Abstract
Intravenous fish oil (FO) has changed the management of intestinal failure associated liver disease (IFALD). This report describes two IFALD patients who received FO for 5 and 10 months, respectively and reports on their 3-year follow-up.
Keywords: cholestasis, parenteral nutrition, fish oil, soybean oil, short bowel syndrome, premature
Introduction
Intestinal failure (IF) is the gastrointestinal tract's inability to absorb adequate fluids and nutrients. This condition results in parenteral nutrition (PN) dependence. While PN is life-sustaining, it can be associated with intestinal failure associated liver disease (IFALD) – a complication that can result in liver failure and the need for transplant. Risk factors for IFALD include prematurity, lack of enteral nutrition (EN), duration of PN, sepsis, and multiple gastrointestinal surgeries. Studies have demonstrated that when standard intravenous soybean oil (SO) is replaced with fish oil (FO) (Omegaven®, Fresenius Kabi, Bad Hamburg, Germany), direct hyperbilirubinemia is more likely to resolve1-10.
FO, which is not approved by the United States Food and Drug Administration (FDA), is only available under compassionate use and research protocols in the United States, and costs significantly more than SO. Because third party payers do not always reimburse for experimental therapies, hospitals and investigators shoulder the majority of the expense. Adding to the cost, investigators may continue FO until PN discontinuation even if direct hyperbilirubinemia has resolved. To date, the longest median follow-up on liver function reported on children who have received FO is 16 months9. There is no data on patients whose FO has been discontinued prior to intestinal adaptation, and limited follow-up on subjects after adaptation2-10.
We present two children who received FO for IFALD. FO emergency use was granted by the FDA, and informed consent was obtained. In order to limit potential toxicities associated with FO and the overall financial cost of the drug, the decision to terminate FO was made by a multi-disciplinary team taking into account risk factors for IFALD and after reversal of cholestasis and EN improvement. To our knowledge, this report provides the longest follow-up to date on children who have received an exclusively FO-based lipid emulsion, and is the only report on children whose FO was discontinued despite the need for ongoing PN.
Case 1
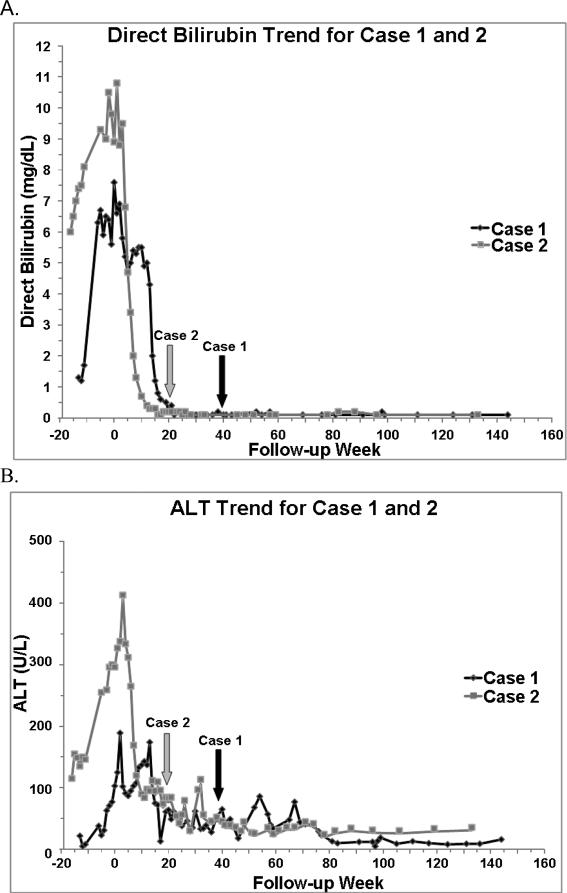
Case 1 is a full-term neonate with gastroschisis and intrauterine growth restriction (birth weight 1.7 kg). A silo was placed, and on day of life (DOL) 2, she developed necrotic bowel requiring resection of her ileocecal valve and all but 15 cm of small bowel. Abdominal closure occurred on DOL 5. Because of anticipated adhesions and multiple abdominal surgeries, a jejunocolostomy was created on DOL 56. Advancement of EN was initially limited by gut dysmotility and short gut. By age 2 months, she developed biochemical IFALD (Figure 1). Her platelet count and coagulation profile, however, were normal. At age 3 months, in an effort to avoid transplant, the patient's SO was replaced with FO (1 gm/kg/d) (EIND 104,766). Prior to transitioning to FO, Case 1 was receiving 121 kcal/kg/d and 3 gm/kg/d of SO (Table 1). By week 24 after FO initiation, her serum direct bilirubin concentration was normal (< 0.2 mg/dL on 2 consecutive measurements separated by 1 week). Her serum transaminase concentrations normalized by week 29 (Figure 1). After 10 months of treatment, when FO was discontinued, 14% of her calories were from EN, and her growth remained suboptimal (Table 2).
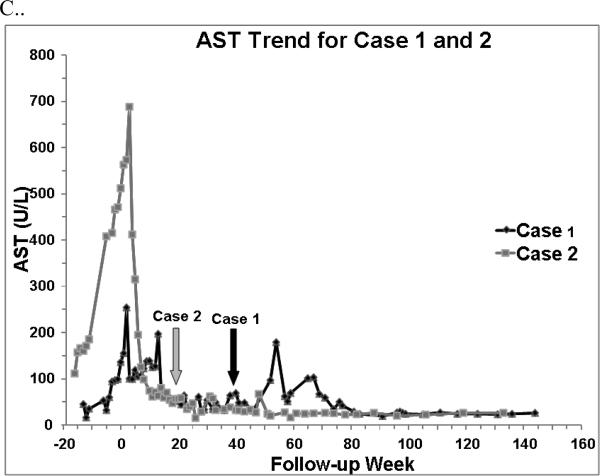
Figure 1.
A. Direct bilirubin trend, B. Alanine aminotransferase (ALT) trend, and C. Asparate aminotransferase (AST) trend for Case 1 and 2. Week 0 represents baseline, when FO (fish oil) was started. Arrows indicate FO discontinuation. A normal direct bilirubin was defined as < 0.2 mg/dL on 2 consecutive measurements. A normal ALT and AST were defined as ≤45 and 36 U/L, respectively on 2 consecutive measurements.
Table 1. Factors Affecting Intestinal Failure Associated Liver Disease in Each Case.
Nutrition and other confounders of intestinal failure associated liver disease at the start of fish oil (FO), FO discontinuation, and 3-year follow-up for Case 1 and 2.
| Case 1 | Case 2 | |
|---|---|---|
| Baseline | ||
| PN Calories (kcal/kg/day) | 121 | 70 |
| Glucose Delivery Rate (mg/kg/min) | 22 | 16 |
| Amino Acids (gm/kg/d) | 3.5 | 1.4 |
| Soybean Oil | 3 gm/kg/day | 2 gm/kg once a week |
| Type of EN | None | Breast milk plus rice cereal |
| EN Calories (kcal/kg/day) | 0 | 59 |
| Total Calories (kcal/kg/d) | 121 | 129+ |
| Number of Gastrointestinal Surgeries† | 4 | 2 |
| Number of Septic Episodes‡ | 0 | 0 |
| At FO Discontinuation | ||
| PN Calories (kcal/kg/day) | 90 | 29 |
| Glucose Delivery Rate (mg/kg/min) | 23 | 3 |
| Amino acids (gm/kg/d) | 2.6 | 1.2 |
| FO (gm/kg/day) | 1 | 1 |
| Type of EN | Puree food plus 340mL ½ strength elemental infant formula | Puree food plus 780mL elemental pediatric formula |
| EN Calories (kcal/kg/day) | 17+ | 70+ |
| Total Calories (kcal/kg/d) | 107+ | 99+ |
| Number of Gastrointestinal Surgeries† | 0 | 0 |
| Number of Septic Episodes during FO‡ | 4 | 1 |
| 3-year Follow-up | ||
| PN Calories (kcal/kg/day) | 83 | 0 |
| Glucose Delivery Rate (mg/kg/min) | 23 | 0 |
| Amino Acids (gm/kg/d) | 3.5 | 0 |
| Soybean Oil (gm/kg/day) | 2 | 0 |
| Type of EN | Pediatric diet Ad Lib | Pediatric diet plus 625mL elemental pediatric formula |
| EN Calories (kcal/kg/day) | Variable | 46+ |
| Total Calories (kcal/kg/d) | 83+ | 46+ |
| Number of Gastrointestinal Surgeries† | 0 | 0 |
| Number of Septic Episodes after FO‡ | 2 | 3 |
Gastrointestinal surgeries do not include gastrostomy tube placement.
Sepsis is defined as a positive blood culture with symptoms suggestive of sepsis and need for intravenous antibiotics.
Table 2.
Growth for Case 1 and 2.
| Case 1 | Case 2 | |
|---|---|---|
| Baseline | ||
| Weight (Z-score)* | -2 | -0.3 |
| Length (Z-score)* | -2.8 | 0 |
| At FO Discontinuation | ||
| Weight (Z-score)* | -1.9 | -0.7 |
| Length (Z-score)* | -1.5 | -1.1 |
| 3-year Follow-up | ||
| Weight (Z-score)* | 0 | -0.5 |
| Length (Z-score)* | -2 | -0.5 |
Z-scores calculated using information from the Center for Disease Control data tables on infant weight- and length-for-age11.
At 3.3 years of age, 3 years after FO initiation (27 months after FO discontinuation), she continues to receive the majority of her calories from PN and 2 gm/kg/d of SO. While her serum bilirubin concentrations, platelet counts, and international ratios are normal, her serum transaminase concentrations occasionally fluctuate above normal (Figure 1). She has demonstrated adequate “catch-up” growth, but her linear growth remains below average (Table 2). No adverse events including bleeding or an essential fatty acid deficiency were associated with FO.
Case 2
Case 2 is an ex-36 week neonate with a birth weight appropriate for gestational age and jejunal atresia with an apple-peel like deformity. On DOL 3, the atresia was repaired with primary anastomosis. Multiple intestinal strictures later developed requiring small bowel resection on DOL 51, leaving her with a partial duodenum, 10 cm of ileum, ileocecal valve, and a full colon in continuity. At 1 month of age, Case 2 developed a direct hyperbilirubinemia, and by 6 months of age she demonstrated severe IFALD manifested by cholestasis, abnormal serum transaminase concentrations, a mild thrombocytopenia, and a liver biopsy demonstrating bridging fibrosis (Figure 1). 2 months prior to the FO start date, Case 2's platelet count ranged from 97,000-130,000. At age 6.5 months, her SO (2 gm/kg once a week) was replaced with FO (1 gm/kg/d), and she received five months of therapy (EIND 104,951). Prior to FO, she was receiving 129 kcal/kg/d and 46% of her caloric intake was from EN (Table 1). While her cholestasis resolved by week 17, her serum aspartate and alanine aminotransferase concentrations corrected after FO discontinuation, by week 28 and 41, respectively (Figure 1). 1 year after FO discontinuation, her platelet count was persistently above 150,000. When FO was terminated, she was placed on PN without SO as she was receiving 70% of her calories from EN.
At 2.4 years of age, almost 2 years after starting FO, Case 2 demonstrated complete intestinal adaptation. At 3.5 years of age, 3 years after FO initiation (2.5 years after FO discontinuation), her liver function tests are normal and her growth is acceptable (Figure 1, Table 2). No adverse events were associated with FO.
Discussion
We present two children with short bowel and IFALD in whom FO served as a means to reverse biochemical IFALD. Case 1 and 2's resolution of direct hyperbilirubinemia, strictly defined as a direct bilirubin < 0.2 mg/dL on 2 consecutive measurements at least 1 week apart, occurred at FO weeks 24 and 17, respectively. Using this information and data from two published studies, we implemented a research protocol (IND 105,326) providing 24 weeks of FO to children at high risk for the complications of IFALD3,4. In the study published by Gura et al the median time to achieve a direct bilirubin < 2 mg/dL was 9.4 weeks for patients with congenital or acquired gastrointestinal disorders with a mean age of 14 weeks and median baseline direct bilirubin of 5.4 mg/dL 4. Subjects received 1 gm/kg/d of FO. In comparison, Diamond et al provided a combination of FO and SO, each dosed at 1 gm/kg/d, to older patients (median age 7.5 months) with more severe cholestasis (median conjugated bilirubin 8.06 mg/dL). 9 out 12 patients demonstrated resolution of cholestasis, defined as a conjugated direct bilirubin of 0 μmol/L, at a median time of 24 weeks. Interestingly, 5 patients were transitioned to FO monotherapy due to lack of response.3
Case 1 is unique—unlike Case 2 and most IFALD patients who receive FO in the United States, Case 1's FO was terminated prior to PN discontinuation. Case 1 maintained normal serum bilirubin concentrations despite ongoing PN, septic episodes, and SO reinitiation. To our knowledge, this is the first report of FO termination and SO reinitation in a patient with extreme SBS and IFALD who has not redeveloped cholestasis. Case 2, on the other hand, maintained normal serum bilirubins and liver functions tests off FO, but did not restart SO because she was receiving sufficient enteral calories (Table 1). A concern is that once FO is terminated, IFALD will return. In comparison to younger children, older children have a decreased risk of IFALD. Hence, FO initiation at an earlier age and prior to advanced IFALD may be advantageous. Once two milestones have been achieved—biochemical resolution of IFALD and EN progress, the liver may be less susceptible to SO and PN toxicities and restarting SO at an older age may be acceptable. Even though FO is more expensive than SO, considering the cost of a transplant and post-transplant care, FO may be a more economically sound alternative to SO12.
Despite more advanced liver disease and initiation of FO at an older age, Case 2 experienced reversal of cholestasis in a shorter time frame than Case 1 (Figure 1). Because FO was not available at our institution until March 2009, Case 2's FO start date was delayed. Explanations for Case 2's quicker reversal are a more appropriate gestational age, more favorable gastrointestinal anatomy, greater enteral intake, enhanced intestinal adaptation, and fewer septic episodes13,14 (Table 1). In both cases, serum bilirubin concentrations normalized before transaminase concentrations as seen in prior reports, and Case 2's transaminases normalized after FO2-4 (Figure 1).
It remains unclear if FO can reverse advanced hepatic histological changes and portal hypertension in the pediatric population15. Case 2 had evidence of portal hypertension at FO initiation and discontinuation as evidenced by clinical exam, liver function tests, and a mild thrombocytopenia (Figure 1). While ultrasounds with doppler would have provided additional information, they were not performed. We can only speculate if longer FO administration would have resulted in a more rapid resolution of Case 2's transaminitis and thrombocytopenia, or if Case 1 would have less fluctuations in her transaminases. Previous reports have demonstrated improved median platelet counts and no change in International Ratios with FO2-4. In the absence of a repeat liver biopsy, it is unknown if Case 2's liver fibrosis may have improved15.
The mechanism by which FO reverses IFALD is likely related to a lower dose of fat (1 gm/kg/d), lack of phytosterols, the anti-inflammatory properties of omega-3 fatty acids and a higher dose of the antioxidant Vitamin E in the form α-tocopherol. SO is dosed up to 4 gm/kg/d and contains high concentrations of phytosterols and inflammatory omega-6 fatty acids, and a lower dose of Vitamin E in the form of γ-tocopherol. Phytosterols reduce biliary flow by antagonizing the farnesoid X receptor, a bile acid nuclear receptor16. Compared to γ-tocopherol, α-tocopherol is more efficient at counteracting free radical damage from lipid perioxidation10. While a change in the composition of the lipid emulsion may be responsible for the resolution of cholestasis, improvement in serum bilirubin concentrations has been noted with SO dose reduction17,18. In attempt to limit her exposure to the hepatoxic effects of SO, Case 2 was prescribed a minimal amount of SO (Table 1). Despite this approach, her IFALD progressed. It remains unclear how lipid composition and dose interact, and which one is more important.
Lipid sparing has raised concerns as premature and IF patients are at high risk for failure-to-thrive and essential fatty deficiencies. While some studies of IFALD children have demonstrated adequate growth with lipid minimization, larger, long-term studies are lacking4,17. While Case 1's growth was suboptimal prior to and during FO, she demonstrated adequate “catch-up” growth with 2 gm/kg/d of SO and improved enteral intake (Table 1). Case 1's persistently negative z-score for length is mostly a reflection of her intestinal failure, genetic potential and history of intrauterine growth restriction14. In contrast, Case 2 demonstrated adequate growth despite lipid sparing during her PN course. In both cases, improved growth is most likely related to improved enteral absorption and, for Case 1, an increase in the dose of her intravenous fat emulsion.
While FO dosed at 1 gm/kg/d has generally not been associated with an essential fatty acid deficiency, less than 1 gm/kg/d of FO or SO can cause a deficiency4,17,19,20. Term neonates require 0.1 gm/kg/d and preterm neonates require 0.25 gm/kg/d of SO to prevent an essential fatty acid deficiency20. Despite 2 gm/kg once a week of SO, standard laboratory evaluations revealed an appropriate triene-tetraene ratio (0.028) for Case 2. This is most likely because she was receiving a fair amount of enteral nutrition in the form of breast milk.
FO provided a bridge to bowel adaptation for Case 2. While children with extreme short gut have been weaned from PN, Case 1 may ultimately require a combined small bowel-liver transplant or isolated small bowel transplant. Her risk factors include poor gastrointestinal anatomy and function necessitating long-term PN, and history of septic episodes and central venous catheter replacements. However, her overall mortality has improved. Until data is available from follow-up and randomized controlled studies, FO's effect on long-term transplant-free survival is unknown. While a randomized controlled trial in a population with advanced IFALD is controversial, this type of trial could provide information on FO and SO minimization efficacy and safety, along with guidelines for FO candidacy and duration. In summary, this report suggests that a limited FO course may help reverse biochemical IFALD. Using FO as a bridge to intestinal rehabilitation or therapy to reverse IFALD so that SO can be restarted at a later date when the liver has matured and can be further protected by EN may be safe and cost-effective.
Acknowledgments
Funding
Kara Calkins, MD has received funding from the NIH K12HD00140, MO1 RR000865, and 2T32GM075776. Stephen Shew, MD has received funding from the NIH K08HD052885. Robert Venick, MD has received funding from the Today's and Tomorrow's Children Fund, University of California, Los Angeles. Intravenous fish oil (Omegaven®, Fresenius Kabi, Bad Hamburg, Germany) was purchased with funds from James Yoo, MD, the Division of Pediatric Surgery, and the Woman's Auxiliary Fund (University of California, Los Angeles).
Abbreviations
- IF
intestinal failure
- PN
parenteral nutrition
- IFALD
intestinal failure associated liver disease
- EN
enteral nutrition
- SO
soybean oil
- FO
fish oil
- FDA
Food and Drug Administration
- DOL
day of life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Contributor Information
Kara Calkins, David Geffen School of Medicine, University of California, Los Angeles, Mattel Children's Hospital Department of Pediatrics, Division of Neonatology and Developmental Biology 10833 Le Conte Avenue, Room B2375 MDCC, Los Angeles, CA 90095 Phone: (310) 825-9330, Fax: (310) 267-0154; KCalkins@mednet.ucla.edu.
Allison Lowe, Mattel Children's Hospital, Department of Pediatrics, University of California, Los Angeles.
Stephen B. Shew, University of California, Los Angeles, Department of Surgery.
James C.Y. Dunn, University of California, Los Angeles, Department of Surgery.
Laurie Reyen, Department of Nursing, Mattel Children's Hospital, University of California, Los Angeles.
Douglas G. Farmer, David Geffen School of Medicine, University of California, Los Angeles, Department of Surgery.
Sherin U. Devaskar, David Geffen School of Medicine, University of California, Los Angeles, Mattel Children's Hospital, Department of Pediatrics.
Robert Venick, David Geffen School of Medicine, University of California, Los Angeles, Mattel Children's Hospital Department of Pediatrics.
References
- 1.Van Aerde J, Duerksen D, et al. Intravenous fish oil emulsion attenuates total parenteral induced cholestasis in new born piglets. Pediatr Res. 1999;45:202–8. doi: 10.1203/00006450-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Puder M, Valim C, et al. Parenteral fish oil improves outcomes in patients with parenteral-associated liver injury. Ann of Surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond IR, Sterescu A, et al. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 4.Gura KM, Lee S, et al. Safety and efficacy of a fish-oil based fat emulsion in the treatment of parenteral associated liver disease. Pediatrics. 2008;121:678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 5.Le HD, de Meijer VE, Robinson, et al. Parenteral fish-oil-based lipid emulsion improves fatty acid profiles and lipids in parenteral nutrition-dependent children. Am J Clin Nutr. 2011;94:749–58. doi: 10.3945/ajcn.110.008557. [DOI] [PubMed] [Google Scholar]
- 6.Ekema G, Falchett D, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (omega-3 fatty acids). J Pediatr Surg. 2008;43:1191–5. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Gura KM, Duggan CP, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;188:197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 8.Lilja HE, Finkel Y, et al. Prevention and reversal of intestinal failure associated liver disease in premature infants with short bowel syndrome using intravenous fish oil in combination with omega-6/9 lipid emulsions. J Pediatr Surg. 2011;46:1361–7. doi: 10.1016/j.jpedsurg.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Angsten G, Finkel Y. Improved outcomes in neonatal short bowel syndrome using parenteral fish oil in combination with ω-6/9 lipid emulsions. JPEN J Parenter Enteral Nutr. 2012 Jan 24;36:587–95. doi: 10.1177/0148607111430507. [DOI] [PubMed] [Google Scholar]
- 10.Goulet O, Antébi H, et al. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2010;34:485–95. doi: 10.1177/0148607110363614. [DOI] [PubMed] [Google Scholar]
- 11.Center for Disease Control http://www.cdc.gov/growthcharts/clinical_charts.htm.
- 12.6th Annual International Pediatric Intestinal Failure Symposium; Chicago, IL. 2010. [Google Scholar]
- 13.Robinson DT, Ehrenkranz RA. Parenteral-associated cholestasis in small for gestational age infants. J Pediatr. 2008;152:59–62. doi: 10.1016/j.jpeds.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Cole CR, Hansen NI, et al. Very low birth weight premature infants with surgical short bowel syndrome: incidence, morbidity, mortality, and growth outcomes at 18 and 22 months. Pediatrics. 2008;122:e573–82. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgibbons S, Jones B, et al. Relationship between biopsy-proven parenteral nutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg. 2010;45:95–99. doi: 10.1016/j.jpedsurg.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BA, Talyor OA, et al. Stigmasterol, a soy lipid derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 17.Cober MP, Killu G, et al. Intravenous fat emulsion reduction for patients with parenteral-associated liver disease. J Pediatr. 2012;160:421–7. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 18.Colomb V, Jobert-Giraud A, et al. Role of lipid emulsions in cholestasis associated with long-term parenteral in children. JPEN. 2000;24:345–50. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 19.Le DL, Meisel J, et al. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81:165–70. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EJ, Simmer K, et al. Essential fatty acid deficiency in parenterally fed preterm infants. J Paediatr Child Health. 1993;29:51–5. doi: 10.1111/j.1440-1754.1993.tb00440.x. [DOI] [PubMed] [Google Scholar]