Abstract
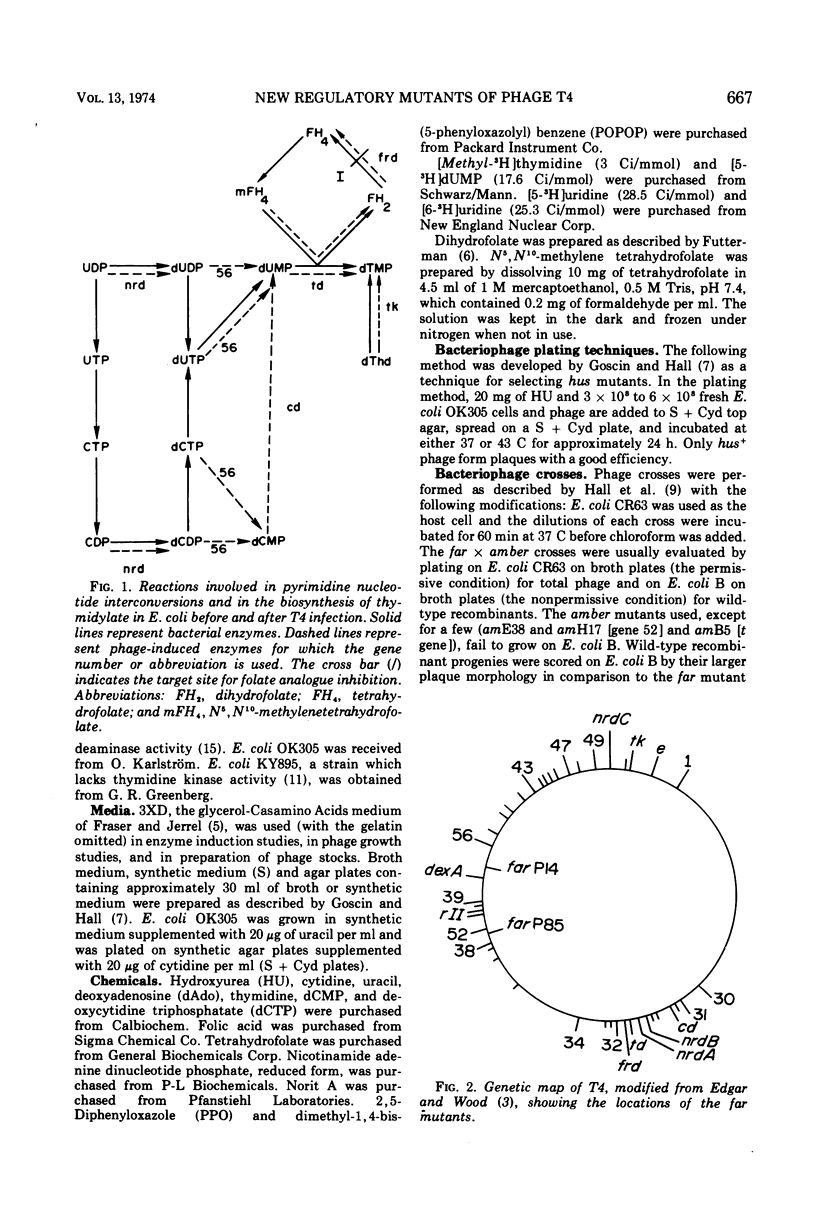
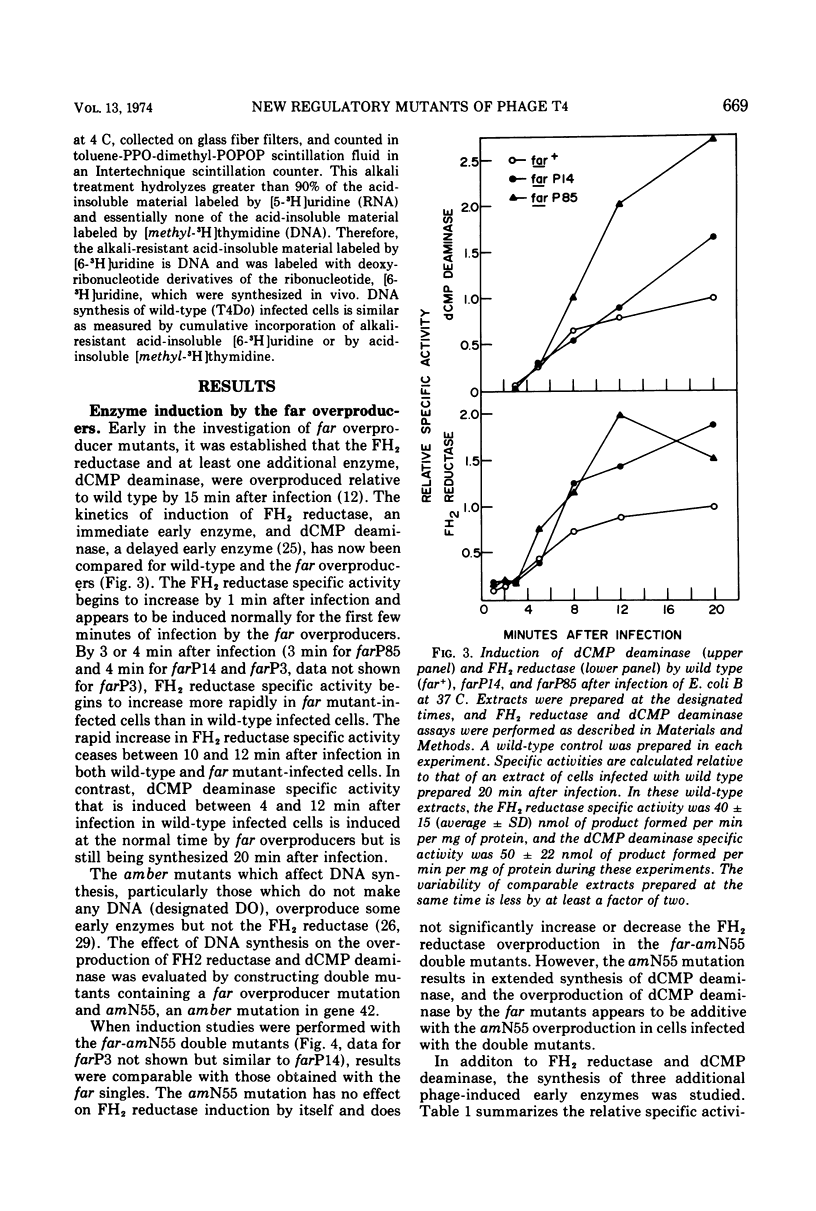
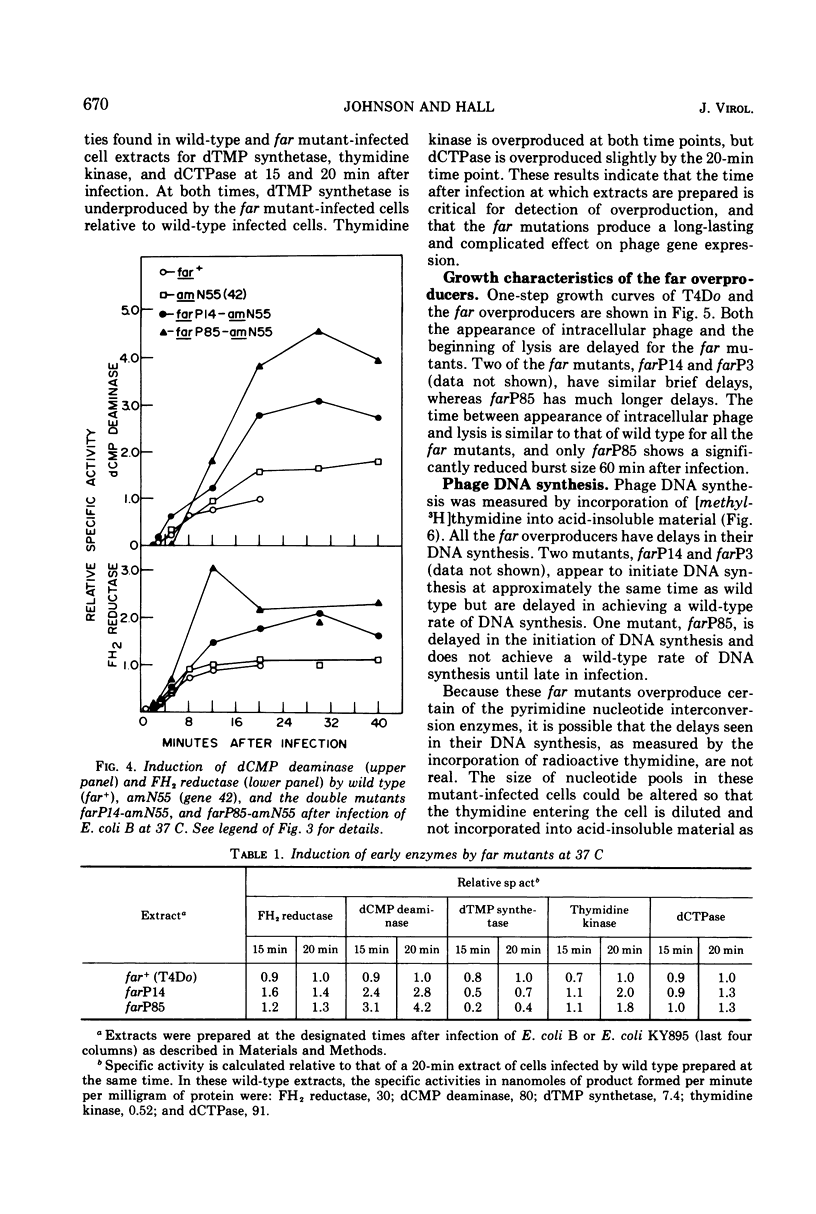
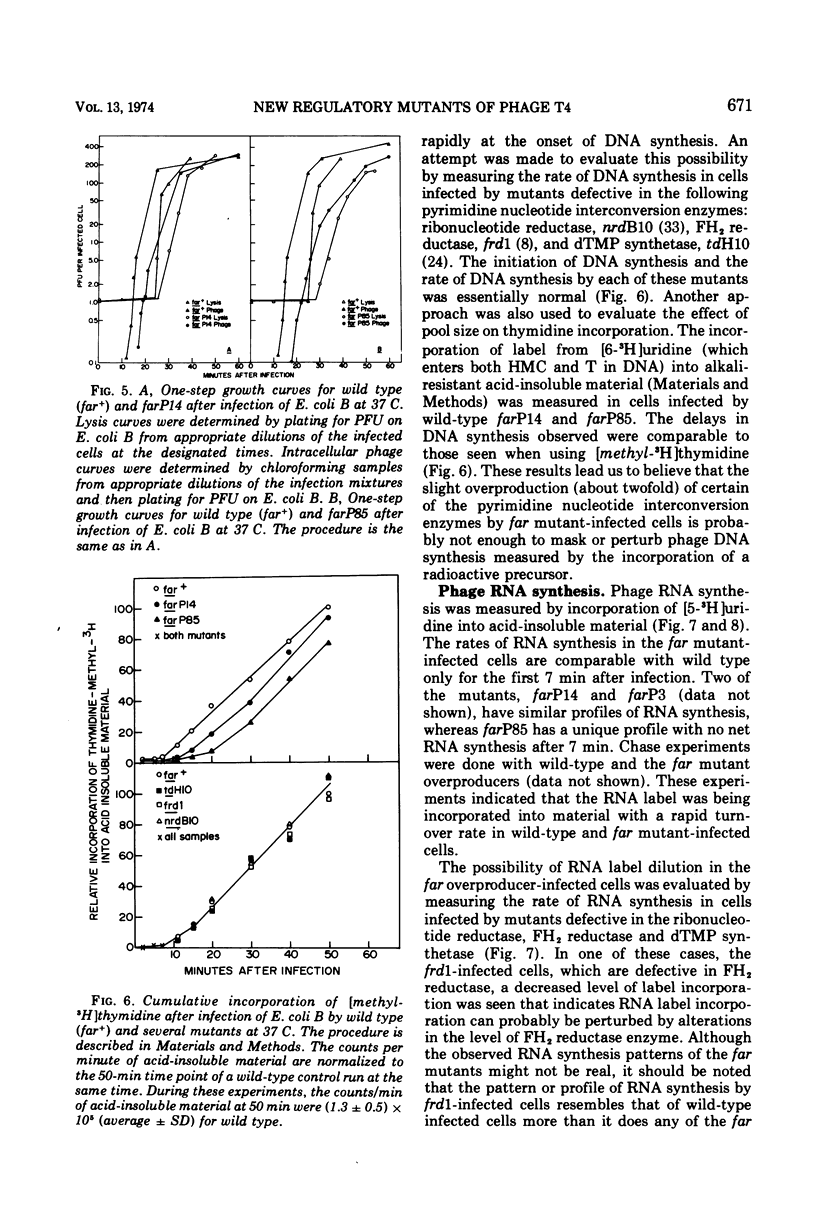
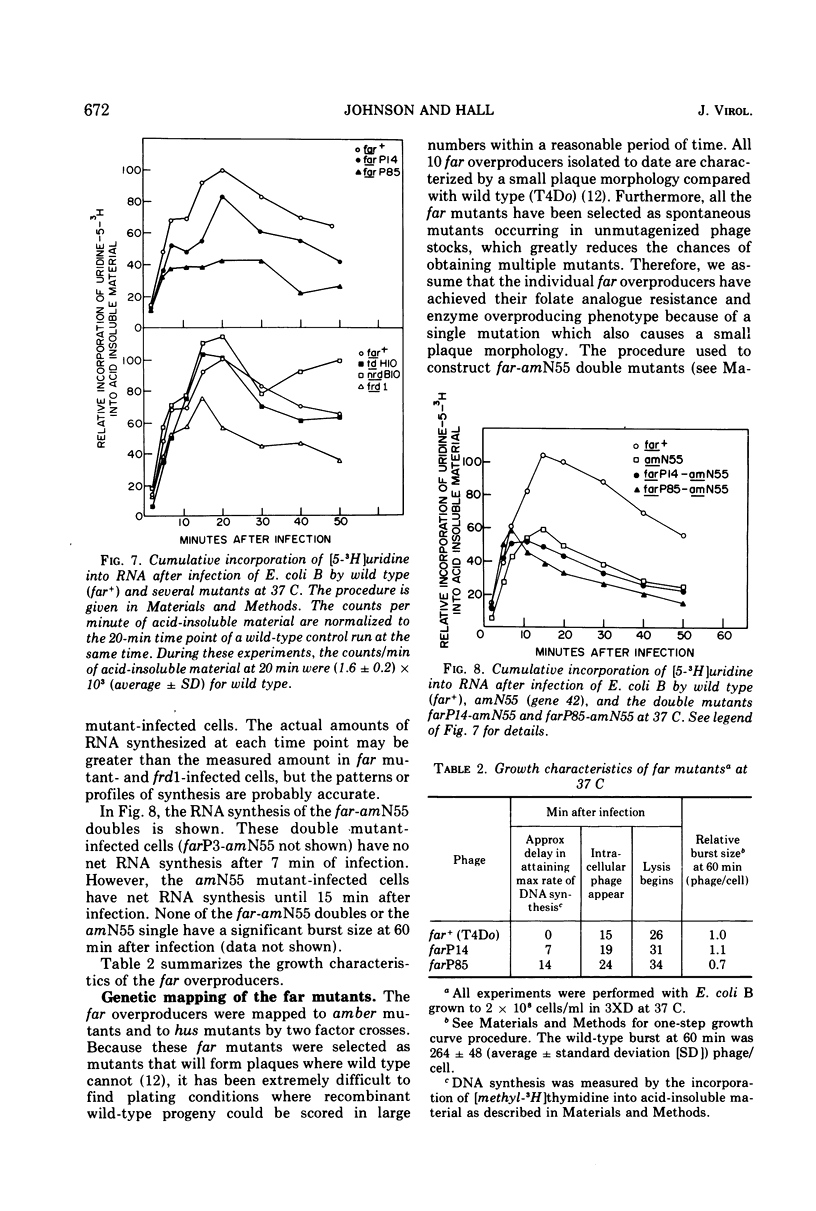
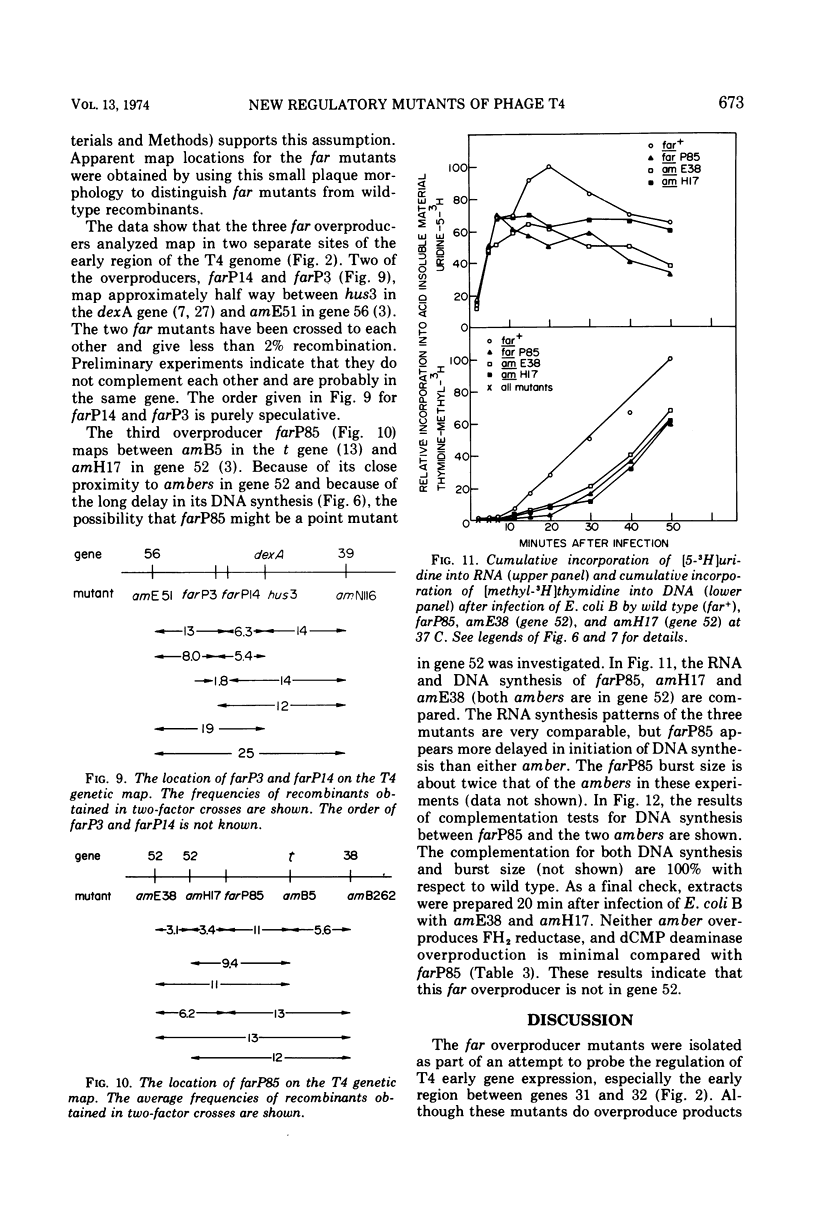
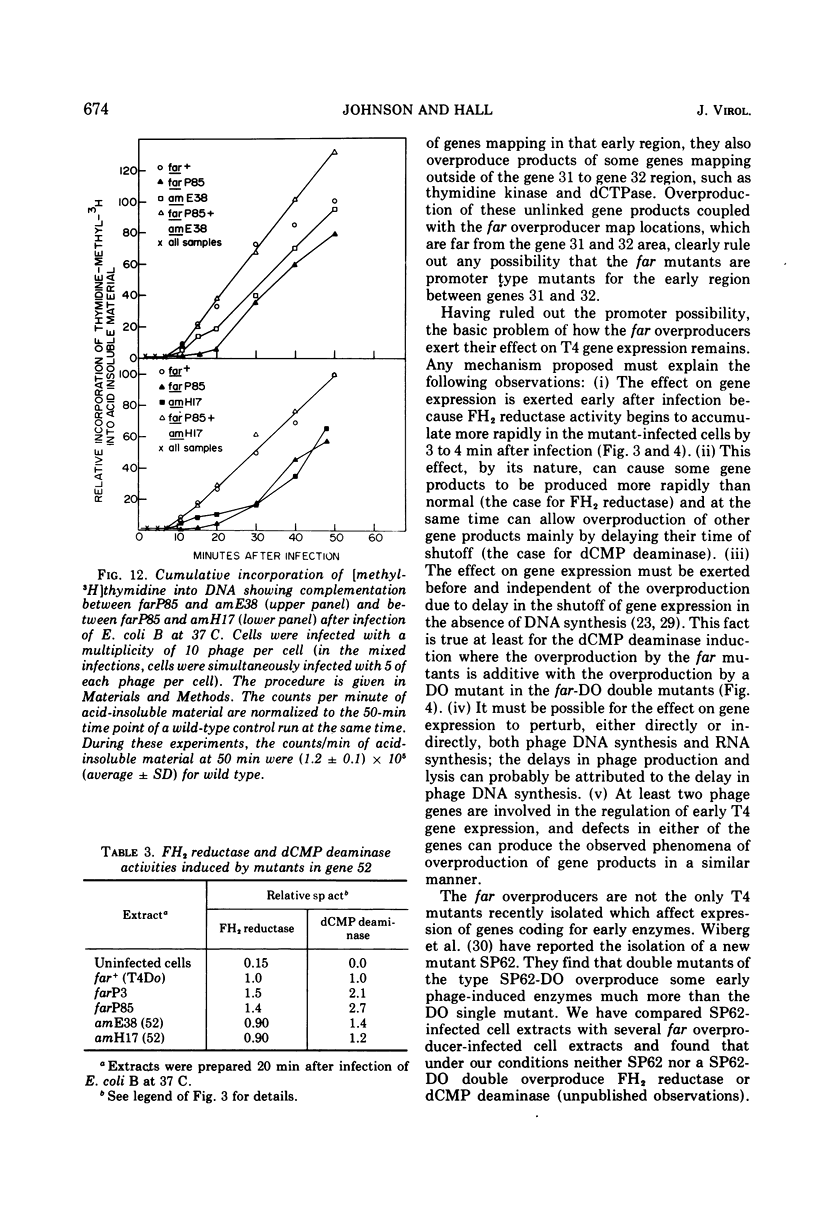
Plating techniques which eliminate T4 plaque formation on Escherichia coli by folate analogue inhibition of dihydrofolate (FH2) reductase (EC 1.5.1.3) allowed the isolation of folate analogue-resistant (far) mutants of T4. One class of far mutants overproduces the phage-induced FH2 reductase. Deoxycytidylate deaminase (EC 3.5.4.12), thymidine kinase (EC 2.7.1.21), and deoxycytidine triphosphatase (EC 3.6.1.12) are also overproduced by 20 min after infection at 37 C. The overproduction of FH2 reductase by these far mutants is not affected by the absence of DNA synthesis. Other types of mutations that affect the synthesis of early enzymes cause overproduction in the absence of DNA synthesis of some of the above enzymes but not of FH2 reductase. Therefore, overproducing far mutants apparently have mutations in previously undescribed genes controlling the expression of the T4 genome. Three of four mutants under study map near gene 56, and one maps near gene 52. All of these mutants show delays in DNA synthesis, phage production, and lysis and appear to show decreased levels of RNA synthesis based on the cumulative incorporation of uridine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. RNA metabolism in T4-infected Escherichia coli. J Mol Biol. 1970 Mar 14;48(2):187–208. doi: 10.1016/0022-2836(70)90156-7. [DOI] [PubMed] [Google Scholar]
- Chace K. V., Hall D. H. Isolation of mutants of bacteriophage T4 unable to induce thymidine kinase activity. J Virol. 1973 Aug;12(2):343–348. doi: 10.1128/jvi.12.2.343-348.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- Goscin L. A., Hall D. H. Hydroxyurea-sensitive mutants of bacteriophage T4. Virology. 1972 Oct;50(1):84–94. doi: 10.1016/0042-6822(72)90348-0. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Isolation and characterization of mutants of bacteriophage T4 resistant to folate analogs. Virology. 1973 Jun;53(2):413–426. doi: 10.1016/0042-6822(73)90221-3. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Kammen H. O. A rapid assay for thymidylate synthetase. Anal Biochem. 1966 Dec;17(3):553–556. doi: 10.1016/0003-2697(66)90192-8. [DOI] [PubMed] [Google Scholar]
- Karlström O. Mutants of Escherichia coli defective in ribonucleoside and deoxyribonucleoside catabolism. J Bacteriol. 1968 Mar;95(3):1069–1077. doi: 10.1128/jb.95.3.1069-1077.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- Naot Y., Shalitin C. Defective concatemer formation in cells infected with deoxyribonucleic acid-delay mutants of bacteriophage T4. J Virol. 1972 Oct;10(4):858–862. doi: 10.1128/jvi.10.4.858-862.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Shalitin C. Role of gene 52 in bacteriophage T4 DNA synthesis. J Virol. 1973 Jun;11(6):862–871. doi: 10.1128/jvi.11.6.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Price A. R., Warner H. R. A structural gene for bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphage nucleotidohydrolase. Virology. 1968 Nov;36(3):523–526. doi: 10.1016/0042-6822(68)90183-9. [DOI] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. Control of the synthesis of T4 phage deoxynucleotide kinase messenger ribonucleic acid in vivo. J Biol Chem. 1972 Dec 10;247(23):7806–7814. [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Galivan J., Maley F. The temporal expression of T2r + bacteriophage genes in vivo and in vitro. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1659–1663. doi: 10.1073/pnas.69.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wovcha M. G., Tomich P. K., Chiu C. S., Greenberg G. R. Direct participation of dCMP hydroxymethylase in synthesis of bacteriophage T4 DNA. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2196–2200. doi: 10.1073/pnas.70.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegian C. D., Mueller M., Selzer G., Russo V., Stahl F. W. Properties of the DNA-delay mutants of bacteriophage T4. Virology. 1971 Dec;46(3):900–919. doi: 10.1016/0042-6822(71)90090-0. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]



