Abstract
Background
Despite the fact that 80% of patients with heart failure are over age 65, recognition of cognitive impairment by physicians in this population has received relatively little attention. The purpose of our study was to evaluate physician documentation (as a measure of recognition) of cognitive impairment at time of discharge in a cohort of older adults hospitalized for heart failure.
Methods
We performed a prospective cohort study of older adults hospitalized with a primary diagnosis of heart failure. Cognitive status was evaluated with the Folstein Mini-Mental State Examination (MMSE) at the time of hospitalization. A score of 21–24 was used to indicate mild cognitive impairment, and a score of ≤20 to indicate moderate to severe impairment. To evaluate physician documentation of cognitive impairment, we used a standardized form with a targeted keyword strategy to review hospital discharge summaries. We calculated the proportion of patients with cognitive impairment documented as such by physicians, and compared characteristics between groups with and without documented cognitive impairment. We then analyzed the association of cognitive impairment, and documentation of cognitive impairment, with 6-month mortality or readmission using Cox proportional hazards regression.
Results
A total of 282 patients completed the cognitive assessment. Their mean age was 80 years of age, 18.8% were nonwhite, and 53.2% were female. Cognitive impairment was present in 132/282 patients (46.8% overall; 25.2% mild, 21.6% moderate-severe). Among those with cognitive impairment, 30/132 (22.7%) were documented as such by physicians. Compared with patients whose cognitive impairment was documented by physicians, those whose impairment was not documented were younger (81.3 years vs. 85.2 years, P<0.05) and had less severe impairment (median MMSE score 22.0 vs. 18.0, P<0.01). After multivariable adjustment, patients whose cognitive impairment was not documented were significantly more likely to experience 6-month mortality or hospital readmission than patients without cognitive impairment.
Conclusions
Cognitive impairment is common in older adults hospitalized for heart failure, yet frequently not documented by physicians. Implementation of strategies to improve recognition and documentation of cognitive impairment may improve the care of these patients, particularly at the time of hospital discharge.
Keywords: heart failure, aging, cognition
INTRODUCTION
Heart failure is overwhelmingly a disease of older adults; approximately 80% of patients in the United States are over 65 years of age 1, 2, and the proportion of patients of very advanced age (≥80 years) has nearly doubled over the last 20 years 3. In addition, heart failure remains a leading cause of hospitalization and early readmissions among Medicare beneficiaries 2, 4. Despite the aging of this population, cognitive impairment may be unrecognized by physicians caring for older patients with heart failure as it falls outside the traditional disease-focused model that dominates heart failure research and clinical care 5–8. Cognitive impairment is among the strongest independent predictors of mortality in older adults hospitalized for heart failure 8. Its recognition has important implications given the complex self-care requirements for optimal heart failure disease management which include symptom monitoring, daily self-weighing, dietary compliance, and adherence with medications 9–11.
Previous work suggests that cognitive impairment is relatively common in older adults hospitalized for heart failure; though estimates vary widely, most studies report a prevalence of at least 25% 10, 12–15. However, the degree to which cognitive impairment is recognized by physicians in older patients is unknown. Recognition at the time of heart failure hospitalization is relevant because it may facilitate targeted interventions – for example, simplifying medical regimens at discharge, tailoring discharge education, or assistance with home medication management – that may improve outcomes including readmissions. With this in mind, the purpose of our study was to assess physician documentation (as a measure of recognition) of cognitive impairment in older adults hospitalized for heart failure, and its association with outcomes.
METHODS
Study Design and Participants
We performed a prospective cohort study of older adults hospitalized for heart failure at two Connecticut Hospitals (Yale-New Haven Hospital and Hospital of Saint Raphael). Participants were enrolled in the COPing with Heart Failure (Comorbidity in Older Patients with heart failure) study, which recruited patients age 65 years and older hospitalized for heart failure between October 31, 2008 and December 22, 2010. The objective of the COPing with Heart Failure study was to assess the prevalence of comorbidities, including cognitive impairment, among non-disabled older adults. Patients were identified at the time of hospitalization by reviewing electronic inpatient census lists for admission diagnoses of heart failure or heart failure-related symptomatology (dyspnea, respiratory failure, or fluid overload). Subsequently, an experienced nurse researcher performed medical record review to confirm a primary admission diagnosis of heart failure, using the Framingham criteria 1. Enrollment occurred during hospitalization between the day after admission and the day of discharge, depending on patient availability. Patients were deemed ineligible if they were non-English speaking, admitted from a nursing home, had isolated right-sided heart failure, or were found to be delirious based on the Confusion Assessment Method 16. Since the goal of the COPing study was to examine the prevalence of comorbidities in a non-disabled cohort of older adults, patients who reported being dependent in ≥3 activities of daily living 2 weeks prior to admission were also excluded. The study was approved by the Yale University School of Medicine Human Investigation Committee. All subjects provided written informed consent.
Clinical Variables
Data were collected through detailed medical record review using a structured abstraction form. Variables collected included age, sex, race (determined through self-report), admission blood pressure, left ventricular ejection fraction (as determined by echocardiogram, Multi Gated Acquisition (MUGA) scan, or left ventriculogram performed within the past 6 months), medical history (chronic renal failure, hypertension, diabetes, coronary artery disease, chronic lung disease), and medications at discharge.
Assessment of Cognitive Impairment
Cognitive impairment was assessed with the Folstein Mini-Mental State Examination (MMSE) 17, which is the most widely utilized instrument to assess cognitive status in older adults 18, 19. The MMSE consists of 11 items that assess domains of orientation, short-term memory, attention, and visual spatial skills, and is scored on a 30-point scale. A score of <25 is generally considered abnormal 20, 21. For the purposes of our study, and consistent with established cut-points, we considered a score of 21–24 to indicate mild cognitive impairment, and a score of ≤20 to indicate moderate to severe impairment 20, 21. The MMSE was administered by a research nurse trained in geriatric assessments.
Physician Documentation of Cognitive Impairment
To evaluate physician documentation of cognitive impairment at the time of discharge, hospital discharge summaries were independently reviewed by two physician investigators (JD, TT) using a standardized form with targeted keywords developed by consensus among the study authors. Cognitive impairment was considered documented if “cognitive impairment” or any of the following terms were present in the discharge summary: dementia, Alzheimer’s disease, memory problems, senile, delirium, confusion, or forgetfulness. Variations in these terms (e.g. cognitively impaired, demented), if present, were counted as well. Cognitive impairment was also considered documented if a new medication intended for treatment of dementia (acetylcholinesterase inhibitor, N-methyl-D-aspartate inhibitor) was initiated during hospitalization and included on the discharge medication list.
Outcomes
Hospital readmission within 6 months of discharge was ascertained through review of electronic medical records. Readmissions to other hospitals (i.e., other than the original, admitting hospital) were not obtainable. Mortality data at 6 months were obtained through review of the Social Security Death Index 22.
Statistical Analysis
To examine characteristics of the study cohort we calculated proportions for categorical variables and means and standard deviations for continuous variables that were normally distributed. For MMSE scores, which were not normally distributed, we generated medians and interquartile ranges. To assess documentation of cognitive impairment, we determined the overall proportion of patients with cognitive impairment that were documented as such by physicians, and analyzed the frequency at which impaired subgroups (mildly abnormal MMSE, moderate-severely abnormal MMSE) were documented. We compared characteristics of patients with and without cognitive impairment, as well as characteristics among patients with cognitive impairment who were documented versus not documented, using the t-test for normally distributed continuous variables and the chi-square test for categorical variables. For between-group comparisons of MMSE scores we generated probability distributions with the Wilcoxon rank-sum test.
To examine 6-month rates of the combined endpoint of mortality or (all-cause) hospital readmission we performed a Cox proportional hazards regression, separating patients into three groups: no cognitive impairment, mild cognitive impairment, and moderate-severe cognitive impairment. We calculated unadjusted hazard ratios for the 6-month combined endpoint using “no cognitive impairment” as the reference group. We then adjusted hazard ratios for baseline differences between groups (age, race, kidney disease, aldosterone receptor antagonist use) in a multivariable model. We also performed a Cox proportional hazards regression to examine the association of documentation of cognitive impairment with the 6-month combined endpoint by separating patients into three groups: no cognitive impairment (reference group), documented cognitive impairment, and cognitive impairment that was not documented. All statistical tests were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
Study sample
Of the 437 patients approached for enrollment, 48 were excluded due to dependency in activities of daily living, 3 were excluded due to delirium, and 104 declined participation, leaving a total of 282 (64.5%) who consented to participation in the study. There were no significant differences between patients who consented versus those who did not consent (mean age 80.0 vs. 80.4 years, P=0.87; female 53.2% vs. 50.3%, P=0.56; nonwhite 18.8% vs. 16.4%, P=0.62). Baseline clinical characteristics of the study sample are listed in Table 1. The most common comorbidities were hypertension (79.1%), diabetes mellitus (63.8%), and coronary artery disease (60.3%).
Table 1.
Study Sample Characteristics
| Total (N=282) | Cognitive Impairment | P Value | ||
|---|---|---|---|---|
| Yes (N=132) | No (N=150) | |||
| Age (mean ± SD) | 80.0±8.0 | 82.2±7.9 | 78.1±7.6 | <0.01 |
| Female | 150 (53.2%) | 65 (49.2%) | 87 (58.0%) | 0.14 |
| Nonwhite race | 53 (18.8%) | 37 (28.0%) | 16 (10.7%) | <0.01 |
| Blood pressure (mmHg ± SD) | ||||
| Systolic | 137.2±26.8 | 140.2±27.7 | 134.5±25.9 | 0.08 |
| Diastolic | 73.5±16.1 | 74.4±17.6 | 72.6±14.6 | 0.35 |
| Left ventricular ejection fraction <50%* | 117 (46.4%) | 55 (48.3%) | 62 (44.9%) | 0.60 |
| Comorbid diseases | ||||
| Chronic Kidney Disease | 113 (40.1%) | 63 (47.7%) | 50 (33.3%) | <0.05 |
| Chronic Lung Disease | 91 (32.3%) | 49 (37.1%) | 42 (28.0%) | 0.10 |
| Diabetes Mellitus | 180 (63.8%) | 85 (64.4%) | 95 (63.3%) | 0.85 |
| Hypertension | 223 (79.1%) | 107 (81.1%) | 116 (77.3%) | 0.44 |
| Coronary artery disease | 170 (60.3%) | 79 (59.9%) | 91 (60.7%) | 0.89 |
| Medications† | ||||
| ACE inhibitor or ARB | 107 (38.4%) | 57 (43.5%) | 50 (33.8%) | 0.10 |
| Beta blocker | 229 (82.1%) | 104 (79.4%) | 125 (84.5%) | 0.27 |
| Loop diuretic | 248 (88.9%) | 118 (90.1%) | 130 (87.8%) | 0.55 |
| Digoxin | 30 (10.8%) | 13 (9.9%) | 17 (11.5%) | 0.67 |
| Aldosterone-receptor antagonist | 48 (17.3%) | 16 (12.3%) | 32 (21.6%) | <0.05 |
Ejection fraction data missing for 30 patients (11%)
Medication data missing for 3 patients (1%)
Prevalence of cognitive impairment
The median MMSE score among the study population was 25 (interquartile range, 22 to 27), and ranged from 5 to 30 with clustering at higher scores (Figure 1). Cognitive impairment was present in 46.8% of patients. Overall, 25.2% met criteria for mild cognitive impairment and 21.6% met criteria for moderate-severe cognitive impairment.
Figure 1.
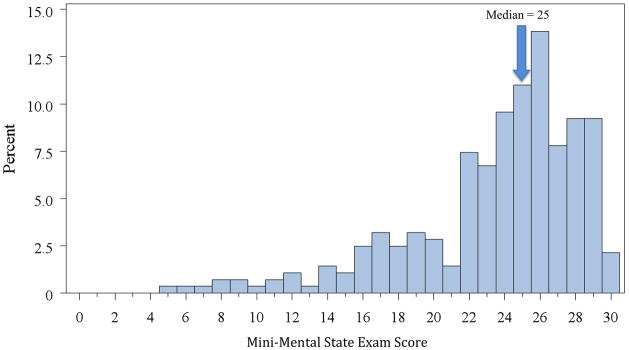
Distribution of MMSE scores among the study sample
As shown in Table 1, compared with patients without cognitive impairment, patients with cognitive impairment were older (82.2 years vs. 78.1 years, P<0.01), more often of nonwhite race (28.0% vs. 10.7%, P<0.01), and had higher rates of chronic kidney disease (47.7% vs. 33.3%, P<0.05) (Table 1). They were also less likely to be prescribed an aldosterone-receptor antagonist at discharge (12.3% vs. 21.6%, P<0.05). There were no significant differences in other discharge medications, or medical comorbidities, between the two groups.
Documentation of cognitive impairment
Of the 132 patients with cognitive impairment, 22.7% were documented as such by physicians at time of discharge. Documentation was less common in patients with mild cognitive impairment than in patients with moderate-severe cognitive impairment (11.3% vs. 39.3%, P<0.01) (Table 2). As shown in Table 3, patients with documented cognitive impairment were older than patients with impairment that was not documented (85.2 vs. 81.3 years, P<0.05), and their median MMSE scores were lower (18.0 vs. 22.0, P<0.01). There were no significant differences in race (37.5% nonwhite in the recognized group vs. 25.0% in the unrecognized group, P=0.17), sex (56.3% female in the recognized group vs. 47.0% in the unrecognized group, P=0.36), or medical comorbidities between patients with and without documented cognitive impairment.
Table 2.
Documentation among Patients with Cognitive Impairment
| Total (N=132) | Documented at Discharge (N=30/132) | |
|---|---|---|
| Mild cognitive impairment | 71 (53.8%) | 8/71 (11.3%)* |
| Moderate-severe cognitive impairment | 61 (46.2%) | 24/61 (39.3%) |
P<0.01 for comparison of documentation of mild vs. moderate-severe impairment
Table 3.
Characteristics of patients with and without documented cognitive impairment
| Cognitive Impairment Documented | P | ||
|---|---|---|---|
| Yes (N=32) | No (N=100) | ||
| Age (mean ± SD) | 85.2±7.0 | 81.3±8.0 | <0.05 |
| Female | 18 (56.3%) | 47 (47.0%) | 0.36 |
| Nonwhite race | 12 (37.5%) | 25 (25.0%) | 0.17 |
| MMSE (median (interquartile range)) | 18.0 (12.0–21.0) | 22.0 (18.0–23.5) | <0.01* |
| Blood pressure (mmHg ± SD) | |||
| Systolic | 142.0±25.8 | 139.7±28.4 | 0.68 |
| Diastolic | 75.3±13.6 | 74.1±18.7 | 0.74 |
| Left ventricular ejection fraction <50%† | 13 (44.8%) | 42 (49.1%) | 0.67 |
| Comorbid diseases | |||
| Chronic Kidney Disease | 17 (53.1%) | 46 (46.0%) | 0.48 |
| Chronic Lung Disease | 13 (40.6%) | 36 (36.0%) | 0.64 |
| Diabetes Mellitus | 21 (65.6%) | 64 (64.0%) | 0.87 |
| Hypertension | 28 (87.5%) | 79 (79.0%) | 0.29 |
| Coronary artery disease | 16 (50.0%) | 63 (63.0%) | 0.19 |
| Medications§ | |||
| ACE inhibitor or ARB | 16 (51.6%) | 41 (41.0%) | 0.30 |
| Beta blocker | 23 (74.2%) | 81 (81.0%) | 0.41 |
| Loop diuretic | 26 (83.9%) | 92 (92.0%) | 0.19 |
| Digoxin | 4 (12.9%) | 9 (9.0%) | 0.53 |
| Aldosterone-receptor antagonist | 4 (12.9%) | 12 (12.1%) | 0.67 |
Wilcoxon-rank sum test
Ejection fraction data missing for 13 patients (12%)
Medication data missing for 3 patients (1%)
Mortality and Hospital Readmission
Patients with mild cognitive impairment (compared with no impairment) were more likely to experience the combined endpoint of mortality or readmission at 6 months, although this difference was not statistically significant (adjusted hazard ratio 1.35, 95% confidence interval [CI] 0.91–2.00, P=0.13). Patients with moderate-severe cognitive impairment were significantly more likely to experience mortality or readmission at 6 months compared with patients with no impairment (adjusted hazard ratio 1.60, 95% CI 1.03–2.48, P=0.04).
Patients with cognitive impairment that was not documented had a significantly higher likelihood of experiencing the combined endpoint of 6-month mortality or readmission compared with patients with no cognitive impairment (adjusted hazard ratio 1.53, 95% CI 1.06–2.20, P=0.02). Among patients with cognitive impairment that was documented, there was no statistically significant difference in the combined endpoint compared with patients with no impairment (adjusted hazard ratio 1.27, 95% CI 0.72–2.25, P=0.41) (Table 4).
Table 4.
Documentation of cognitive impairment and risk of mortality or readmission
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Cognitive impairment documented | 1.42 (0.86–2.37) | 0.17 | 1.27 (0.72–2.25) | 0.41 |
| Cognitive impairment not documented | 1.60 (1.14–2.25) | <0.01 | 1.53 (1.06–2.20) | 0.02 |
Outcomes at 6 months. Reference group: no cognitive impairment
Adjusted for independently significant factors (age, race, kidney disease, aldosterone receptor antagonist)
DISCUSSION
In our study of older adults hospitalized for heart failure we found that cognitive impairment was common (present in 47% of the study sample) and yet documented in only a minority of patients. As expected, documentation improved as the severity of cognitive impairment increased, but even among patients with moderate-severe impairment it was documented in fewer than half of the patients. While prior studies have demonstrated that cognitive impairment is present in a significant subset of hospitalized older adults for heart failure 12–14, our findings extend this work by demonstrating that cognitive impairment, while common, is infrequently documented by physicians. An additional important finding of our study was that patients with cognitive impairment that was not documented were significantly more likely to experience mortality or hospital readmission at 6 months compared with patients without cognitive impairment. This finding builds on previous research in patients with heart failure that has demonstrated associations between cognitive impairment and mortality 8, 23 as well as hospital readmission 23. Whether recognition and documentation of cognitive impairment in patients with heart failure with implementation of appropriate supportive services can alter patients’ trajectories should be examined in future studies.
Cognitive impairment is highly prevalent in older adults with a variety of chronic diseases, including COPD 24, 25 and cancer 26, 27. We chose to focus our study on heart failure for several reasons. First, the self-care requirements for optimal disease management in heart failure are extensive; typical discharge instructions include daily weighing, fluid restriction, symptom monitoring, and compliance with a low-sodium diet and multidrug regimens 9, 28. Cognitive impairment may interfere with any one of these necessary tasks – for example, doses of diuretics may be missed, or changes in symptoms (dyspnea, weight gain) may not be recognized until they are severe. Patients may also not remember to report ongoing problems at routine medical encounters 13. Notably, patients hospitalized for heart failure have the highest rates of early readmission after discharge 4, 29. While causes of early readmission are complex, unrecognized cognitive impairment may contribute to this problem as patients may not be optimally empowered to manage their heart failure after transition to the home setting 30. Recognizing cognitive impairment may allow physicians to simplify medication regimens or individualize discharge education 31, 32. It also may allow the implementation of more resource-intensive monitoring strategies, such as visiting nurse services, for patients who may benefit the most.
Currently, national quality guidelines recommend assessment of cognition among hospitalized older adults 33. While the demands of inpatient care can make formal assessment of cognitive status challenging, the MMSE can be administered in less than ten minutes, and shorter instruments such as the Mini-Cog 34 can be administered in three minutes. Measurement can also be incorporated into nursing protocols 35 which has the potential to facilitate evaluation. Another barrier to the assessment of cognition may be the current organization of care, in which documentation of cognitive impairment is reimbursed at a lower rate than other medical conditions 36, which could potentially be addressed through modification of the existing reimbursement system to capture the complexity of caring for cognitively impaired patients 36.
There are several limitations to our study. We utilized hospital discharge summaries to assess physician documentation of cognitive impairment, and it is possible that impairment was documented elsewhere but not reported in the discharge summary. However, the discharge summary is the primary means of communicating details of the inpatient hospitalization to the outpatient clinicians assuming care, including clinical assessment, diagnostic evaluation, and post-hospital follow-up. Reflecting its importance in transitions of care, the discharge summary is mandated on all patients within 30 days of discharge 37 and was available on 100% of patients in our study. An additional limitation is that education level was not available on all participants and we were therefore unable to adjust the MMSE based on this 38. However, in a subgroup of 121 patients for whom information about educational attainment was available, only 8% of patients had a low education level (<9th grade), and there was no association between low education and cognitive impairment in this subgroup. The prevalence of diabetes and chronic kidney disease in our population was higher than in several large registries of older adults with heart failure 39, 40; however, we do not believe that these comorbidities would influence documentation of cognitive impairment. Also, as the mean age of our study population was 80 years, and the majority (81.2%) were white, the applicability of our findings to younger or minority patients with heart failure is limited and these populations merit further investigation. Finally, we excluded patients with disabilities in activities of daily living or delirium, both of which may be associated with higher rates of cognitive impairment (and its recognition).
In conclusion, cognitive impairment is present in a substantial number of older patients hospitalized for heart failure, yet infrequently documented by physicians at the time of hospital discharge. Presence of cognitive impairment and lack of documentation are associated with increased 6 month mortality or readmission. Future studies are needed to determine whether efforts to improve recognition and documentation at the time of inpatient hospitalization may inform individually tailored heart failure care and influence meaningful outcomes including hospital readmission, mortality, and quality of life.
Acknowledgments
Funding Sources
Dr. Dodson is supported by a training grant in Geriatric Clinical Epidemiology from the NIH/NIA (T32 AG019134) and a Clinical Research Loan Repayment award from the NIH/NHLBI.
Dr. Chaudhry is supported by a Beeson Career Development Award from the NIH/NIA (K23 AG030986).
Footnotes
Disclosures
John A. Dodson: None
Tuyet-Trinh N. Truong: None
Virginia R. Towle: None
Gerard Kerins: None
Sarwat I. Chaudhry: None
All authors had access to the data and a role in the writing of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham study. J Am Coll Cardiol. 1993;4:A6–A13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–43. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–85. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 7.Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007;93:665–71. doi: 10.1136/hrt.2005.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–16. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron J, Worrall-Carter L, Page K, et al. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–15. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 10.Dickson VV, Tkacs N, Riegel B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–31. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal Cognitive Assessment Tool in outpatients >=65 years of age with heart failure. Am J Cardiol. 2011;107:1203–7. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–9. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lien CTC, Gillespie ND, Struthers AD, McMurdo MET. Heart failure in frail elderly patients: Diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail. 2002;4:91–8. doi: 10.1016/s1388-9842(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 14.Zuccalà G, Marzetti E, Cesari M, et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Debette S, Bauters C, Leys D, et al. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13:205–8. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 16.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Vogels RLC, Oosterman JM, Van Harten B, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55:1764–70. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 19.Pressler SJ. Cognitive functioning and chronic heart failure: A review of the literature (2002-July 2007) J Cardiovasc Nurs. 2008;23:239–49. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 20.Young J, Meagher D, MacLullich A. Cognitive assessment of older people. BMJ. 2011;343:d5042. doi: 10.1136/bmj.d5042. [DOI] [PubMed] [Google Scholar]
- 21.Mungas D. In-office mental status testing: A practical guide. Geriatrics. 1991;46:54–66. [PubMed] [Google Scholar]
- 22.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2:2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLennan SN, Pearson SA, Cameron J, Stewart S. Prognostic importance of cognitive impairment in chronic heart failure patients: Does specialist management make a difference? Eur J Heart Fail. 2006;8:494–501. doi: 10.1016/j.ejheart.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35:913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 25.Almagro P, Calbo E, Ochoa de Echagucen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121:1441–8. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 26.Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer. 1997;79:835–42. [PubMed] [Google Scholar]
- 27.Kurita GP, Sjøgren P, Ekholm O, et al. Prevalence and predictors of cognitive dysfunction in opioid-treated patients with cancer: A multinational study. J Clin Oncol. 2011;29:1297–303. doi: 10.1200/JCO.2010.32.6884. [DOI] [PubMed] [Google Scholar]
- 28.Riegel B, Carlson B, Moser DK, et al. Psychometric testing of the Self-Care of Heart Failure Index. J Card Fail. 2004;10:350–60. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekman I, Fagerberg B, Skoog I. The clinical implications of cognitive impairment in elderly patients with chronic heart failure. J Cardiovasc Nurs. 2001;16:47–55. doi: 10.1097/00005082-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Cameron J, Ski CF, Thompson DR. Cognitive impairment in chronic heart failure and the need for screening. Am J Cardiol. 2011;107:1547–8. doi: 10.1016/j.amjcard.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Strömberg A. The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7:363–369. doi: 10.1016/j.ejheart.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55:S293–S301. doi: 10.1111/j.1532-5415.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 34.Borson S, Scanlan J, Brush M, et al. The Mini-Cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–32. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 36.Fillit H, Geldmacher DS, Welter RT, et al. Optimizing coding and reimbursement to improve management of Alzheimer’s disease and related dementias. J Am Geriatr Soc. 2002;50:1871–8. doi: 10.1046/j.1532-5415.2002.50519.x. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed January 10, 2012];Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Standards. Available at: http://www.jointcommission.org/standardsinformation/standards.aspx.
- 38.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. J Am Med Assoc. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 39.Fonarow GC, Adams KF, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure. J Am Med Assoc. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 40.Curtis LH, Greiner MA, Hammill BG, et al. Representativeness of a national heart failure quality-of-care registry. Circ Cardiovasc Qual and Outcomes. 2009;2:377–84. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]


