Abstract
Background:
Over 15 000 new oesophago-gastric cancers are diagnosed annually in the United Kingdom, with most being advanced disease. We identified and quantified features of this cancer in primary care.
Methods:
Case–control study using electronic primary-care records of the UK patients aged ⩾40 years was performed. Cases with primary oesophago-gastric cancer were matched to controls on age, sex and practice. Putative features of cancer were identified in the year before diagnosis. Odds ratios (ORs) were calculated for these features using conditional logistic regression, and positive predictive values (PPVs) were calculated.
Results:
A total of 7471 cases and 32 877 controls were studied. Sixteen features were independently associated with oesophago-gastric cancer (all P<0.001): dysphagia, OR 139 (95% confidence interval 112–173); reflux, 5.7 (4.8–6.8); abdominal pain, 2.6 (2.3–3.0); epigastric pain, 8.8 (7.0–11.0); dyspepsia, 6 (5.1–7.1); nausea and/or vomiting, 4.9 (4.0–6.0); constipation, 1.5 (1.2–1.7); chest pain, 1.6 (1.4–1.9); weight loss, 8.9 (7.1–11.2); thrombocytosis, 2.4 (2.0–2.9); low haemoglobin, 2.4 (2.1–2.7); low MCV, 5.2 (4.2–6.4); high inflammatory markers, 1.7 (1.4–2.0); raised hepatic enzymes, 1.3 (1.2–1.5); high white cell count, 1.4 (1.2–1.7); and high cholesterol, 0.8 (0.7–0.8). The only PPV >5% in patients ⩾55 years was for dysphagia. In patients <55 years, all PPVs were <1%.
Conclusion:
Symptoms of oesophago-gastric cancer reported in secondary care were also important in primary care. The results should inform guidance and commissioning policy for upper GI endoscopy.
Keywords: oesophago-gastric cancer, primary care, symptoms, diagnosis, positive predictive values
Oesophago-gastric tumours account for 6% of all UK cancers, with 15 500 new diagnoses each year (8173 oesophageal cancer; 7610 gastric cancer); two-thirds are in men, and 92% of new cases occur in those aged ⩾55 years (Office for National Statistics, 2010). Worldwide, there are almost 1.5 million new cases annually, two-thirds gastric and one-third oesophageal. In Western countries, gastric cancer is decreasing in incidence, but the increase in oesophageal cancer outweighs this fall, leading to an overall increase (NHS Information Centre, 2010). Mortality is very high, with 5-year survival, approximately 7% for oesophageal and 12% for gastric cancers (Rachet et al, 2009).
There are presently no screening tests available for oesophago-gastric cancer, other than surveillance of patients with Barrett’s oesophagus, though this accounts for <5% of all new oesophageal cancer diagnoses (Hvid-Jensen et al, 2011). Therefore, diagnosis largely depends upon presentation with symptoms, generally to primary care (Hamilton, 2010). However, symptoms are often vague and are also common in benign conditions. Indeed, dyspepsia is experienced by 40% of the population, though only 5% report it to their doctor (National Prescribing Centre, 2006). Furthermore, treatment of dyspepsia with acid suppressants may hide symptoms of gastric cancer and delay diagnosis (Talley et al, 1993). In the United Kingdom, selection of patients for investigation is guided by publications from the National Institute for Health and Clinical Excellence (NICE) (NICE, 2004, 2005). These recommendations were based almost entirely upon secondary-care studies (that is, the referred population), and describe several ‘alarm’ symptoms (Meineche-Schmidt and Jorgensen, 2002; Varadarajulu et al, 2005; Vakil et al, 2006). Even so, the yield of upper gastrointestinal cancer with alarm symptoms is only 4%, many of these cancers being at an advanced stage (Kapoor et al, 2005). Liberalising access to endoscopy in an attempt to increase the number of early-stage cancers is an option, but this strategy could increase the number of endoscopies up to 10-fold, with no increase in the number of cancers diagnosed (Agreus et al, 2001).
In addition, public awareness of dysphagia as an alarm symptom for oesophageal cancer is poor and may contribute to patient delay in seeking advice (Tentzeris et al, 2011). An awareness campaign for the United Kingdom is currently under consideration. Thus, it is timely to study the features of oesophago-gastric cancer as presented to primary care. It is also logical to study the two cancer sites collectively, as they share a common diagnostic test – endoscopy. The aim of this study was to identify and quantify the clinical features of oesophago-gastric cancer in primary care, ultimately to improve selection of patients for investigation.
Materials and Methods
This was a case–control study using data from the General Practice Research Database (GPRD) in the United Kingdom. The GPRD maintains an anonymised copy of medical records of participating practices; these contain full details of the patient, including all consultations, recorded symptoms, investigations and diagnoses. The data are subject to stringent checks on validation and quality, and they are regarded as high quality in terms of accuracy, completeness and validity of diagnoses (Khan et al, 2010). We have previously used similar methods for several cancer diagnostic studies (Hamilton and Kernick, 2007; Dommett et al, 2012).
Identification of cases and controls
A list of 42 (18 oesophageal, 24 gastric) tumour diagnostic codes (available from the authors) was collated from the GPRD master code library. This has approximately 100 000 codes covering all events in primary care. GPRD staff identified all patients ⩾40 years with an oesophago-gastric tumour diagnosed between 1 January 2000 and 31 December 2009, and with at least 1 year of data meeting their quality standards before diagnosis. For each case, the GPRD identified up to five controls, matched by year of birth, sex and practice, using a computer-generated random sequence. We excluded cases and controls with no consultations in the year before diagnosis of the case (the index date). We included primary oesophago-gastric cancers that had metastasised but excluded cancers from other sites that had spread to the oesophagus or stomach. Controls were excluded if they had ever had oesophago-gastric cancer.
Selection of possible features of oesophago-gastric cancer
We studied all previously described diagnostic features of oesophago-gastric cancer identified in a literature review, supplemented by literature from relevant cancer websites. The GPRD’s code list has many synonyms for similar symptoms, often including additional description such as severity or duration. These synonyms were identified and merged. The dyspepsia variable merged codes with either the word ‘dyspepsia’ or ‘indigestion’ the reflux variable included ‘regurgitation’ as well as ‘reflux’ the variable ‘epigastric pain’ required a precise anatomical description, whereas the variable ‘abdominal pain’ incorporated all other abdominal pain variables without a precise anatomical description. Occurrences of features in the year before the index date were identified. These were only retained if they occurred in ⩾5% of cases or controls. For laboratory tests, we used the local laboratory range to identify abnormal results; we considered patients without a test to be equivalent to those with a normal result. We merged all hepatic enzyme results into a composite variable, deemed abnormal if any enzyme was raised; similarly, abnormal erythrocyte sedimentation rate, plasma viscosity and C-reactive protein were collated into a single variable called raised inflammatory markers. To test for any recording bias between cases and controls, we also identified all codes for fractures (making the assumption that the fracture rate would be approximately equal).
Analysis
All analyses were performed using Stata (version 11) (Statacorp, 2010), and our main analytical method was conditional logistic regression. Variables associated with oesophago-gastric cancer with a P-value <0.1 in the univariable regressions entered multivariable analyses. Multivariable regressions were performed in stages, initially collecting similar variables together, such as those reflecting abdominal pain. Significant variables then entered a second stage, grouping variables into abdominal symptoms, other symptoms and investigations. For these two stages, a threshold P-value of <0.05 was used. The final model was derived from all variables surviving the earlier staged regressions, and used a threshold P-value of <0.01. All rejected variables were checked to see if they contributed to the final model, and 11 clinically plausible interactions were investigated. Stratified analyses were performed as an exploratory analysis of the timing of symptoms. We also examined the possibility that the symptom patterns differed between oesophageal and gastric cancers by repeating the two multivariable models in the single sites: where the odds ratios (ORs) for features differed materially, an interaction term between the symptom and cancer site was added to the final model.
Calculation of positive predictive values (PPVs)
We calculated PPVs for the risk of oesophago-gastric cancer in patients consulting in primary care using Bayes’ theorem (Knottnerus 2002). In this, the posterior odds of disease=the prior odds × the likelihood ratio. For the prior odds, we used the age-specific national incidence rate of oesophago-gastric cancer for 2008 (Cancer Research UK 2008). As all the 7471 cases analysed had consulted in primary care, but only 32 877 of 36 212 (90.8%) controls had consulted in the study period, we divided the posterior odds by 0.908 to give predictive values for the consulting population. This analysis was performed in two age groups over and under 55 years to mirror the current UK national guidance (NICE, 2004, 2005).
Results
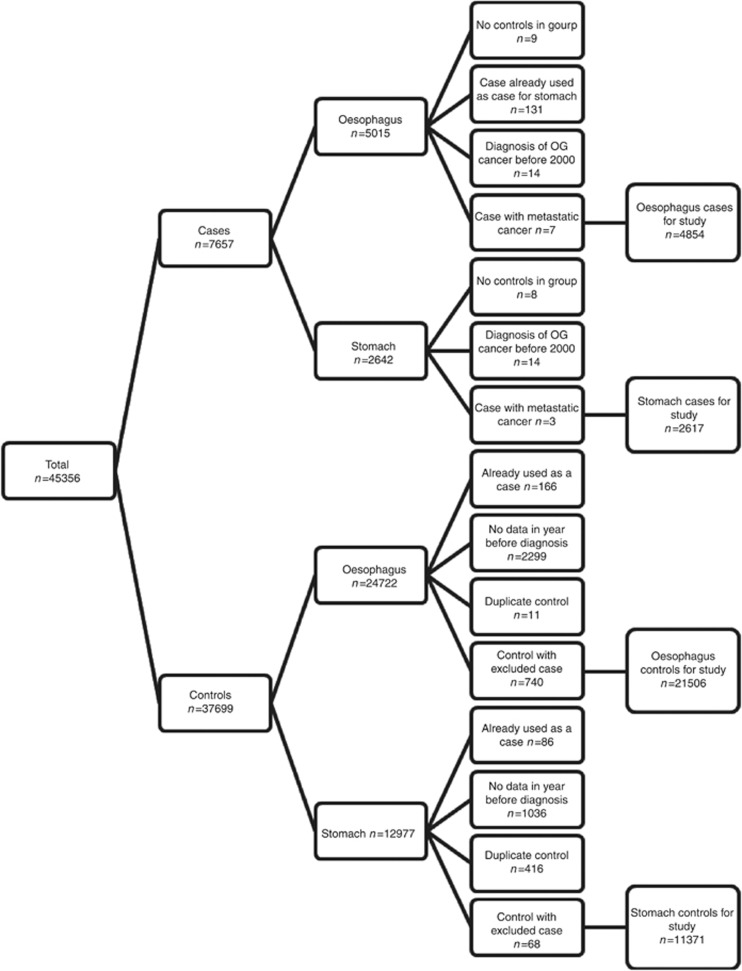
We were initially supplied with 7657 cases and 37 699 controls: this includes 2968 cases in whom fewer than five controls were available. The application of exclusions is shown in Figure 1.
Figure 1.
Application of exclusion criteria for cases and matched controls.
The demographic features of patients are shown in Table 1, and their clinical features are shown in Table 2. In the year before diagnosis, cases presented to primary care more frequently than controls: median number of consultations 26 (interquartile range 15–42) vs 15 (7–28); P<0.001 in Mann–Whitney test. For the cases and controls, respectively, 130 (1.7%) and 521 (1.6%) had a record of a fracture. Barrett’s oesophagus was recorded in 209 (2.8%) of cases, and 51 (0.2%) of controls.
Table 1. Characteristics of patients in primary care with oesophago-gastric cancer and matched controls. Figures are in numbers (percentages).
|
Oesophageal cancer
|
Gastric cancer
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls n=21506 | Cases | Controls n=11371 | |||||
| Age in years | Total n=4854 | Females (n=1680) | Males (n=3174) | Total (n=2617) | Females (n=992) | Males (n=1625) | ||
| 40–54 | 387 (8.0) | 94(5.6) | 291 (9.2) | 1539 (7.2) | 130 (5.0) | 49 (4.9) | 80 (4.9) | 497 (4.4) |
| 55–69 | 1712 (35.2) | 468 (27.9) | 1243 (39.1) | 7473 (34.8) | 671 (25.6) | 218 (22) | 453 (27.9) | 2887 (25.4) |
| 70–84 | 2230 (45.9) | 814 (48.5) | 1416 (44.6) | 10296 (47.9) | 1437 (54.9) | 523 (52.7) | 914 (56.2) | 6431 (56.6) |
| ⩾85 | 532 (10.9) | 304 (18.1) | 224 (7.1) | 2198 (10.2) | 382 (14.6) | 202 (20.4) | 178 (11.0) | 1556 (13.7) |
Table 2. Frequency of selected features in cases and controls in the whole study population.
| Clinical feature | Cases, n (%), n=7471 | Controls, n (%), n=32877 | Likelihood ratio (95% CI) | PPV as a percentagea (95% CI) |
|---|---|---|---|---|
|
Symptoms
| ||||
| Dysphagia | 2420 (32.4) | 185 (0.6) | 57.6 (49.7–66.7) | 1.5 (1.3–1.8) |
| Dyspepsia | 1294 (17.3) | 764 (2.3) | 7.5 (6.8–8.1) | 0.2 (0.19–0.22) |
| Nausea or vomiting | 979 (13.1) | 637 (1.9) | 6.8 (6.1–7.4) | 0.18 (0.17–0.2) |
| Abdominal pain | 905 (12.1) | 1310 (4.0) | 3.0 (2.8–3.3) | 0.08 (0.08–0.09) |
| Reflux | 842 (11.3) | 513 (1.6) | 7.2 (6.5–8.0) | 0.19 (0.18–0.22) |
| Chest pain | 727 (9.7) | 1589 (4.8) | 2.0 (1.9–2.2) | 0.05 (0.05–0.06) |
| Epigastric pain | 617 (8.3) | 266 (0.8) | 10.2 (8.9–11.8) | 0.28 (0.24–0.32) |
| Loss of weight | 615 (8.2) | 276 (0.8) | 9.8 (8.5–11.3) | 0.26 (0.23–0.31) |
| Constipation | 608 (8.1) | 1073 (3.3) | 2.5 (2.3–2.8) | 0.07 (0.06–0.07) |
|
Investigations
| ||||
| Low haemoglobin | 2045 (27.4) | 3353 (10.2) | 2.7 (2.6–2.8) | 0.07 (0.07–0.08) |
| Abnormal hepatic enzymes | 1272 (17.0) | 3479 (10.6) | 1.6 (1.5–1.7) | 0.04 (0.04–0.05) |
| Raised inflammatory markers | 1010 (13.5) | 1421 (4.3) | 3.1 (2.9–3.4) | 0.08 (0.08–0.09) |
| Raised cholesterol | 920 (12.3) | 5180 (15.8) | 0.8 (0.7–0.8) | 0.02 (0.02–0.02) |
Abbreviations: CI=confidence interval; PPV=positive predictive value. aPPV in the consulting population.
Multivariable analysis results are shown in Table 3: of cases, only 26.6% had none of the features present in this Table. Sixteen features were associated with oesophago-gastric cancer: all P-value <0.001. No interaction terms with age or sex were found, but interactions with dysphagia with loss of weight and dysphagia with nausea and/or vomiting were antagonistic. The only symptom with a markedly different OR between the two separate cancer sites was dysphagia (oesophageal cancer, OR 230 (confidence interval (CI) 180–300); gastric cancer, OR 20 (CI 14–29)). When expressed as an interaction term in the unified model, the interaction OR for dysphagia was 0.27 (CI 0.21–0.33): P<0.001. The ORs for the remaining symptoms were remarkably similar.
Table 3. Multivariable analysis of the clinical features of oesophago-gastric cancer.
| Variable | Odds ratio (95% CI) |
|---|---|
|
Symptoms
| |
| Dysphagia | 139 (112–173) |
| Loss of weight | 8.9 (7.1–11.2) |
| Epigastric pain | 8.8 (7.0–11) |
| Dyspepsia | 6.0 (5.1–7.1) |
| Reflux | 5.7 (4.8–6.8) |
| Nausea or vomiting | 4.9 (4.0–6.0) |
| Abdominal pain | 2.6 (2.3–3.0) |
| Chest pain | 1.6 (1.4–1.9) |
| Constipation | 1.5 (1.2–1.7) |
|
Investigations
| |
| Thrombocytosis | 2.4 (2.0–2.9) |
| Low haemoglobin | 2.4 (2.1–2.7) |
| Low mean red cell volume | 5.2 (4.2–6.4) |
| Leucocytosis | 1.4 (1.2–1.7) |
| Raised inflammatory markers | 1.7 (1.4–2.0) |
| Abnormal hepatic enzymes | 1.3 (1.2–1.5) |
| Raised cholesterol | 0.8 (0.7–0.8) |
|
Interaction terms
| |
| Dysphagia and loss of weight | 0.2 (0.06–0.5) |
| Dysphagia and nausea or vomiting | 0.1 (0.06–0.2) |
Abbreviation: CI=confidence interval.
Stratified analyses were performed at 3, 6 and 9 months before diagnosis on the final multivariable model. No differences between the models were found at 3 and 6 months before diagnosis. Three symptoms, dysphagia, dyspepsia and nausea and/or vomiting, were associated with cancer at 9 months before diagnosis, ORs 120 (CI 15–980), 5.5 (CI 2.3–13) and 3.7 (CI 1.5–9), respectively.
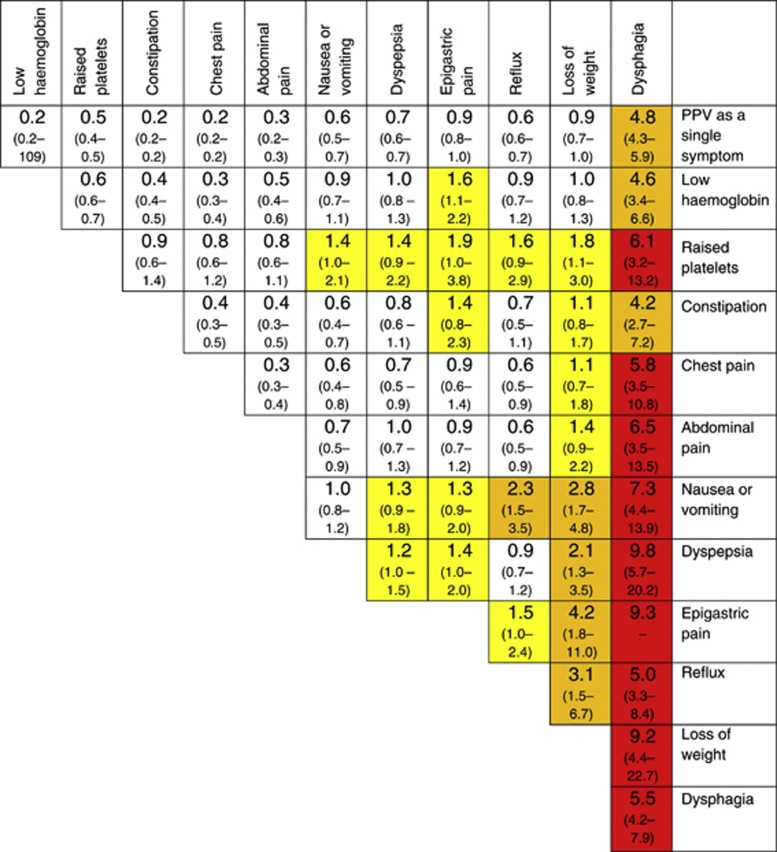
Figure 2 shows PPVs for oesophago-gastric cancer for the symptoms in Table 3, plus low haemoglobin and thrombocytosis for patients aged ⩾55 years. All PPVs for symptom combinations in patients aged <55 years were <1% (data not shown). The highest PPV in this age group was for dysphagia, 0.8% (CI 0.4–1.5%).
Figure 2.
PPVs (95% CIs) for oesophago-gastric cancer in men and women aged >55 years for individual risk markers and for pairs of risk markers in combination. The top figure in each cell is the PPV (95% CIs) when both features are present. CIs have not been calculated when any cell in the 2 × 2 table was <5 (invariably, this was because too few controls had both features). The yellow shading is for features with a PPV >1% the amber for PPV >2.0% and the red for PPV >5.0%. The cells along the diagonal relate to the PPV when the same feature has been reported twice. Thus, the dysphagia/dysphagia intersect is the PPV for oesophago-gastric cancer when a patient has attended at least twice with dysphagia.
Discussion
This is the first study quantifying the risk of oesophago-gastric cancer in primary care that incorporates single and multiple symptoms, as well as laboratory results. Most of the symptoms that have been reported from secondary-care studies and from patient groups were also strongly associated with oesophago-gastric cancer in this primary-care study. This was particularly so for dysphagia, with a risk of cancer of 4.8%. No other isolated symptom had a risk >1%, even when we restricted the analysis to patients >55 years of age to match current guidance. In combination, however, several symptom pairs carried higher risks, especially when one of the symptoms was loss of weight. In contrast, no symptom in patients <55 years had a risk >1%, even dysphagia. The features in the multivariable analysis reflect the symptomatology that was apparent for at least 6 months before diagnosis, and a small subset of the associations was present for even longer than 9 months before diagnosis.
Strengths and limitations of the study
This study is large, and uses primary care data. This is crucial: selection of patients for investigation is performed by clinicians in primary care, so primary-care data must be used to illuminate the selection process. The GPRD is considered by many to be the gold standard of longitudinal patient databases from primary care. It has been used in nearly 1000 research papers published in peer-reviewed journals, and its validity has been well documented (Herrett et al, 2010; Khan et al, 2010). The patient population in the database is also broadly representative of the UK population. In addition, laboratory results are transmitted directly to the database, allowing us to use the local normal range to identify abnormal results, as well as minimising transcription errors. A further strength was our decision to study oesophageal and gastric cancers together; it was logical to answer the question of what clinical features should prompt consideration of endoscopy, as both cancers share the same investigation. In any case, the associations between symptoms and the individual cancers were remarkably similar (other than for dysphagia). A previous primary-care study agrees with this finding, suggesting that dysphagia should be taken seriously as a warning of oesophageal malignancy (Jones et al, 2009).
We could not check the accuracy of diagnosis in the cases by histology or determine the staging. This is less important than it appears: primary-care recording of cancer diagnoses is excellent, especially because maintaining a cancer registry became one of the items in the Quality and Outcomes Framework. Staging data may have allowed us to identify clinical features, particularly associated with early-stage cancer: although superficially attractive, it is actually valuable to identify all cancers, including those that cannot be cured, as treatment may still be beneficial.
The main limitation of the study is that we had to rely upon the accurate recording by GPs of symptoms. Under-recording of symptoms or signs may have led to some features that may be genuinely associated with oesophago-gastric cancer not being identified, such as upper gastrointestinal bleeding. When calculating PPVs, under-recording is only important if the proportion of symptom under-recording was markedly higher in either cases or controls. We have no reason to believe this is the case (Hamilton, 2009).
Comparison with existing literature
The prevalence of the common symptoms of oesophago-gastric cancer in cases was lower than in previous secondary-care studies (Ojala et al, 1982; Bodger et al, 2000; Fransen et al, 2007; Berrill et al, 2011). This probably reflects different symptom experience early in the disease, especially in primary care. There are two possible alternative explanations. The first is under-recording of symptoms as discussed earlier, and the second is that most previous studies directly interviewed patients after diagnosis. Such methods may be subjected to recall bias, thus reporting higher rates of symptoms than studies using indirect methods, such as ours. Again, this may matter less than it appears: unvoiced symptoms are a well-recognised feature of primary care, and can only be combated by using checklists, a procedure rarely used in primary care. The risk from dysphagia in primary care has previously been estimated as 5.4% in men and 2.7% in women (Jones et al, 2007). Our figure of 4.8% (with little difference between the sexes) is similar. Another primary-care study using a different data set has reported associations for six features of cancer (dysphagia, haematemesis, abdominal pain, loss of weight, loss of appetite and anaemia), though without reporting PPVs (Hippisley-Cox and Coupland, 2011). Our finding of a strong association between a raised platelet count and cancer has been described before for ovarian and lung cancers, though not for oesophago-gastric tumours (Hamilton et al, 2005; Stone et al, 2012).
Implications for clinical practice and future research
Selection of patients for investigation – generally by endoscopy – has always been difficult. The ‘alarm’ symptoms, such as loss of weight or dysphagia, are shown in this study to have a sufficiently high PPV to warrant endoscopy, though in the case of weight loss, additional symptoms are needed to raise the PPV >1%. Our figures support current NICE guidance on such symptoms (NICE, 2005). However, using alarm symptoms alone to identify patients for endoscopy will miss over half of patients with cancer, as only 32% of our cases had reported dysphagia, and 8% reported loss of weight. A previous primary-care study agrees with this, suggesting that a symptom-based approach, focusing on single symptoms, such as dysphagia, is likely to miss 40% of current oesophago-gastric cancers, some of which will be at a curable stage (Jones et al, 2007).
It has long been recognised that certain ‘low-risk but not no-risk’ symptoms are associated with cancer – particularly dyspepsia. The risk is even lower in younger patients, though the decision to use a threshold age of 55 years for investigation was not based on primary-care literature, and is hotly contested, especially by patient advocacy groups. There is no generally agreed view of a particular level of risk that warrants cancer investigation: patients, commissioners of care and clinicians will all have a voice in such a decision, which will need to include a health-economic dimension. If a 2% risk is chosen, endoscopy would be offered to over 55 patients, for most patients with loss of weight and a second gastrointestinal symptom, as well as all patients with dysphagia. It is only if a lower figure of (say) 1% risk is accepted that a much larger group of patients are selected for endoscopy. This would include most combinations of dyspepsia with a second symptom, plus combinations with epigastric pain. Indeed, there must be considerable overlap between these two symptoms, with many clinicians using them interchangeably (we assigned these symptoms according to the GP’s records, but each GP will have their own personal definition of the two terms).
By lowering the threshold for investigation, many more patients would be identified (as dyspepsia, in particular, was common in cases). This would bring considerable investigative costs. Until prevention by reduction of alcohol, smoking or obesity reduces the incidence – and this is not likely in the short term – or a screening programme is developed – and again it is difficult to conceive of what this could be – or a biomarker is uncovered, then the only solution to the UK marked excess mortality from oesophago-gastric cancer will be a considerable expansion of testing. Currently, there is almost a threefold variation within England, with European rates even higher (Department of Health, 2012).This will mean commissioners and clinicians accepting the financial and organisational costs, as well as patients accepting a high initial ‘false-positive rate’ – that is, being selected for endoscopy, yet transpiring not to have cancer. This is not our decision as authors to make, though our research shows that it is naive to believe that more appropriate selection of patients will identify a higher number of cancers without increasing use of investigations.
Conclusion
Although current mortality from oesophago-gastric cancer is very high in the United Kingdom, other European countries have better outcomes from cancer (Abdel-Rahman et al, 2009). It is not known if these better outcomes arise from better access to investigations or public awareness of symptoms, or both. This study has two main uses: first, it can guide GPs in the selection of patients for urgent investigation, and second, it suggests that selection of patients for investigation using only alarm symptoms will have a limited effect.
Acknowledgments
We thank for the contribution provided by the Discovery Programme Steering Committee comprising: Roger Jones (Chair), Jonathan Banks, Alison Clutterbuck, Jon Emery, Joanne Hartland, Sandra Hollinghurst, Maire Justice, Jenny Knowles, Helen Morris, Greg Rubin. This study was funded by the NIHR Programme Grants for Applied Research funding scheme. The study was approved by the Independent Scientific Advisory Committee (ISAC).
Author contributions
SS performed the data manipulation and analyses, under the supervision of WH and TJP. SS and WH drafted the article. All the six authors made revisions.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Abdel-Rahman M, Stockton D, Rachet B, Hakulinen T, Coleman M (2009) What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer 101(S2): S115–S124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agreus L, Svardsudd K, Talley NJ, Jones MP, Tibblin G (2001) Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population based study. Am J Gastroenterol 96: 2905–2914 [DOI] [PubMed] [Google Scholar]
- Berrill JW, Turner J, Hurley J, Swift J, Dolwani S, Green JT (2011) Referral criteria for open access gastroscopy: do they inevitably delay the diagnosis of cancer? Gut 60(Suppl 1): A101–A102 [Google Scholar]
- Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV (2000) Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia guidelines (1996). Aliment Pharmacol Ther 14: 413–420 [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2008) "Incidence Statistics." Retrieved 3 June 2011, from http://publications.cancerresearchuk.org/downloads/Product/cs_pdf_incidence_feb_2008.pdf
- Department of Health (2012) “NHS atlas of variation 2011 Map 42: rate of activity for gastroscopy (upper GI endoscopy) per population by PCT.”
- Dommett R, Redaniel M, Stevens M, Hamilton W, Martin R (2012) Features of childhood cancer in primary care: a population-based nested case-control study. Br J Cancer 106: 982–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen G, Janssen M, Muris J, Mesters I, Knottnerus J (2007) Measuring the severity of upper gastrointestinal complaints: does GP assessment correspond with patients' self-assessment? Fam Pract 24: 252–258 [DOI] [PubMed] [Google Scholar]
- Hamilton W (2009) The CAPER studies: five case–control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 101(S2): S80–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W (2010) Cancer diagnosis in primary care. Br J Gen Pract 60(571): 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Kernick D (2007) Clinical features of primary brain tumours: a case–control study using electronic primary care records. BJGP 57: 695–699 [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Peters TJ, Round A, Sharp D (2005) What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 60: 1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69(1): 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C (2011) Identifying patients with suspected gastro-oesophageal cancer in primary care: derivation and validation of an algorithm. BJGP 61: 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid-Jensen F, Pedersen L, Mohr Drewes A, Toft Sorensen H, Funch-Jensen P (2011) Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 365(15): 1375–1383 [DOI] [PubMed] [Google Scholar]
- Jones R, Charlton J, Latinovic R, Gulliford MC (2009) Alarm symptoms and identification of non-cancer diagnoses in primary care: cohort study. BMJ 339: b3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Latinovic R, Charlton J, Gulliford MC (2007) Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ 334(7602): 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N, Bassi A, Sturgess R, Bodger K (2005) Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut 54: 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NF, Harrison SE, Rose PW (2010) Validity of diagnostic coding within the General Practice Research Database: a systematic review. BJGP 60(572): e128–e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knottnerus JA (2002) The Evidence Base of Clinical Diagnosis. BMJ Books: London [Google Scholar]
- Meineche-Schmidt V, Jorgensen T (2002) 'Alarm symptoms' in patients with dyspepsia: a three year prospective study from general practice. Scand J Gastroenterol 37(9): 999–1007 [DOI] [PubMed] [Google Scholar]
- National Prescribing Centre (2006) “The management of dyspepsia in primary care:MeReC Briefing; 2005-06.” 32. Retrieved 12 June 2012, 2012, from http://www.npc.nhs.uk/merec/therap/dysp/resources/merec_bulletin_vol16_no3.pdf
- Nhs Information Centre (2010) The National Oesophago-gastric cancer audit 2010. Third Annual Report. Information Centre: London, NHS [Google Scholar]
- Nice (2004) “Dyspepsia:managing dyspepsia in adults in primary care.” Retrieved 17 April 2012, 2012, from www.nice.org.uk/CG017fullguideline
- Nice (2005) “CG27 Referral for suspected cancer: full guideline”
- Office for National Statistics (2010) Cancer Statistics registrations: registrations of cancer diagnosed in 2008, England. Natl Stat
- Ojala K, Sorri M, Jokinen K, Kairaluoma MI (1982) Symptoms and diagnostic delay in patients with carcinoma of oesophagus and gastric cardia: a retrospective study of 225 patients. Postgrad Med J 58(679): 264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachet B, Maringe C, Nur U, Quaresma M, Shah A, Woods LM, Ellis L, Walters S, Forman D, Steward J, Coleman MP (2009) Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol 10(4): 351–369 [DOI] [PubMed] [Google Scholar]
- Statacorp (2010) Stata Statistical Software: Release 11. College Station, TX, Stata Corporation
- Stone RL, Nick AM, Mcneish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupaimoole R, Armaiz-Pena GN, Pecot CV, Coward J, Deavers MT, Vasquez HG, Urbauer D, Landen CN, Hu W, Gershenson H, Matsuo K, Shahzad MMK, King ER, Tekedereli I, Ozpolat B, Ahn EH, Bond VK, Wang R, Drew AF, Gushiken F, Collins K, Degeest K, Lutgendorf SK, Chiu W, Lopez-Berestein G, Afshar-Kharghan V, Sood AK (2012) Paraneoplastic Thrombocytosis in Ovarian Cancer. N Engl J Med 366(7): 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Weaver AL, Tesmer DL, Zinsmeister AR (1993) Lack of discriminant value of dyspepsia subgroups in patients referred for upper endoscopy. Gastroenterology 105: 1378–1386 [DOI] [PubMed] [Google Scholar]
- Tentzeris V, Lake B, Cherian T, Milligan J, Sigurdsson A (2011) Poor awareness of symptoms of oesophageal cancer. Interact Cardiovasc Thorac Surg 12(1): 32–34 [DOI] [PubMed] [Google Scholar]
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ (2006) Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 131: 390–401 [DOI] [PubMed] [Google Scholar]
- Varadarajulu S, Eloubeidi MA, Patel RS (2005) The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 61(7): 804–808 [DOI] [PubMed] [Google Scholar]