activity (mean ± SEM, n = 4) ± NleB and TMG. HeLa cells were co-transfected with luciferase reporters and NleB-HA or control plasmids for 60 h, and treated with TMG for 8 h before treating with TNF for 20′. Asterisks indicate significantly different luciferase activity, t-test. F. AP-1 luciferase activity (mean ± SEM, n = 4) ± NleB and TMG. G. Colonization (log10 CFUs/g colon) of indicated C. rodentium strains (7 d post-gavage) in C57BL/6J mice (n = 6–7). Asterisks indicate significantly different colonization magnitude as compared with WT; Kruskal-Wallis test. H. NF-κB luciferase activity as a function of C. rodentium infection and GAPDH-Myc over-expression (mean ± SEM, n = 4). HeLa cells were co-transfected with GAPDH-Myc or Myc-epitope control plasmids with NF-κB luciferase reporter plasmids for 48 h and then infected with the indicated C. rodentium strains for 3 h. Asterisks indicate significantly different luciferase activity between GAPDH-Myc or Myc-epitope transfection.
GAPDH that had been previously labeled in vitro with O-GlcNAc by NleB had significantly lower affinity for TRAF2, as compared with unlabeled GAPDH (Fig. 6C), while adding excess UDP-GlcNAc to the ELISA had no discernible impact on TRAF2-GAPDH affinity (Fig. 6C). Thus, the glycosyltransferase activity of NleB appears to disrupt the TRAF2-GAPDH interaction, as GAPDH O-GlcNAcylation by NleB significantly reduced the affinity between GAPDH and TRAF2 in vitro (Fig. 6) and prevented their association in vivo (Fig. 4).
We next examined whether NleB-mediated GAPDH O-GlcNAcylation is essential for NleB to inhibit NF-κB pathway activation. By contrast to WT NleB, NleB(AAA) did not prevent IκBα degradation stimulated by TNF (Fig. 5E). NleBΔ202 mutant also failed to block IκBα degradation and p65 nuclear translocation (Fig. 6D). These data are consistent with our findings that TRAF2 polyubiquitination was not impaired by the glycosyltransferase-deficient mutant NleB(AAA) upon TNF stimulation, by contrast with WT NleB (Fig. 4D & 5E).
WT NleB completely abolished TRAF2 polyubiquitination induced by TNF in TMG-treated cells, whereas NleB(AAA) had no impact (Fig. 5E, lanes 7 vs. 8). Although TMG treatment alone did not prevent IκBα degradation and NF-κB activation, it stabilized IκBα in the presence of WT NleB, but not in the presence of NleB(AAA) (Fig. 5E). NleB exhibited a more robust inhibition of NF-κB in cells treated with TMG as compared with cells not treated with TMG (Fig. 6E). NF-κB activity was promoted by TMG treatment, consistent with previous reports (Kawauchi et al., 2009). Neither NleB nor TMG altered AP-1 activation (Fig. 6F), indicating that NleB-mediated GAPDH O-GlcNAcylation is specifically involved in the NF-κB signaling pathway.
To evaluate whether the O-GlcNAc transferase activity of NleB is important to C. rodentium virulence, we infected mice with C. rodentium strains expressing either WT NleB (ΔnleB/pnleB) or the O-GlcNAc transferase-deficient NleB mutant, NleB(AAA) [ΔnleB/pnleB(AAA)]. Mice infected with ΔnleB/pnleB(AAA) C. rodentium strain showed a ~100-fold reduction in colonization magnitude after 7 days, as compared with C. rodentium expressing WT NleB (Fig. 6G). While the reduction in colonization was not as great as compared with the impact of deleting the entire nleB gene, this result demonstrated that a major role of C. rodentium host colonization mediated by NleB is due to its O-GlcNAc transferase activity.
Consistent with this phenotype, the NleB(AAA) mutant failed to suppress NF-κB activation during C. rodentium infection of HeLa cells, as compared with WT and ΔnleB/pnleB C. rodentium, even in the absence of exogenous TNF (Fig. 6H). Over-expressing GAPDH ectopically in cells that were subsequently infected with C. rodentium strains expressing NleB was able to restore partially NF-κB luciferase activity (Fig. 6H). By contrast, GAPDH over-expression did not increase NF-κB activity in cells infected with ΔnleB C. rodentium, suggesting a potential saturation of the interaction between GAPDH and endogenous TRAF2. Because GAPDH over-expression also did not significantly increase basal NF-κB activity in uninfected cells, our data suggest that the O-GlcNAc transferase activity of NleB is essential for its ability to inhibit NF-κB activation and to enhance C. rodentium colonization and virulence through a mechanism involving the modification of GAPDH (Fig. 7). Further, these data suggest that GAPDH might play a role as a general stress sensor by participating in NF-κB signaling.
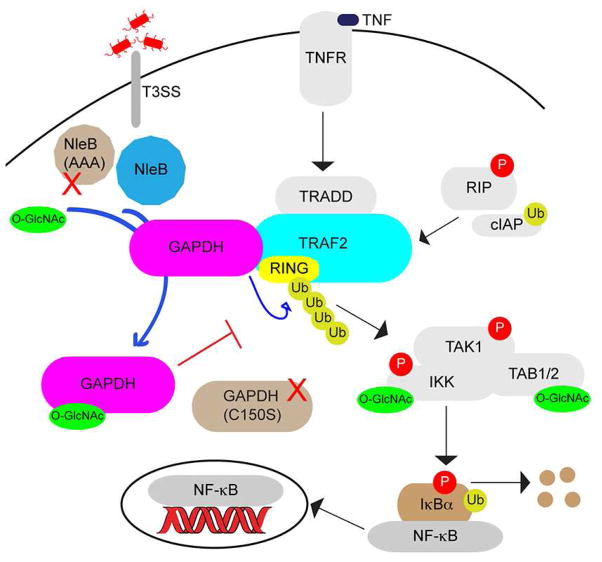
Figure 7. Working model for the role of GAPDH in the NF-κB signaling pathway and its targeting by NleB to attenuate TRAF2 activation.
After TNF stimulation through TNFR, GAPDH is induced to the TRAF2 complex, promoting TRAF2 polyubiquitination and activating the NF-κB pathway through IKK. Drug treatments and mutagenesis studies show that this recruitment is dependent upon GAPDH enzyme activity. During infection, NleB binds GAPDH and modifies it with O-GlcNAc. O-GlcNAcylated GAPDH is unable to bind TRAF2, reducing downstream NF-κB activation. NleB(AAA) is deficient in O-GlcNAc transferase activity and unable to inhibit GAPDH binding to TRAF2. Whether NleB directly impacts host cell metabolic pathways and the identification of other substrates for its O-GlcNAcylation activity remain to be determined.
Discussion
Several studies have suggested GAPDH may be involved in regulating innate immunity. GAPDH is enriched with the NF-κB family member c-Rel and with other NF-κB signaling molecules (Bouwmeester et al., 2004). Mookherjee et al also predicted that GAPDH interacts with molecules in the NF-κB pathway (Mookherjee et al., 2009). GAPDH binding to TRAF2 was enhanced under stress conditions, similar to the interaction between Siah1 and GAPDH (Hara et al., 2005). Our data suggest that GAPDH may be a co-activator of TRAF2, perhaps acting at an early step in the TNF/NF-κB signaling pathway, before E1 and E2 enzymes are recruited to TRAF2. How GAPDH facilitates TRAF2 polyubiquitination is unknown and will require significant future structure-function experiments. We raise the possibility that there may be a network of factors recruited to TRAF2 upon TNFR stimulation that is important to regulating TRAF2 activation, among these, GAPDH.
While TNF activates the NF-κB, p38, and JNK signaling pathways through TRAF2, the role of TRAF2 ubiquitination is more essential to downstream JNK, than to either p38 or NF-κB signaling (Habelhah et al., 2004), possibly due to the redundant role of TRAF2 and TRAF5 in NF-κB activation (Tada et al., 2001, Yeh et al., 1997). Our data show that GAPDH-mediated TRAF2 ubiquitination is required for NF-κB activation, but not for JNK and p38 activation. GAPDH may catalyze a specific form of TRAF2 ubiquitination or may promote a different modification that somehow enhances the TRAF2 polyubiquitination activity required for downstream NF-κB activation.
We found that the GAPDH C150 residue is essential to the GAPDH-TRAF2 interaction. This is consistent with previous work, which also showed that C150 is involved in the GAPDH activities that are independent of glycolysis (Hara et al., 2005). Although it is not clear how C150 contributes to GAPDH involvement in these processes, this active site residue is redox-sensitive and is often modified under stress conditions.
Various studies have also shown that cellular signaling and protein glycosylation are tightly linked (Wellen and Thompson, 2012). O-GlcNAcylation requires UDP-GlcNAc, the final product of the hexosamine biosynthetic pathway. Evidence that O-GlcNAcylation is important in regulating immune signaling pathways is emerging. Enhanced glucose metabolism induces O-GlcNAcylation of NF-κB components and increases NF-κB activity (Kawauchi et al., 2009, Yang et al., 2008). For example, O-GlcNAcylation of TAB1 after IL-1β stimulation is essential for TAK1 activation and NF-κB activation (Pathak et al., 2012). In agreement with these studies, we found that inhibiting OGA increased NF-κB activity after TNF stimulation.
We showed that GAPDH is O-GlcNAcylated by the T3SS effector NleB, a glycogenin-related glycosyltransferase. O-GlcNAcylated GAPDH fails to interact with TRAF2 in vivo. GAPDH O-GlcNAcylation by OGT on T227 has been reported before (Park et al., 2009). However, we did not detect OGT-mediated GAPDH O-GlcNAcylation under our experimental conditions. We are currently identifying NleB-mediated O-GlcNAcylation site(s) on GAPDH to clarify the precise mechanism by which GAPDH functions in the NF-κB signaling pathway.
We have considered the possibility that NleB activity could be somehow coordinated with changes to host cell metabolism associated with infection (Borregaard and Herlin, 1982, Cramer et al., 2003). However, our preliminary efforts to address this complex issue indicated that NleB-mediated GAPDH O-GlcNAcylation is largely insensitive to different glucose concentrations, likely due to the maintenance of relatively high concentrations of UDP-GlcNAc. Therefore, NleB does not appear to share the same mechanism as OGT, which by contrast, is sensitive to glucose concentrations (Hart et al., 2011). However, inhibiting OGA with TMG augmented GAPDH O-GlcNAcylation by NleB, leaving open the possibility that NleB co-ordinately regulates protein O-GlcNAcylation in concert with the mammalian enzymes OGT and OGA.
Clostridial toxins glucosylate and inhibit GTPases of the Rho and Ras superfamily (Kelly and LaMont, 2008). Legionella pneumophila Lgt proteins glucosylate the eukaryotic elongation factor 1A to block protein synthesis (Belyi et al., 2006). Like NleB, these toxins all belong to GT-A family of glycosyltransferases and contain at least one Rossmann-like fold and the DXD catalytic motif. We have yet to characterize the substrate specificity of NleB and it is possible that NleB may be able to utilize other nucleotide sugars to glycosylate GAPDH. It remains to be determined if NleB interacts with other host proteins, and if so, whether NleB O-GlcNAcylates these proteins. We note that several other proteins detected in GAPDH immunoprecipitates appear to be more heavily O-GlcNAcylated in the presence, than in the absence, of NleB (Fig. 5D).
The difference in colonization magnitude between the ΔnleB strain and ΔnleB/pnleB(AAA) is not unexpected. NleB contains an additional DAD motif in its N-terminus, which could also contribute to the ability of NleB to control TNF-induced NF-κB activity. It will be interesting to examine the contribution of other NleB functional domains to bacterial virulence. It is also possible that NleB has functions that are independent of its glycosyltransferase activity and its targeting of the NF-κB pathway.
The impact of NleB on NF-κB is tightly linked with its O-GlcNAc transferase activity directed against GAPDH. GAPDH fails to interact with TRAF2 in the presence of NleB, resulting in attenuated TRAF2 polyubiquitination. This is similar to the phenotype of cells expressing GAPDH C150S, which also fails to bind TRAF2 and fails to promote TRAF2 polyubiquitination. Furthermore, the NleB(AAA) mutant is unable to O-GlcNAcylate GAPDH and does not inhibit NF-κB activation. Our discovery that NleB utilizes UDP-GlcNAc to disrupt TRAF2 complex formation represents a mechanism by which enteric pathogens inhibit host innate immunity.
Experimental procedures
Bacterial strains, plasmids, reagents, and oligonucleotides
Reagents were used according to manufacturer’s recommendations and are described in the supplemental information. Bacterial strains, plasmids, and oligonucleotide sequences are also described in supplemental information.
RNA interference
Three pmol of scrambled siRNAs or siRNAs directed against GAPDH were transfected into HeLa cells using Lipofectamine 2000. The transfection mixture was replaced with complete growth media after 6 h and cells were incubated for a further 60 h prior to harvest. Knockdown specificity was confirmed using immunoblotting. GAPDH siRNA sense-strand sequences were: UTR-1: 5′-AGCACA2GAG2A2GAGAGAGAC3T; UTR-2: 5′-CATGTAC2ATCA2UA3GTAC3TG; UTR-3: CTC2TCACAGT2GC2ATGTAGA3.
Affinity columns and protein identification by LC-ESI-MS/MS
Both affinity column and mass spectrometry experiments were conducted essentially as described before (Gao et al., 2009). Bands excised from protein gels were digested in-gel with trypsin at 37 °C overnight. Spectra were obtained in the positive ion mode with a nano ESI-Q-Tof micro mass spectrometer.
In vitro pulldown assays
1.0 mg of purified NleB-FLAG or NleC-FLAG were immobilized on FLAG M2-conjugated beads and then incubated with 1.0 mg GAPDH for 4 h at 4 °C. Beads were centrifuged and washed three times with 20 mM HEPES, pH 7.9, 150 mM KCl, 0.2 mM EDTA, 0.1 % NP-40, 10 % glycerol, 1.0 mM DTT). SDS-PAGE sample buffer was added and samples were analyzed using immunoblotting.
O-GlcNAcylation assays
NleB-FLAG or NleC-FLAG proteins (500 ng) were incubated with 500 ng GAPDH at 4 °C for 18 h ± 1 mM UDP-GlcNAc. O-GlcNAcylated proteins were detected using the Click-iT O-GlcNAc Enzymatic Labeling System (Invitrogen). Alternatively, proteins were incubated in 50 mM Tris–HCl, pH 7.5, 1 mM DTT, 10 mM MnCl2, 1 mM UDP-GlcNAc for 4 h at room temperature.
GAPDH enzyme activity
GAPDH activity in HeLa cells was measured by using a fluorescence-based assay kit (KDalert™ GAPDH) according to the manufacturer’s recommendations. Briefly, 1.0*104 cells were treated ± 10 μM IA for 40′. Cells were resuspended in ice-cold Cell Lysis Buffer and incubated for 20′. Aliquots were transferred to 96-well plates and changes in fluorescence were measured every 4′ in a fluorescence plate reader. GAPDH activity was determined through comparison to protein standards. For in vitro GAPDH enzymatic activity assays, 0.1 μg GAPDH was pre-incubated with 0.1 μg NleB-FLAG or NleC-FLAG with 1 mM IA and or 0.1 mM UDP-GlcNAc.
Luciferase assays
Luciferase assays were conducted as described (Gao et al., 2009). HeLa cells were co-transfected at a ratio of 10:1 (1.0 μg total DNA) with a luciferase reporter construct (NF-κB or AP-1) together with the renilla luciferase pTKRL plasmid, cultured for 48 h, in the presence or absence of TNF. Cells were lysed with passive lysis buffer and lysates were analyzed according to the protocol from Dual-Luciferase Kit. The fold-induction was calculated as [relative FU stimulated)/(relative FU unstimulated] samples. Luciferase assays were performed in triplicate with at least three independently transfected cell populations.
TRAF2 ubiquitination assay
Ubiquitination assays were performed using enzymes obtained from Boston Biochem, supplemented with NleB (50 nM), and UDP-GlcNAc (1 mM). His-E1(100 nM), Ubc/Uev1a (100 nM), His-TRAF2 (50 nM), ubiquitin (2 μM), and ATP (2 mM) were incubated in 5 mM MgCl2, 50 mM HEPES, pH 7.5, 10 mM NaCl, 1 mM DTT) at 30 °C for 1 h, in the pres ence or absence of GAPDH (50 nM), NleB (50 nM), and UDP-GlcNAc (1 mM). Reactions were terminated by adding SDS-PAGE loading buffer and boiled at 95 °C for 5′. TRAF2 ubiquitination was determined using immunoblotting with α-ubiquitin antibody.
ELISAs
TNF concentrations in mouse sera were measured using a mouse TNF Quantikine ELISA Kit according to the manufacturer’s instructions. Protein binding studies were conducted as previously described, (Pham et al., 2012).
Mouse infections
All animal experiments were performed according to Institutional Animal Care and Use Committee-approved protocols (Animal Welfare Assurance #A3237–01) and conducted as previously described (Gao et al., 2009).
Bioinformatics
Probabilistic searches using profile-Hidden Markov Models were done using the HHPred/HHSearch package (Soding et al., 2005). Multiple sequence alignments were obtained using the PROMALS3D software (Pei et al., 2008), seeded with NleB and their glycogenin-related homologs.
Statistics
RT-PCR, luciferase, ELISA, and enzyme activity data were analyzed statistically using one-way ANOVA with Bonferonni’s multiple comparison tests. C. rodentium colonization data were analyzed using the Kruskal-Wallis test. P values < 0.05 were considered significant.
Supplementary Material
Highlights.
The glycolysis enzyme GAPDH interacts with the TNF receptor associated factor, TRAF2
The C. rodentium and E. coli virulence effector NleB binds GAPDH
NleB uses its N-acetyl-D-glucosamine (O-GlcNAc) transferase activity to modify GAPDH
O-GlcNAcylation of GAPDH disrupts TRAF2-GAPDH complexes and attenuates NF-κB signaling
Acknowledgments
The project described was supported by Grant Numbers AI076227 and AI093913 from the National Institute of Allergy and Infectious Diseases (NIAID). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALVAREZ SE, HARIKUMAR KB, HAIT NC, ALLEGOOD J, STRUB GM, KIM EY, MACEYKA M, JIANG H, LUO C, KORDULA T, MILSTIEN S, SPIEGEL S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–8. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELYI Y, NIGGEWEG R, OPITZ B, VOGELSGESANG M, HIPPENSTIEL S, WILM M, AKTORIES K. Legionella pneumophila glucosyltransferase inhibits host elongation factor 1A. Proc Natl Acad Sci U S A. 2006;103:16953–8. doi: 10.1073/pnas.0601562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORREGAARD N, HERLIN T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–7. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUWMEESTER T, BAUCH A, RUFFNER H, ANGRAND PO, BERGAMINI G, CROUGHTON K, CRUCIAT C, EBERHARD D, GAGNEUR J, GHIDELLI S, HOPF C, HUHSE B, MANGANO R, MICHON AM, SCHIRLE M, SCHLEGL J, SCHWAB M, STEIN MA, BAUER A, CASARI G, DREWES G, GAVIN AC, JACKSON DB, JOBERTY G, NEUBAUER G, RICK J, KUSTER B, SUPERTI-FURGA G. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- BUGAREL M, BEUTIN L, FACH P. Low-density macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): a new approach for molecular risk assessment of STEC isolates. Appl Environ Microbiol. 2010;76:203–11. doi: 10.1128/AEM.01921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G, GOEDDEL DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- CRAMER T, YAMANISHI Y, CLAUSEN BE, FORSTER I, PAWLINSKI R, MACKMAN N, HAASE VH, JAENISCH R, CORR M, NIZET V, FIRESTEIN GS, GERBER HP, FERRARA N, JOHNSON RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAN P, KENNY B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol. 2009;12:101–9. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENG W, VALLANCE BA, LI Y, PUENTE JL, FINLAY BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48:95–115. doi: 10.1046/j.1365-2958.2003.03429.x. [DOI] [PubMed] [Google Scholar]
- FUJIKI R, HASHIBA W, SEKINE H, YOKOYAMA A, CHIKANISHI T, ITO S, IMAI Y, KIM J, HE HH, IGARASHI K, KANNO J, OHTAKE F, KITAGAWA H, ROEDER RG, BROWN M, KATO S. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–60. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO X, WAN F, MATEO K, CALLEGARI E, WANG D, DENG W, PUENTE J, LI F, CHAUSSEE MS, FINLAY BB, LENARDO MJ, HARDWIDGE PR. Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog. 2009;5:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABELHAH H, TAKAHASHI S, CHO SG, KADOYA T, WATANABE T, RONAI Z. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. EMBO J. 2004;23:322–32. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARA MR, AGRAWAL N, KIM SF, CASCIO MB, FUJIMURO M, OZEKI Y, TAKAHASHI M, CHEAH JH, TANKOU SK, HESTER LD, FERRIS CD, HAYWARD SD, SNYDER SH, SAWA A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–74. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- HARRISON GJ, VAN WIJHE MH, DE GROOT B, DIJK FJ, GUSTAFSON LA, VAN BEEK JH. Glycolytic buffering affects cardiac bioenergetic signaling and contractile reserve similar to creatine kinase. Am J Physiol Heart Circ Physiol. 2003;285:H883–90. doi: 10.1152/ajpheart.00725.2002. [DOI] [PubMed] [Google Scholar]
- HART GW, SLAWSON C, RAMIREZ-CORREA G, LAGERLOF O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Q, LAN F, ZHENG Z, XIE F, HAN J, DONG L, XIE Y, ZHENG F. Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating GAPDH at threonine 237 and decreasing its nuclear translocation. J Biol Chem. 2011;286:42211–20. doi: 10.1074/jbc.M111.296905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAUCHI K, ARAKI K, TOBIUME K, TANAKA N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 2009;106:3431–6. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY CP, LAMONT JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- KELLY M, HART E, MUNDY R, MARCHES O, WILES S, BADEA L, LUCK S, TAUSCHEK M, FRANKEL G, ROBINS-BROWNE RM, HARTLAND EL. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect Immun. 2006;74:2328–37. doi: 10.1128/IAI.74.4.2328-2337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZARUS MB, NAM Y, JIANG J, SLIZ P, WALKER S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564–7. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, MUSHEGIAN A. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Science. 2003;12:1418–31. doi: 10.1110/ps.0302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOKHERJEE N, LIPPERT DN, HAMILL P, FALSAFI R, NIJNIK A, KINDRACHUK J, PISTOLIC J, GARDY J, MIRI P, NASEER M, FOSTER LJ, HANCOCK RE. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–96. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- NADLER C, BARUCH K, KOBI S, MILLS E, HAVIV G, FARAGO M, ALKALAY I, BARTFELD S, MEYER TF, BEN-NERIAH Y, ROSENSHINE I. The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog. 2010;6:e1000743. doi: 10.1371/journal.ppat.1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON HJ, PEARSON JS, BADEA L, KELLY M, LUCAS M, HOLLOWAY G, WAGSTAFF KM, DUNSTONE MA, SLOAN J, WHISSTOCK JC, KAPER JB, ROBINS-BROWNE RM, JANS DA, FRANKEL G, PHILLIPS AD, COULSON BS, HARTLAND EL. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog. 2010;6:e1000898. doi: 10.1371/journal.ppat.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J, HAN D, KIM K, KANG Y, KIM Y. O-GlcNAcylation disrupts glyceraldehyde-3-phosphate dehydrogenase homo-tetramer formation and mediates its nuclear translocation. Biochim Biophys Acta. 2009;1794:254–62. doi: 10.1016/j.bbapap.2008.10.003. [DOI] [PubMed] [Google Scholar]
- PATHAK S, BORODKIN VS, ALBARBARAWI O, CAMPBELL DG, IBRAHIM A, VAN AALTEN DM. O-GlcNAcylation of TAB1 modulates TAK1-mediated cytokine release. EMBO J. 2012;31:1394–404. doi: 10.1038/emboj.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON JS, RIEDMAIER P, MARCHES O, FRANKEL G, HARTLAND EL. A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-kappaB for degradation. Mol Microbiol. 2011;80:219–30. doi: 10.1111/j.1365-2958.2011.07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEI J, KIM BH, GRISHIN NV. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008;36:2295–300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAM TH, GAO X, TSAI K, OLSEN R, WAN F, HARDWIDGE PR. Functional differences and interactions between the E. coli type III secretion system effectors NleH1 and NleH2. Infect Immun. 2012 doi: 10.1128/IAI.06358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMAN MM, MCFADDEN G. Modulation of NF-kappaB signalling by microbial pathogens. Nat Rev Microbiol. 2011;9:291–306. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN N, HARA MR, KORNBERG MD, CASCIO MB, BAE BI, SHAHANI N, THOMAS B, DAWSON TM, DAWSON VL, SNYDER SH, SAWA A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol. 2008;10:866–73. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SODING J, BIEGERT A, LUPAS AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–8. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TADA K, OKAZAKI T, SAKON S, KOBARAI T, KUROSAWA K, YAMAOKA S, HASHIMOTO H, MAK TW, YAGITA H, OKUMURA K, YEH WC, NAKANO H. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–4. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- VAN ANTWERP DJ, MARTIN SJ, KAFRI T, GREEN DR, VERMA IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- WELLEN KE, THOMPSON CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–6. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- WENTZEL P, EJDESJO A, ERIKSSON UJ. Maternal diabetes in vivo and high glucose in vitro diminish GAPDH activity in rat embryos. Diabetes. 2003;52:1222–8. doi: 10.2337/diabetes.52.5.1222. [DOI] [PubMed] [Google Scholar]
- WICKHAM ME, LUPP C, MASCARENHAS M, VAZQUEZ A, COOMBES BK, BROWN NF, COBURN BA, DENG W, PUENTE JL, KARMALI MA, FINLAY BB. Bacterial genetic determinants of non-O157 STEC outbreaks and hemolytic-uremic syndrome after infection. J Infect Dis. 2006;194:819–27. doi: 10.1086/506620. [DOI] [PubMed] [Google Scholar]
- WICKHAM ME, LUPP C, VAZQUEZ A, MASCARENHAS M, COBURN B, COOMBES BK, KARMALI MA, PUENTE JL, DENG W, BRETT FINLAY B. Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 2007;9:400–7. doi: 10.1016/j.micinf.2006.12.016. [DOI] [PubMed] [Google Scholar]
- YANG WH, PARK SY, NAM HW, KIM DO H, KANG JG, KANG ES, KIM YS, LEE HC, KIM KS, CHO JW. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008;105:17345–50. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEH WC, SHAHINIAN A, SPEISER D, KRAUNUS J, BILLIA F, WAKEHAM A, DE LA POMPA JL, FERRICK D, HUM B, ISCOVE N, OHASHI P, ROTHE M, GOEDDEL DV, MAK TW. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–25. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- ZHENG Y, VALDEZ PA, DANILENKO DM, HU Y, SA SM, GONG Q, ABBAS AR, MODRUSAN Z, GHILARDI N, DE SAUVAGE FJ, OUYANG W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.