Abstract
Background:
In this study, we evaluated the possibility that KRAS mutational status might be predictive of oxaliplatin (OXA) efficacy. We also explored the role of excision repair cross complementing group-1 (ERCC-1).
Methods:
Ninety anti-epidermal growth factor receptor-naive advanced colorectal cancer patients were retrospectively analysed. In all patients KRAS mutational status was assessed. In 60 patients mRNA ERCC-1 expression was also investigated. Response rate (RR) and progression-free survival (PFS) after FOLFOX-6±bevacizumab were evaluated according to KRAS status and mRNA ERCC-1 expression.
Results:
Among 90 patients 47% wild-type (wt) and 53% mutated (mt) KRAS tumours were found. Response rate was 26% in the wt KRAS group, whereas it was 56% in the mt KRAS group; the difference is statistically significant in the total sample (P=0.008) and when only patients receiving FOLFOX-6±bevacizumab as first-line are considered (P=0.01). Progression-free survival was longer in mt than in wt KRAS patients over all patients (10 vs 8 months, respectively, P=0.001) and in those treated as first-line (10 vs 8 months, respectively, P=0.0069). Mt KRAS patients experienced a longer survival (24 vs 18 months; P=0.01). ERCC-1 mRNA expression was not found to correlate with FOLFOX activity in our analysis.
Conclusion:
Our results suggest that activating mutation of KRAS oncogene may predict response to OXA. Basal expression of ERCC-1 mRNA does not explain the high efficacy of FOLFOX-6 in mt KRAS patients.
Keywords: oxaliplatin, KRAS, EGFR, ERCC-1
Cetuximab (CET) and panitumumab (PAN), anti-epidermal growth factor receptor (anti-EGFR) antibodies, are effective in the treatment of metastatic colorectal cancer both alone and in combination with chemotherapy (CT) (Cunningham et al, 2004; Van Cutsem et al, 2007; Bokemeyer et al, 2009; Van Cutsem et al, 2009; Douillard et al, 2010; Peeters et al, 2010). In an unselected population, the combination of an irinotecan (IRI)-based CT with an anti-EGFR antibody results in an increase in response rate (RR) and progression-free survival (PFS) compared with CT alone (Van Cutsem et al, 2009, Peeters et al, 2010). On the other side, in the OPUS study the combination of oxaliplatin (OXA)-based CT with CET did not reach a statistically significant improvement of RR, although a trend toward a better outcome was recorded (46% vs 36% P=0.06); furthermore there was no difference in terms of PFS (Bokemeyer et al, 2009). Nonetheless, in the OPUS study, when patients were retrospectively analysed according to KRAS gene mutational status, the addition of CET resulted in a significant benefit in terms of RR (61% vs 37% P=0.01) and PFS (7.7 vs 7.2 m; P=0.01) in patients with wild-type (wt) KRAS in comparison with those carrying an activating mutation of the same oncogene (Bokemeyer et al, 2009). The same effect was observed with the combination of OXA-based CT and PAN (Douillard et al, 2010).
When OXA-based CT is combined with an anti-EGFR antibody, a detrimental effect has been reported in patients carrying an activating mutation of KRAS oncogene (Bokemeyer et al, 2009; Douillard et al, 2010). Moreover, in the OPUS study a trend toward an improved PFS was unexpectedly observed in mutated (mt) patients compared with wt KRAS patients treated with FOLFOX alone (Bokemeyer et al, 2009). This advantage did not reach statistical significance (HR 1.404; P=0.16), but it is nonetheless interesting, given the exploratory nature of the analysis and the large number of patients censored. On the contrary, when FOLFIRI CT in combination with anti-EGFR antibodies is considered, no detrimental effect in mt KRAS patients has ever been registered, thus generating the hypothesis that the KRAS mutation might be relevant in the response to CT and, consequently, that the choice of backbone CT to combine with anti-EGFR antibodies may be influential.
The scenario has recently become much more complex, as the combination of an OXA-based CT with CET has not improved RR and PFS even in wt KRAS patients in two large randomized studies (COIN and NORDIC trials) (Maughan et al, 2011; Tveit et al, (2012)). Several hypotheses have been expressed about this topic, but it cannot be excluded that the lack of selection based on EGFR overexpression in these studies might have a role. Interestingly, retrospective analysis of a subset of EGFR-positive patients in the COIN study has shown a detrimental effect of the combination of OXA-based CT and CET as it has been observed in the OPUS study.
The possibility that KRAS mutational status could affect response to standard CT was investigated in a retrospective analysis of a complex, three-arm, phase III study intended to evaluate the administration of different sequences of cytotoxic agents before targeted agents became available. No difference was found in overall survival (OS) between OXA- and IRI-based CT in wt or mt KRAS patients, but the results are controversial as most patients were exposed to all the drugs tested and the endpoint cannot distinguish the separate activity of each treatment (Seymour et al, 2007; Richman et al, 2009).
We retrospectively analysed our series of patients affected by advanced CRC treated with first- or second-line FOLFOX-6, mainly in the years before anti-EGFR antibodies became available. Response rate in relation to KRAS mutational status was our primary endpoint. Progression-free survival and OS were secondary endpoints. For a subset of patients also immunohistochemistry data on EGFR expression were retrieved. Following preliminary results of other authors (Shirota et al, 2001), we considered the possibility that response to OXA in advanced CRC might be influenced by expression of the excision repair cross complementing group-1 (ERCC-1), a pathway also involved in KRAS mutagenesis (Yang et al, 2007). We therefore included in our study the determination of ERCC-1 expression, using real-time PCR (RT–PCR).
Patients and methods
Patients
Clinical records of patients affected by metastatic CRC and treated at our institution before anti-EGFR antibodies were introduced between May 2006 and April 2009 were reviewed. All patients had undergone to colorectal surgery, so normal colonic mucosa was available in addition to tumour tissue. Eligibility criteria included as follows: FOLFOX-6 regimen as first- or second-line therapy, no anti-EGFR treatment, availability of stored tissue sample sufficient for quality-controlled mutation analysis, evaluation of response according to the RECIST criteria, no serious concomitant illness (uncontrolled hypertension, recent myocardial infarction, unstable angina, grade ≥2 New York Heart Association heart disease, uncontrolled diabetes, renal or liver failures), which could have affected treatment duration or survival. Concomitant treatment with bevacizumab was not considered an exclusion criteria. Only patients who were given computerised tomography at regular intervals, not longer than 3 months, were considered. Patients who received adjuvant FOLFOX-4 CT were excluded. Consent for CT was obtained by all patients; separate consent for the molecular analysis was obtained by the patient or, in case of death, by his relatives. All eligible patients were consecutively included.
KRAS assessment
Tumour was identified in haematoxylin- and eosin-stained sections of formalin-fixed, paraffin-embedded archivial blocks. DNA was extracted by 5 μm sections of paraffin-embedded tissue, containing 70% tumour at least, using the QIAamp DNA mini Kit (Qiagen, Milan, Italy). KRAS codons 12 and 13 were amplified in one PCR; KRAS codon 61 was amplified separately. Thermal cycling conditions were: 95 °C for 12 min followed by 40 cycles of 95 °C for 10 s, 55 °C for 20 s and 72 °C for 20 s. PCR conditions were as follows: primer concentration 200 nmol l−1, MgCl2 concentration 2 mmol l−1; 30 ng of genomic DNA and 12.5 μl of Eppendorf Prime mastermix (Eppendorf, Milan, Italy) in a final reaction volume of 25 μl. PCR products were electrophoresed in a 2.5% agarose gel, stained with ethidium bromide and visualised under UV light. Thereafter, 5 μl of PCR product was treated with ExoSAP-IT (GE Healthcare, Milan, Italy) following the manufacturer’s protocol, amplified with the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Milan, Italy) using the same primers of the amplification, and sequenced with an ABI PRISM 3100-Avant Genetic Analyser (Applied Biosystems).
mRNA extraction and ERCC-1 expression
After being deparaffined, three 10-μm slides were digested overnight at 55 °C in 200 μl of TENS 1 × (10 mℳ Tris pH 7.4, 10 mℳ EDTA, 100 mℳ NaCl and 1% SDS) with 100 mg ml−1 proteinase K, and RNA was then extracted by the RNAsi mini kit (Qiagen), following the manufacturer’s protocol. We assessed the quantity and quality of the RNA spectrophometrically (E260, E260/E280 ratio, spectrum 220–320 nm; Biochrom, Cambridge, UK) and by separation on an Agilent 2100 Bioanalyzer (Palo Alto, CA, USA). RNA was treated with RQ1 RNase-Free DNase (Promega, Milan, Italy) and concentrations of the various samples were determined by spectrophotometer. The amplification and quantification of ERCC-1 mRNA and ACTB mRNA (taken as the internal reference gene) were performed using the iScript one-step RT–PCR kit for probes (Bio-Rad, Milan, Italy) following the manufacturer’s protocol. The sequences of the primers and probes used are as follows: for ERCC-1, forward 5′-GGGAATTTGGCGACGTAATTC-3′, reverse 5′-GCGGAGGCTGGAACAG-3′, probe (FAM)-5′-CACAGGTGCTCTGCCCAGCACATA-3′(TAMRA); for ACTB, forward 5′-TGAGCGCGGCTACAGCTT-3′, reverse 5′-TCCTTAATGTCAGCACGATTT-3′, probe (FAM)-5′-ACCACCACGGCCGAGCGG-3′(TAMRA). All primers were used to study intron spanning to avoid contamination with genomic DNA. Thermocycler conditions were as follows: 50 °C to 10 min and 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 min and 60 °C for 35 min. The relative levels of expression of the target gene (ERCC-1), compared with the internal reference gene (ACTB), were expressed as 2-ΔCt, where ΔCt is the difference between two absolute measurements: the value of Ct (cycle threshold at which the fluorescence curve reaches an exponential) of the interest gene and the value of Ct internal reference gene (ACTB).
We performed the ERCC-1 expression on tumour and normal colonic mucosa of each patient and in colonic mucosa of 30 healthy controls. Relative mRNA expression (tumour/normal ratio) was calculated as (ERCC-1/β-actin in tumour)/(ERCC-1/β-actin in paired normal tissue). Excision repair cross complementing group-1 mRNA expression did not show a statistically significant difference in three different measurements. We found that the median of relative ERCC-1 expression was 6.21 × 10−3 (range, from 0.18 to 220.67)±45.51. This value was established as the cutoff value for ERCC-1 expression. In addition, we found that ERCC-1 mRNA expression in the colonic mucosa of 30 healthy controls was not significantly different to the ERCC-1 expressed in normal colonic mucosa of patients. Each assay was performed in triplicate and data were processed using the CFX96 optical system software (Bio-Rad).
Endpoint of the study
This was a retrospective study. As OS is generally influenced by many factors (molecular markers, previous and subsequent lines of therapy, etc.) and PFS could differ if the interval for radiological assessment for progression is not prescheduled, we selected objective RR as our primary endpoint, calculated as the sum of observed complete and partial responses. Patients experiencing stable disease (SD) or progression were classified as non-responders. Responses were evaluated in accordance with the RECIST guidelines and were assessed by investigators who at the time of data collection were blinded to KRAS mutational status. Progression-free survival and OS were chosen as secondary endpoints. Progression-free survival was calculated from the start of FOLFOX regimen until clinical or radiological progression, second colorectal primary, death from any cause or last follow-up. Overall survival was calculated from the start of FOLFOX regimen to death due to any cause.
Statistical analysis
We assumed a KRAS mutation rate of about 50% (45–55%). Considering that the RR of OXA-based CT in KRAS non-selected patients is about 40% and that in the OPUS study RR was 61% among mt KRAS patients and 37% among wt KRAS patients, we postulated an absolute difference of 30% in RR between mt and wt KRAS patients (60% vs 30%). To demonstrate this difference with a study power of 80%, a sample size of at least 40 patients per group was required. Progression-free survival and OS were estimated using the Kaplan–Meier method. Differences in the distribution of categorical variables were assessed using Pearson’s χ2 test. Cox multiple regression analysis for PFS and OS was used to assess the role of variables that resulted to be significant at univariate analysis. Tested variables included gender (male vs female), age (<65 vs ≥65 years), grade of tumour differentiation (well vs moderately differentiated and undifferentiated), number of metastatic sites (<2 vs ≥2), PS (ECOG; 0 vs ≥1) and KRAS status (KRAS wt vs KRAS mt). The significance level was set at P 0.05 for each test. Statistical analysis was carried out using SPSS package version 15 (SPSS Inc., Chicago, IL, USA).
As there are no data concerning the relationship between KRAS mutational status and ERCC-1 expression, all statistical analyses of ERCC-1 should be considered exploratory.
Results
Patients’ characteristics
Patients’characteristics are summarised in Table 1. In this study, we included 90 patients whose stored tumour samples contained sufficient quality/quantity DNA for the mutation frequency analysis. In 60 of these patients, the material was also sufficient for ERCC-1 determination by PCR. In 68 out of 90 (75%) patients, data concerning EGFR were also available. Using a cutoff point of 1%, 56 out of 68 (82% 28 out of 38 mt KRAS and 28 out of 30 wt KRAS) of our patients were EGFR-positive, whereas with a cutoff point of 10%, 22 out of 68 (32% 14mt KRAS and 8 wt KRAS) of our patients were EGFR-positive.
Table 1. Patient characteristics (n 90).
|
Wt KRAS (42 patients 47%)
|
||||
|---|---|---|---|---|
| Characteristics | No./total | % | Mt KRAS (48 patients, 53%) | |
|
Gender
| ||||
| M | 26/42 | 62 | 30/48 | 62 |
| F | 16/42 | 38 | 18/48 | 38 |
| Median age | 64 | 62 | ||
|
Tumour site
| ||||
| Right colon | 11/42 | 26 | 13/48 | 27 |
| Trasversum | 1/42 | 3 | 2/48 | 5 |
| Descending colon | 3/42 | 7 | 6/48 | 12 |
| Sigmoid—rectum | 27/42 | 64 | 27/48 | 56 |
|
Metastatic sites
| ||||
| Liver | 35/42 | 83 | 38/48 | 80 |
| Lung | 17/42 | 40 | 21/48 | 44 |
| Lymphonodes | 5/42 | 12 | 8/48 | 17 |
| Peritoneum | 6/42 | 14 | 7/48 | 15 |
| Other | 4/42 | 10 | 2/48 | 4 |
|
FOLFOX
| ||||
| First line | 22/42 | 52 | 27/48 | 56 |
| Second line | 20/42 | 48 | 21/48 | 44 |
|
PS (ECOG)
| ||||
| 0 | 33/42 | 78 | 39/48 | 81 |
| 1 | 9/42 | 22 | 8/48 | 17 |
| 2 | 0/42 | 0 | 1/48 | 2 |
| Synchronous metastases | 34/42 | 80 | 36/48 | 75 |
| Metachronuos metastases | 8/42 | 20 | 12/48 | 25 |
| Median time to relapse (m) | 32 | 29 | ||
| Surgery for primary tumour | 39/42 | 93 | 44/48 | 91 |
| Adjuvant CT (5-FU+folinic acid) | 7/42 | 16 | 5/48 | 10 |
|
Third- and forth-line CT
| ||||
| Capecitabine | 7/42 | 16 | 27/48 | 56 |
| Anti-EGFR therapies | 18/42 | 43 | 3/48 | 6 |
|
EGFR expression (68 patients; cutoff point 1%)
| ||||
| Overexpression | 28/30 | 93 | 28/38 | 73 |
| Underexpression | 2/30 | 7 | 10/38 | 27 |
Abbreviations: CT=chemotherapy; ECOG=Eastern Cooperative Oncology Group; EGFR=epidermal growth factor receptor; F=female; FOLFOX=fluorouracil, leucovorin and oxaliplatin; FU=fluorouracil; M=male; Mt=muatted; PS=performance status; Wt=wild type.
At the beginning of treatment all patients were in good physical condition with performance status 0–1. Forty-two (47%) and forty-eight (53%) patients had wt and mt KRAS tumours, respectively. Median age was similar in both groups, 64 in wt KRAS and 62 in mt KRAS patients. Most patients were males, with similar gender distribution in the two groups. More than 90% of patients in both groups underwent surgery for primary tumour. As patients receiving adjuvant FOLFOX-4 were excluded, most of our patients had synchronous metastases (80% among wt KRAS and 75% among mt KRAS patients, respectively). Twenty-two of forty-two wt KRAS patients received FOLFOX-6 as first-line therapy (two of them received concomitant bevacizumab) and 20 patients received it as second-line treatment (one with concomitant bevacizumab). In the mt KRAS population, 27 and 21 out of 48 patients received FOLFOX-6 as first-line and second-line treatment, respectively, three patients received concomitant bevacizumab in front-line. Patients treated with FOLFOX-6 as second-line had received FOLFIRI in first-line.
Chemotherapy activity according to KRAS mutational status
In wt KRAS patients, we observed one CR, 10 PR (RR 26%, 95% CI: 10.9–36.7), 19 SDs and 12 progressive diseases (PD). In mt KRAS patients two CR, 25 PR (RR 56%, 95% CI: 38.6–66.2), 14 SD and 7 PD were recorded. Response rate was significantly higher in mt than in wt KRAS patients (HR: 2.148, 95% CI: 1.222–3.781; P=0.008; Table 2). Although the value of subgroups analysis is influenced by small sample size, a significantly higher RR (70% vs 27%) in favour of mt patients was also seen in patients receiving FOLFOX-6 as first-line therapy (HR: 2.580, 95% CI: 1.250–5.327; P=0.01). In patients treated with FOLFOX-6 as second-line therapy the trend towards a higher RR in the mt subgroup (38% vs 25% HR: 1.524, 95% CI: 0.598–3.880; P=0.37) was not significant.
Table 2. Chemotherapy activity according to mutational status.
| Characteristics (no.) | RR no (%) | PFS (m) | OS (m) |
|---|---|---|---|
| KRAS wt (42) | 11 (26) | 8 | 18 |
| KRAS mt (48) | 27 (56) | 10 | 24 |
| P=0.008 | P=0.001 | P=0.01 | |
|
First line
| |||
| KRAS wt (22) | 6 (27) | 8 | 18 |
| KRAS mt (27) | 19 (70) | 10 | 29 |
| P=0.001 | P=0.006 | P=0.01 | |
|
Second line
| |||
| KRAS wt (20) | 5 (25) | 8 | — |
| KRAS mt (21) | 8 (38) | 8 | — |
| P=0.375 | P=0.067 | — | |
Abbreviations: mt=mutated; OS=overall survival; PFS=progression-free survival; RR=response rate; wt=wild type.
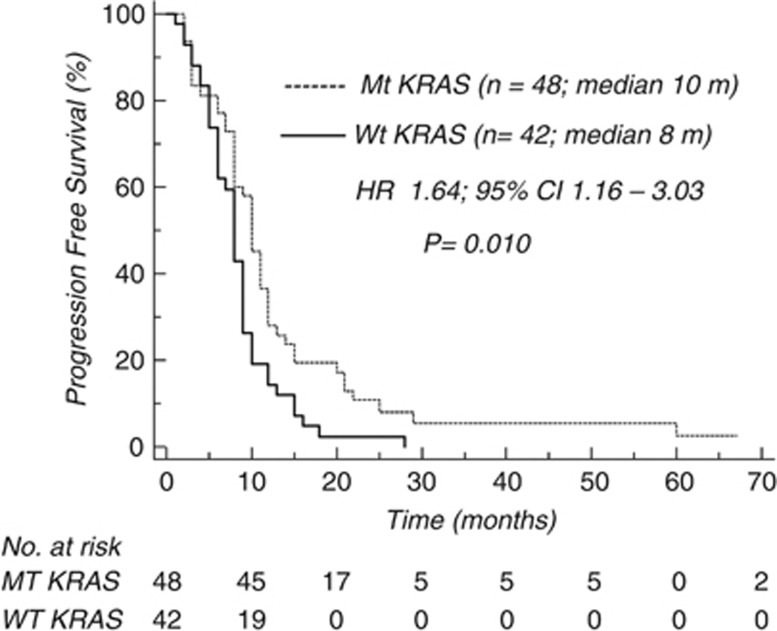
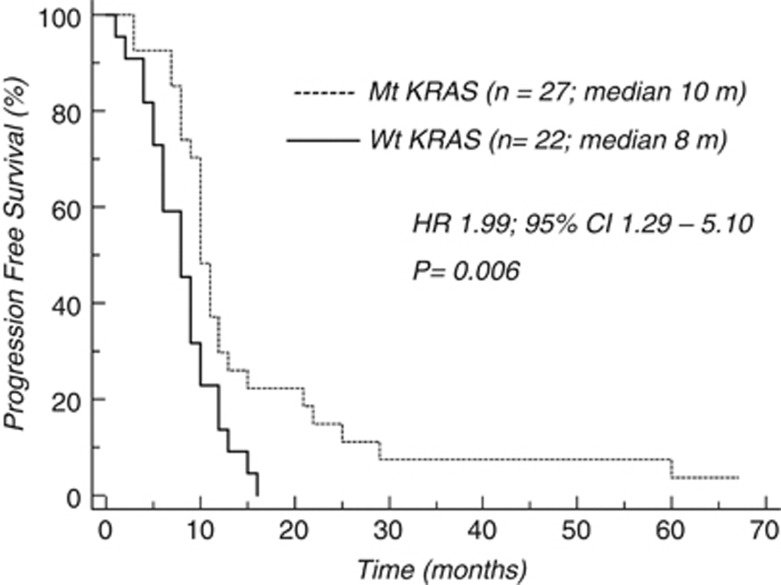
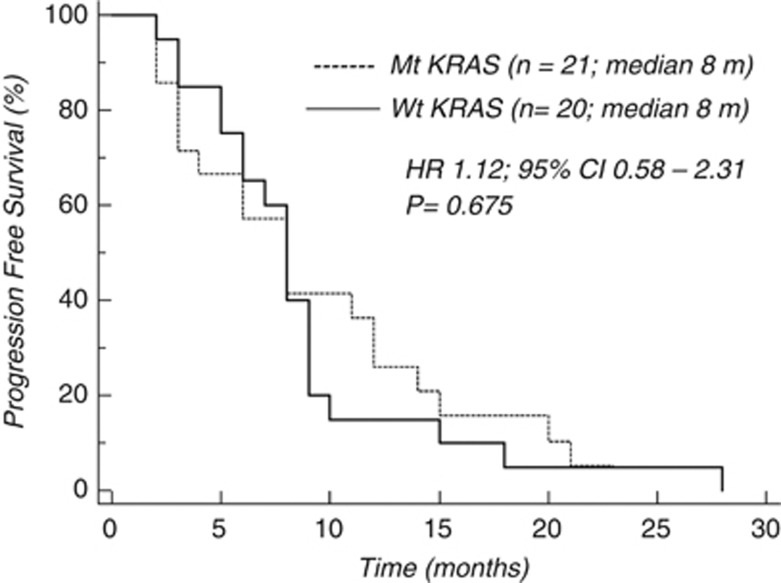
In the whole population, median PFS was 10 and 8 months in mt and wt KRAS patients, respectively (HR 1.645, 95% CI: 1.161–3.030; P=0.01; Figure 1). The difference in favour of mt KRAS patients remained significant in the subgroup of patients who received FOLFOX-6 as first-line treatment (10 vs 8 months; HR 1.999, 95% CI: 1.296–5.109; P=0.0069; Figure 2), whereas it was not significant in the subgroup who received this treatment as second-line therapy (Figure 3).
Figure 1.
Chemotherapy activity (PFS) according to KRAS mutational status (whole population).
Figure 2.
Chemotherapy activity (PFS) according to KRAS mutational status (first line).
Figure 3.
Chemotherapy activity (PFS) according to KRAS mutational status (second line).
Median OS of wt KRAS patients was 18 months, whereas it was 24 months for mt KRAS patients (HR 1.64, 95% CI: 1.13–2.89; P 0.01). Among patients receiving FOLFOX as front-line CT, median OS of wt KRAS patients was 18 months, whereas it was 29 months for mt KRAS patients (HR 1.9, 95% CI: 1.21–4.54; P 0.01).
Among the other clinical variables considered, median PFS and OS were significantly improved in PS (ECOG) <1 patients and in patient with <2 metastatic sites both in the whole population and first-line population. At multivariate analysis (KRAS status, PS and number of metastatic sites), KRAS mt status maintained an independent prognostic value in first-line population (PFS P=0.02; OS P=0.03), whereas in the whole population it remained an independent prognostic variable only when OS was considered (OS P=0.03; PFS P=0.08).
Chemotherapy activity according to ERCC-1 expression
According to the cutoff value, the ERCC-1 gene was overexpressed in 30 out of 60 patients, 16 of whom were mt. This means that the distribution of patients with over and underexpression of ERCC-1 is similar in mt and wt KRAS subgroups. The efficacy of FOLFOX-6 was no different in patients with higher ERCC-1 levels from those with low levels of the gene. Response rate was 53% (95% CI: 35.5–71.2) and 40% (95% CI: 22.5–57.5) in patients overexpressing ERCC-1 in comparison with those underexpressing the ERCC-1 gene, respectively (HR: 1.333, 95% CI: 0.768–2.314; P=0.307); PFS was 9 and 8.5 months, respectively (HR 1.094, 95% CI: 0.633–1.950; P=0.71). In ERCC-1 overexpressing patients, however, RR was higher in mt than in wt patients (40% vs 13%, respectively, HR: 3.000, 95% CI: 1.090–8.254; P=0.033); PFS was also longer in the same group of patients (10 vs 8 months; HR 2.231; 95% CI: 1.299–8.251; P=0.01; Table 3). A similar, though not significant, trend was observed in patients underexpressing ERCC-1.
Table 3. Chemotherapy activity according to ERCC-1 expression.
| Characteristics (no.) | RR no. (%) | PFS (m) |
|---|---|---|
| Overexpression ERCC (30 patients) | 16 (53)* | 9° |
| KRAS wt (14 patients) | 4 (13)ˆ | 8Δ |
| KRAS mt (16 patients) | 12 (40)ˆ | 10Δ |
| P̂=0.03 | ΔP=0.01 | |
| Underexpression ERCC-1 (30 patients) | 12 (40)* | 8.5° |
| KRAS wt | 3 (10) | 8 |
| KRAS mt | 9 (30) | 10 |
| P=0.06 | P=0.06 |
Abbreviations: ERCC-1=excision repair cross complementing group-1; RR=response rate; PFS=progression-free survival; wt=wild type; mt=mutated.
*P=0.30, °P=0.71.
Discussion
In mt KRAS patients, the combination of an anti-EGFR antibody and FOLFOX has consistently achieved worse results than FOLFOX alone in some phase II and phase III studies, whereas the same observations are not found when the CT administered is FOLFIRI. (Bokemeyer et al, 2009; Douillard et al, 2010). This unexpected observation has prompted suggestions that there may be a detrimental effect of anti-EGFR antibodies on OXA activity in the presence of KRAS mutation. Moreover, following two large phase III studies (NORDIC and COIN) in which the combination of OXA-based regimens with CET failed to improve PFS and OS, the idea that the combination of an anti-EGFR antibody with OXA is not the best schedule for advanced colorectal cancer patients is gaining ground (Maughan et al, 2011; Tveit et al, (2012)). The reason for this supposed negative interaction is not clear.
The extent of the interaction, however, cannot be assessed if the activity of OXA (without an anti-EGFR) in different mutational conditions is not established. In a previous retrospective analysis of a large phase III study other authors reported that KRAS mutational status does not influence OS (Richman et al, 2009). However, in this study patients in both arms were sequentially exposed to all cytotoxic drugs and >50% received post study treatment, so that OS does not seem an appropriate endpoint to evaluate the impact of OXA-based CT on patients with different mutational status.
In our series of patients not treated with any anti-EGFR antibody, we found a significantly higher RR and PFS in mt than in wt KRAS patients. The advantage is more evident in patients who received FOLFOX-6 as first-line treatment than in those who received it as second-line therapy, but it is sufficiently large to suggest a benefit for mt KRAS patients, even if our results refer to a small retrospective series. The reason for the lack of significance in second-line therapy might be simply related to the low number of patients, but the possibility that molecular changes associated with disease progression might have a role cannot be excluded.
Although retrospective, this is the first report analysing activity of FOLFOX-6 in relation to KRAS mutational status and showing a longer survival in mt KRAS patients in comparison with wt KRAS patients. The benefit is statistically significant in univariate, as well as in multivariate analysis, but it needs to be confirmed in larger series of patients, as it is in contrast with other reports. Actually, the prognostic role of KRAS mutation is not clear, as the only study evaluating the impact of KRAS mutational status on OS in patients eligible for best supportive care alone failed to demonstrate any prognostic role (Karapetis et al, 2008). On the other hand, all observations suggesting a poorer survival for mt compared with wt KRAS patients result from large studies in which mt KRAS patients also received anti-EGFR therapies (Bokemeyer et al, 2009; Douillard et al, 2010; Maughan et al, 2011; Tveit et al, 2012).
In vitro studies suggest that the repair of OXA-induced DNA damage has an important role in resistance to platinum derivatives (Shellard et al, 1993; Reed et al, 1998; Altaha et al, 2004). The NER pathway is mainly involved in this process, in which the endonuclease encoded by the ERCC-1 gene is the rate-limiting step (Hanawalt et al, 2002; Rosell et al, 2007; Li et al, 2000). In several studies on different tumours, the overexpression of ERCC-1 has been related to resistance to platinum-based therapy (Ferry et al, 2000; Britten et al, 2000; Cobo et al, 2007; Breen et al, 2008; Benhar et al, 2002). It has also been shown that CET may potentiate the effect of OXA in responsive tumour cell lines by downregulating NER-related mechanisms involved in resistance and promoting apoptosis (Balin-Gauthier et al, 2008).
Based on these observations, we evaluated whether different sensitivity to OXA of mt and wt KRAS patients might depend on a different ERCC-1 expression in relation to KRAS mutational status; we also investigated the difference in OXA activity between patients with an overexpression of ERCC-1 and those without. We found that KRAS mutational status did not affect the basal level of mRNA ERCC-1 expression; despite the small size of the sample, however, in ERCC-1 overexpressing patients RR and PFS were significantly higher in mt KRAS patients, whereas this difference did not reach statistical significance in patients with mRNA ERCC-1 underexpression. This apparently surprising results might indicate that in patient with constitutive higher sensitivity to OXA (low ERCC-1) the role of KRAS mutational status becomes less important when compared with patients with a constitutively reduced sensitivity to OXA (high ERCC-1). Even if our clinical data come from a small-sized sample, they suggest that the basal level of ERCC-1 is not related to OXA efficacy, but anyway it may have a role. In fact, in vitro preliminary results from our group support the possibility of an interaction between KRAS mutational status, OXA efficacy and ERCC-1 expression, as mt KRAS cells are not able to overexpress ERCC-1 in response to OXA therapy when compared with wt KRAS cells (Orlandi et al, 2012). On the other hand, ERCC-1 in response to OXA is positively regulated by EGFR in wt KRAS cells (Balin-Gauthier et al, 2008). Taken together with inability of mt KRAS cells to overexpress ERCC-1 in response to OXA, this suggests that in wt KRAS patients response to OXA-based therapy would be poorer when EGFR is overexpressed whereas it is unlikely to occur in mt KRAS patients.
In the control arm of COIN, NORDIC and PRIME trials, the RRs to OXA-based CT were 57%, 47% and 48% in wt KRAS patients, whereas it were 46%, 40% and 40% in mt KRAS patients, respectively. Although differences were not statistically significant, they converge toward a trend opposite to results of our study. The inverse relationship between efficacy of OXA-based therapy and EGFR overexpression, above hypothesised, might explain the high RR to OXA-based CT in the control arm of COIN and NORDIC trials, as patients in these studies were not selected for EGFR overexpression. The lack of this kind of selection might also account for the difference in RR of wt and mt KRAS patients between COIN, NORDIC, and PRIME trials and our study.
In conclusion, our study suggests that KRAS mutational status might be a predictive biomarker of response to OXA-based CT. Other studies are needed to identify subgroups with different RR and/or prognosis among mt KRAS patients treated with OXA-based combination therapy; this is particularly important in some clinical settings as conversion therapy. Basal ERCC-1 expression is not related to KRAS mutation, but the possibility that the enzyme might be poorly induced by OXA in mt tumors cannot be excluded.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Altaha R, Liang X, Yu JJ, Reed E (2004) Excision repair cross-complementing-group 1: gene expression and platinum resistance. Int J Mol Med 14: 959–970 [PubMed] [Google Scholar]
- Balin-Gauthier D, Delord JP, Pillaire MJ, Rochaix P, Hoffman JS, Bugat R, Cazaux C, Canal P, Allal BC (2008) Cetuximab potentiates oxaliplatin-induced cytotoxic effect through a defect in NER and DNA replication initiation. Br J Cancer 98: 120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar M, Engelberger D, Levitzki A (2002) Cisplatin-induced activation of the EGFR receptor. Oncogene 21: 8723–8731 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin and oxaliplatin with or without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27: 663–671 [DOI] [PubMed] [Google Scholar]
- Breen D, Barlesi F (2008) The place of excision repair cross complementation 1 (ERCC-1) in surgically treated non-small cell lung cancer. Eur J Cardiothorax Surg 33: 805–811 [DOI] [PubMed] [Google Scholar]
- Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D (2000) ERCC1 expression as a molecular marker of cisplatin resistance in human cervical tumor cells. Int J Cancer 89: 453–457 [PubMed] [Google Scholar]
- Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G, Muñoz MA, Felip E, Alberola V, Camps C, Domine M, Sanchez JJ, Sanchez-Ronco M, Danenberg K, Taron M, Gandara D, Rosell R (2007) Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small cell lung cancer. J Clin Oncol 25: 2747–2754 [DOI] [PubMed] [Google Scholar]
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Eng J Med 351: 337–345 [DOI] [PubMed] [Google Scholar]
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31): 4697–4705 [DOI] [PubMed] [Google Scholar]
- Ferry KV, Hamilton TC, Johnson SW (2000) Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol 60: 1305–1313 [DOI] [PubMed] [Google Scholar]
- Hanawalt PC (2002) Subpathways of nucleotide excision repair and their regulation. Oncogene 21: 8949–8956 [DOI] [PubMed] [Google Scholar]
- Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutation and benefit from cetuximab in advanced colorectal cancer. N Eng J Med 359: 1757–1765 [DOI] [PubMed] [Google Scholar]
- Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D, Cross CL, Bostick-Bruton F, Reed E (2000) Association between the level of ERCC1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res 20: 645–652 [PubMed] [Google Scholar]
- Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP on behalf of the MRC Trial Investigators (2011) Addition of cetuximab to oxaliplatin based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377: 2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi A, Di Salvatore M, Basso M, Bagalà C, Strippoli A, Plastino F, Dadduzio E, Di Lascio S, Quirino M, Cassano A, Astone A, Barone C (2012) ERCC1, KRAS mutation and oxaliplatin sensitivity: old dogs and new tricks. J Clin Oncol 30(Suppl 4)): abstract 489. [Google Scholar]
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28(31): 4706–4713 [DOI] [PubMed] [Google Scholar]
- Reed E (1998) Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev 24: 331–344 [DOI] [PubMed] [Google Scholar]
- Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P (2009) KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol 27: 5931–5937 [DOI] [PubMed] [Google Scholar]
- Rosell R, Mendez P, Isla D, Taron M (2007) Platinum resistance related to a functional NER pathway. J Thorax Oncol 2: 1063–1066 [DOI] [PubMed] [Google Scholar]
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, Smith DB, Shepherd S, Maraveyas A, Ferry DR, Meade AM, Thompson L, Griffiths GO, Parmar MK, Stephens RJ FOCUS Trial Investigators; National Cancer Research Institute Colorectal Clinical Studies Group (2007) Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomized controlled trial. Lancet 370: 143–152 [DOI] [PubMed] [Google Scholar]
- Shellard SA, Fichtinger-Schepman AM, Lazo JS, Hill BT (1993) Evidence of differential cisplatin-DNA adduct formation, removal and tolerance of DNA damage in three human lung carcinoma cell lines. Anticancer Drugs 4: 491–500 [DOI] [PubMed] [Google Scholar]
- Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ (2001) ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 9(23): 4298–4304 [DOI] [PubMed] [Google Scholar]
- Tveit KM, Guren T, Glimelius B, Pfeiffer P, Halfdan S, Pyrrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E, Fokstuen T, Hansen F, hofsli E, Birkemeyer E, Johnsson A, Starkhammar H, Yilmaz MK, Keldsen, Erdal AB, Dajani O, Dahl O, Christoffersen T (2012) Phase III trial of Cetuximab with continuous or intermittent fluorouracil, leucovorin and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer. The NORDIC-VII study. J Clin Oncol 30(15): 1755–1762 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Eng J Med 360: 1408–1417 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25: 1658–1664 [DOI] [PubMed] [Google Scholar]
- Yang G, Curley D, Bosenberg MW, Tsao H (2007) Loss of Xeroderma Pigmentosum C (Xpc) enhances melanoma photocarcinogenesis in Ink4a-Arf-deficient mice. Cancer Res 67(12): 5649–5657 [DOI] [PubMed] [Google Scholar]