Abstract
Objective
To test the hypothesis that resistin is associated with insulin resistance and inflammation in pediatric patients with chronic kidney disease (CKD).
Study design
This study is a cross-sectional analysis of 319 children in the Chronic Kidney Disease in Children cohort, a large cohort of children with stage II–IV CKD. Univariate and multivariate regression modeling was used to evaluate the association of serum resistin level with glomerular filtration rate (GFR), demographic data, and cardiovascular risk factors, including inflammatory cytokines, insulin resistance, and serum lipids.
Results
In univariate analyses, serum resistin level was negatively correlated with GFR (P < .01). Increased serum resistin was associated with elevated inflammatory cytokines, including interleukin (IL)-6 (P < .01), IL-10 (P < .01), and tumor necrosis factor-α (P < .01). Resistin level was not associated with insulin resistance, although it was positively correlated with serum triglycerides (P < .01) and negatively correlated with high-density lipoprotein cholesterol (P < .01). In multivariate analysis, GFR (β = −0.01; P < .001), IL-6 (β = 0.18; P < .001), IL-10 (β = 0.09; P = .01), and pubertal status (β = 0.18; P < .01) were significantly associated with serum resistin level.
Conclusion
These results indicate that serum resistin level increases with GFR decline and is involved in the inflammatory milieu present in CKD.
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in children and young adults with end-stage renal disease. 1 Given that CVD in children is often subclinical, biomarkers predictive of cardiovascular morbidity are needed to improve long-term outcomes in pediatric patients with chronic kidney disease (CKD). Resistin is a 12.5-kDa protein belonging to a family of cysteine-rich proteins known as resistin-like molecules. Since its discovery in 2001, resistin has generated much interest because of its association with known risk factors for CVD, including insulin resistance2–4 and inflammation.5–9 Therefore, it has been suggested that elevated serum resistin level may represent a novel risk factor for CVD.10
Serum resistin levels are elevated in patients with CKD.5,6,11,12 The few studies performed in adults with CKD have failed to identify a relationship between serum resistin level and insulin resistance6,7; however, resistin has been found to be associated with tumor necrosis factor (TNF)-α6,7 and interleukin (IL)-6,5,6,8,9 suggesting that the proinflammatory effects of resistin may be more important in increasing cardiovascular risk.
In the present study, we hypothesized that resistin isassociated with insulin resistance and inflammation inpediatric patients with renal insufficiency. We tested this hypothesis in a cross-sectional study of patients enrolled in the Chronic Kidney Disease in Children (CKiD) study, a prospective observational study of children with mild to moderate CKD. We analyzed the association between resistin level and markers of insulin resistance and inflammation, as well as other potential CVD risk factors in children with CKD.
Methods
The CKiD study is a longitudinal, observational study of a cohort of 586 children with CKD conducted at 46 pediatric nephrology centers in North America. Institutional Review Board approval was obtained at each participating center. Specific details on the design and methods of this study have been published previously.13 The goals of the CKiD study include to identify risk factors for the progression of CKD as well as to describe the prevalence of novel and traditional risk factors for CVD in this patient population. Inclusion criteria were age 1–16 years and a glomerular filtration rate (GFR) of 30–90 mL/min/1.73 m2 as estimated by the Schwartz equation. Among study participants, 319 patients had serum available for analysis of resistin levels. The present study is a cross-sectional analysis of baseline serum resistin levels obtained in this cohort at year 2 of the CKiD study.
Serum resistin analysis was carried out at the Metabolic Phenotyping Core at the University of Texas Southwestern Medical Center, Dallas using serum samples from the CKiD study. Original samples were collected locally, shipped, and stored at −80°C at the National Institutes of Health’s Biologic Repository. The Human Resistin ELISA Kit (Millipore, Billerica, Massachusetts) was used to determine total serum resistin.
In addition to resistin, fasting insulin, TNF-α, IL-6, and IL-10 levels from the year 2 visit were determined utilizing the same laboratory. A bead-based multiplex assay (Luminex 100; Bio-Rad, Hercules, California) was used to determine cytokine concentrations. This assay has been validated and shown to have excellent linearity, precision, and sensitivity.14–16 The homeostasis model assessment of insulin resistance (HOMA-IR) was used as a measure of insulin resistance. HOMA-IR score was calculated by dividing the product of serum insulin (mU/mL) and glucose (mmol/mL) by a factor of 22.5.
Other laboratory data obtained for analysis included fasting lipid profiles (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) and urine protein-to-creatinine ratio. GFR was calculated using plasma iohexol disappearance curves (iGFR), as described previously.13 Demographic and clinical data, including age, sex, body mass index (BMI), pubertal status, and casual blood pressure, were collected at the same time that serum resistin levels and other laboratory measurements were obtained.
For statistical analysis, continuous variables are reported as median and IQR, because most variables were not normally distributed. Univariate analyses were performed to explore the relationship between the dependent variable (serum resistin level) with clinical and demographic data. Comparisons between categorical variables were performed using the Wilcoxon ranksum test or Kruskal-Wallis test. For all continuous variables, Pearson correlation coefficients were calculated along with their associated P values to identify variables with a statistically significant association with serum resistin level. Laboratory values, including serum resistin, inflammatory markers, indices of insulin resistance, and lipid profiles, were log-transformed to achieve normality and ful-fill assumptions of linear regression modeling. Multivariate regression analysis was performed using all variables with a P value of <.10 in univariate analyses. Variables with a P value of <.05, selected using backward elimination, were considered statistically significant and included in a final regression model. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results
Characteristics of the study cohort are summarized in the Table. The cohort was predominately Caucasian and male; approximately one-half (48%) were prepubertal. Median iGFR was 45 mL/min/1.73m2. The majority of patients had a nonglomerular cause of CKD. The most common non-glomerular causes of CKD were obstructive uropathy, renal dysplasia, and reflux nephropathy (21%, 17%, and 16% of the entire cohort, respectively). Among glomerular etiologies, focal and segmental glomerulosclerosis and hemolytic uremic syndrome were most prevalent, representing 7% and 4% of the cohort, respectively. Obesity was seen in 15% of the cohort; hypertension, in 15% as well.
Table.
Baseline characteristics of the CKiD cohort
| Characteristic | CKiD cohort (n = 319) |
|---|---|
| Age, years | 12.4 (8.9, 15.6) |
| Sex, % | |
| Male | 57 |
| Female | 43 |
| Ethnicity, % | |
| Caucasian | 72 |
| African-American | 13 |
| Other | 15 |
| Cause of CKD, % | |
| Glomerular | 21 |
| Nonglomerular | 79 |
| Pubertal status, % | |
| Prepubertal | 48 |
| Pubertal | 52 |
| Duration of CKD, years | 7.6 (4.3–1.7) |
| iGFR, mL/min/1.73 m2 | 45 (34–58) |
| Height percentile | 25 (8–51) |
| Weight percentile | 45 (21–77) |
| BMI percentile | 60 (33–88) |
| Systolic blood pressure percentile | 59 (29–82) |
| Diastolic blood pressure percentile | 61 (39–85) |
| HDL, mg/dL | 48 (41–56) |
| LDL, mg/dL | 104 (84–121) |
| Triglycerides, mg/dL | 107 (74–143) |
| IL-6, pg/mL | 1.9 (1.1–3.6) |
| IL-10, pg/mL | 2.3 (1.6–4.3) |
| TNF-α, pg/mL | 5.0 (3.6–8.2) |
| Resistin, ng/mL | 18.6 (13.7–27.1) |
| Glucose, mg/dL | 89 (83–95) |
| Insulin, mU/L | 7.6 (4.2–12.2) |
| HOMA-IR | 1.7 (0.9–2.8) |
| Protein-to-creatinine ratio | 0.44 (0.18–1.1) |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Continuous variables are presented as median (IQR); categorical variables, as percentage. Pre-pubertal is defined as Tanner stage 1; pubertal, as Tanner stage >1.
Serum Resistin Level and Patient Demographics
Serum resistin level did not differ significantly by sex or race. The median serum resistin level was higher in children with a glomerular etiology compared with those with a nonglomerular etiology (21.3 ng/mL vs 17.9 ng/mL; P = .03). Pubertal children had higher serum resistin levels than prepubertal children (median 20.3 ng/mL vs 16.7 ng/mL; P = .01). Serum resistin level increased with age (r = 0.15; P < .01). There was no relationship between BMI z score based on age and sex and serum resistin level.
Serum Resistin Level, Markers of Inflammation, and Renal Function
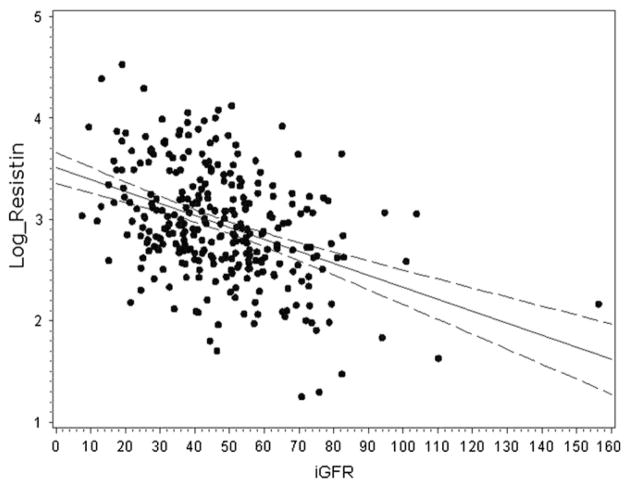
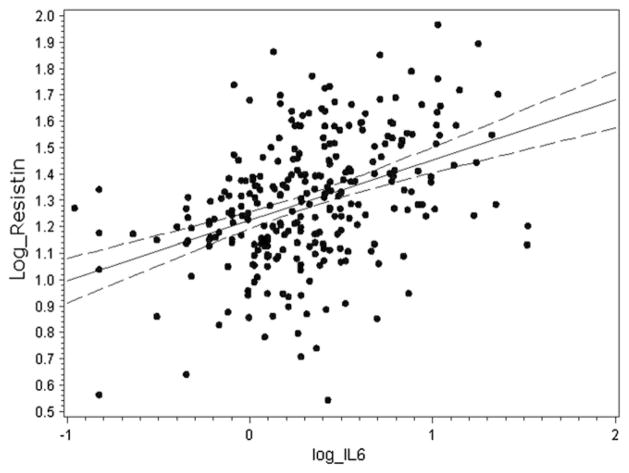
In our cohort, serum resistin level was negatively correlated with iGFR (Figure 1) and positively correlated with urine protein-to-creatinine ratio (r = 0.3; P < .001). The median serum resistin level was 23.2 ng/mL (IQR, 17.0–35.9 ng/mL) in children with CKD grade IV–V, 18.6 ng/mL (IQR, 14.1–28.3) in children with CKD grade III, and 13.9 ng/mL (IQR, 8.7–21.2) in children with CKD grade I–II (P < .001). There was no significant correlation between iGFR and IL-6 (r = −0.009, P = .88), IL-10 (r = −0.056; P = .35), or TNF-α (r = 0.048; P = .50). Resistin level was also associated with markers of inflammation. Specifically, IL-10 (r = 0.37; P < .001), IL-6 (Figure 2), and TNF-α (r = 0.18; P = .009) were all increased in patients with higher serum resistin levels.
Figure 1.
Correlation of resistin (ng/mL) and iGFR (mL/min/ 1.73 m2) levels in children with CKD (r = −0.41; P < .001). Serum resistin values were log-transformed.
Figure 2.
Correlation of resistin (ng/mL) and IL-6 (pg/mL) levels in children with CKD (r = 0.40; P < .001). Both resistin and IL-6 values were log-transformed.
Serum Resistin Level, Insulin Resistance, and Dyslipidemia
On univariate analysis, elevated serum resistin level was significantly associated with high triglycerides (r = 0.17; P = .002) and low high-density lipoprotein cholesterol (r = −0.22; P < .001). Serum resistin level was not significantly correlated with low-density lipoprotein cholesterol, insulin, or HOMA-IR score (Figure 3).
Figure 3.
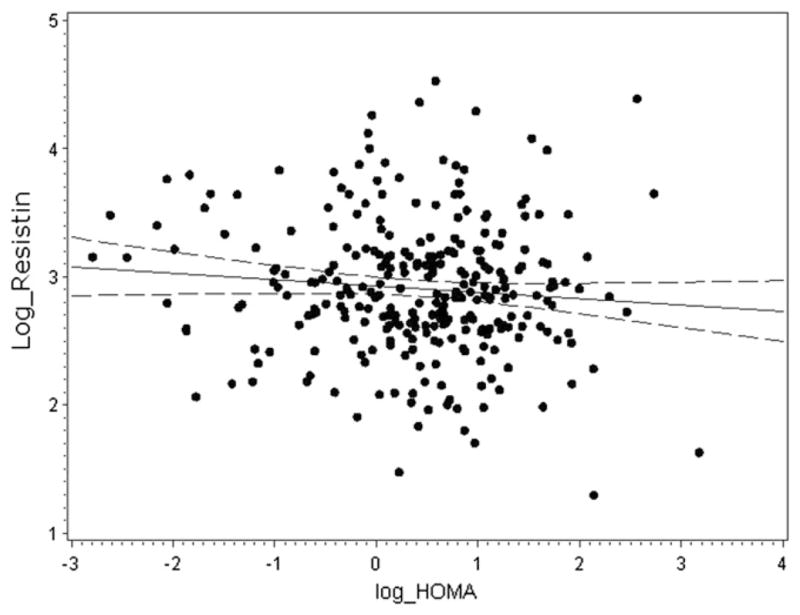
Correlation of resistin (ng/mL) and HOMA-IR (mU/L) values in children with CKD (r = −0.09; P = .13). Both resistin and HOMA-IR values were log-transformed.
Multivariate Analysis
iGFR, IL-6, IL-10, and puberty were statistically significant and thus included in the final model. There was an increase in mean serum resistin level of 11.3% per 10-mL decrease in iGFR (95% CI, 8.0%–14.6%). Mean serum resistin level increased by 1.75% (95% CI, 1.08%–2.41%) and 0.86% (95% CI, 0.19%–1.55%) per 10% increase IL-6 and IL-10, respectively. Postpubertal status was correlated with increased serum resistin level, with pubertal children having an adjusted mean increase in serum resistin level of 20.1% (95% CI, 7.5%–34.2%).
Discussion
We have demonstrated that serum resistin level increases with progressively declining kidney function in children with CKD. Given resistin’s molecular weight of only 12.5 kDa, it would be expected to be freely filtered at the glomerular basement membrane. Decreased filtration as a cause of elevated resistin levels in patients with renal insufficiency has been suggested in several adult studies.5,6,17 Our data confirm the association of renal function with serum resistin level reported by Nusken et al11 and Buyan et al12 in much smaller pediatric cohorts.
Decreased clearance might not be the only cause of elevated resistin levels, however. CKD per se has been recognized as a state of chronic inflammation,18,19 and recent data suggest that resistin is involved in inflammatory processes. Resistin is expressed primarily in macrophages20,21 and stimulates production of IL-6 and TNF-α.22,23 Furthermore, Axelsson et al6 showed a significant independent association of resistin with IL-6 in adults with CKD.
Similarly, the present study demonstrated an independent association of inflammatory markers with serum resistin. Stenvinkel et al24 demonstrated that IL-6 was predictive of progression of carotid artery atherosclerosis, and several other studies have shown that IL-6 is associated with increased mortality in patients receiving hemodialysis.25–27 Resistin might have a similar relationship to patient outcomes, due in part to its proinflammatory characteristics. Resistin induces smooth muscle cell proliferation in vitro28 and has been found in human endarterectomy lesions.29 However, even though some studies have demonstrated an association with cardiovascular outcomes such as hypertension and coronary artery calcification,8,9 others have not reached similar conclusions.30,31 More prospective data are needed to determine the predictive value of resistin in cardiovascular morbidity in children with CKD.
We found an association between serum resistin level and IL-10, a cytokine with anti-inflammatory properties, including down-regulation of IL-6 and TNF-α.18 This paradoxical finding is likely attributable to increased production of IL-10 in response to proinflammatory factors rather than to a direct action of resistin per se. This is consistent with the findings of Goldstein et al,32 who reported elevated levels of both IL-6 and IL-10 in children receiving hemodialysis. Furthermore, immunocompetent hemodialysis patients have demonstrated high in vitro secretion of IL-10 by mono-nuclear cells in response to inflammatory stimuli.33
In contrast to previous adult studies,34–37 inflammation was not associated with GFR decline in our cohort. This can be explained by the fact that previous studies demonstrating this association included patients either undergoing hemodialysis or close to starting hemodialysis, whereas our study excluded those with severe renal impairment (GFR <30 mL/min/1.73 m2). In addition, the most common causes of adult renal failure, diabetes and hypertension, may be related to systemic inflammation per se,38 whereas most of our pediatric cohort had congenital nonglomerular etiologies. Consistent with this, IL-6 levels were not markedly different than values reported in a recent study of 147 healthy children using a similar multiplex assay (0.99–2.61 pg/mL).39 However, resistin levels were significantly higher than previously reported levels in 201 pediatric controls (5.83–6.75 ng/mL) using an immunoassay technique.40 Thus, resistin may be a more sensitive marker of the proinflammatory state in children with CKD than traditional cytokines.
Our study demonstrated an independent association of pubertal status with resistin levels. This association has been reported by previous studies in children without CKD,40,41 suggesting that resistin level is correlated with maturational development rather than with anthropometric parameters, such as age and BMI. The correlation of resistin with testosterone and estradiol reported by Gerber et al40 further supports this finding.
Resistin has generated much interest due to its putative association with insulin resistance in murine models. In mice with diet-induced obesity, serum resistin levels were significantly elevated and correlated with body fat, insulin, glucose, and triglyceride levels.3 Many human studies have failed to corroborate this proposed link between insulin resistance and resistin, however.6,8,12,17,31 Our findings confirm that resistin in not associated with insulin resistance or BMI. Furthermore, the association of resistin with dyslipidemia was lost in multivariate analysis.
This study has several strengths, including the large size of the CKiD cohort, standardized methods of obtaining clinical and laboratory data, and precise measurement of GFR using iohexol clearance. Nevertheless, some limitations should be mentioned. First, because of the study’s cross-sectional design, we are unable to draw any causal inferences from the data. Continuing longitudinal analysis of the CKiD cohort will evaluate the predictive value of resistin in cardiovascular outcomes. Second, the CKiD cohort does not include children with advanced CKD and end-stage renal disease, a population with a significantly elevated risk of cardiovascular mortality. Finally, we did not evaluate resistin or other inflammatory cytokines in healthy children, which precludes any direct comparison of our cohort with control subjects. Although comparisons can be made to historical controls, there is some inherent variability because of the methodological differences between studies. In our study, serum resistin level was measured by ELISA, the gold standard used in previous studies. Cytokine levels were measured by a multiplex bead array assay, which has the advantage of analyzing multiple cytokines simultaneously. Most previous studies used ELISA for this, however. Although differences in absolute values using multiplex assays have been reported among manufacturers,42 several previous studies have demonstrated excellent correlations with ELISA.14,16 Therefore, although absolute values of inflammatory cytokines must be interpreted with caution, overall trends and correlations should not be affected by assay variability.
Acknowledgments
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Research grant DK076957 (to M.M.). The Chronic Kidney Disease in Children study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, National Institute of Child Health and Human Development, and National Heart, Lung, and Blood Institute (grants U01-DK-66143, U01-DK-66174, U01-DK-82194, and U01-DK-66116).
Glossary
- BMI
Body mass index
- CKiD
Chronic Kidney Disease in Children
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- GFR
Glomerular filtration rate
- HOMA-IR
Homeostasis model assessment of insulin resistance
- IL
Interleukin
- TNF
Tumor necrosis factor
Footnotes
The authors declare no conflicts of interest.
Data used in this study were presented in abstract form at the American Society of Nephrology’s annual meeting, Denver, CO, November 18, 2010.
References
- 1.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–7. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 2.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 3.Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–9. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 4.McTernan PG, Fisher FM, Valsamakis G, Chetty R, Harte A, McTernan CL, et al. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab. 2003;88:6098–106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- 5.Malyszko J, Malyszko JS, Kozminski P, Pawlak K, Mysliwiec M. Elevated resistin is related to inflammation and residual renal function in haemodialysed patients. Nephrology (Carlton) 2007;12:246–53. doi: 10.1111/j.1440-1797.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 6.Axelsson J, Bergsten A, Qureshi AR, Heimburger O, Barany P, Lonnqvist F, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69:596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 7.Yaturu S, Reddy RD, Rains J, Jain SK. Plasma and urine levels of resistin and adiponectin in chronic kidney disease. Cytokine. 2007;37:1–5. doi: 10.1016/j.cyto.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Curhan GC, Forman JP. Plasma resistin levels associate with risk for hypertension among nondiabetic women. J Am Soc Nephrol. 2010;21:1185–91. doi: 10.1681/ASN.2009101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen G, Horl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Semin Dial. 2009;22:373–7. doi: 10.1111/j.1525-139X.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 11.Nusken KD, Kratzsch J, Wienholz V, Stohr W, Rascher W, Dotsch J. Circulating resistin concentrations in children depend on renal function. Nephrol Dial Transplant. 2006;21:107–12. doi: 10.1093/ndt/gfi084. [DOI] [PubMed] [Google Scholar]
- 12.Buyan N, Bideci A, Ozkaya O, Ortac E, Bakkaloglu S, Gonen S, et al. Leptin and resistin levels and their relationships with glucose metabolism in children with chronic renal insufficiency and undergoing dialysis. Nephrology (Carlton) 2006;11:192–6. doi: 10.1111/j.1440-1797.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–15. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–9. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins TB, Pasi BM, Pickering JW, Jaskowski TD, Litwin CM, Hill HR. Determination of cytokine responses using a multiplexed fluorescent microsphere immunoassay. Am J Clin Pathol. 2002;118:346–53. doi: 10.1309/N0T6-C56B-GXB2-NVFB. [DOI] [PubMed] [Google Scholar]
- 16.Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. J Immunol Methods. 2002;260:207–18. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 17.Kielstein JT, Becker B, Graf S, Brabant G, Haller H, Fliser D. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis. 2003;42:62–6. doi: 10.1016/s0272-6386(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 18.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia: the good, the bad, and the ugly. Kidney Int. 2005;67:1216–33. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–52. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 20.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–35. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 21.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR-γ activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 22.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 23.Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Stenvinkel P, Heimburger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis. 2002;39:274–82. doi: 10.1053/ajkd.2002.30546. [DOI] [PubMed] [Google Scholar]
- 25.Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–14. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–44. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–8. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 28.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–40. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 29.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–8. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Pilz S, Weihrauch G, Seelhorst U, Wellnitz B, Winkelmann BR, Boehm BO, et al. Implications of resistin plasma levels in subjects undergoing coronary angiography. Clin Endocrinol (Oxf) 2007;66:380–6. doi: 10.1111/j.1365-2265.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 31.Diez JJ, Iglesias P, Fernandez-Reyes MJ, Aguilera A, Bajo MA, Alvarez-Fidalgo P, et al. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol (Oxf) 2005;62:242–9. doi: 10.1111/j.1365-2265.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein SL, Currier H, Watters L, Hempe JM, Sheth RD, Silverstein D. Acute and chronic inflammation in pediatric patients receiving hemodialysis. J Pediatr. 2003;143:653–7. doi: 10.1067/S0022-3476(03)00534-1. [DOI] [PubMed] [Google Scholar]
- 33.Girndt M, Kohler H, Schiedhelm-Weick E, Schlaak JF, Meyerzum Buschenfelde KH, Fleischer B. Production of interleukin-6, tumor necrosis factor-α and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney Int. 1995;47:559–65. doi: 10.1038/ki.1995.70. [DOI] [PubMed] [Google Scholar]
- 34.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–8. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 35.Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39:954–60. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 36.Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, et al. Balance between IL-1α, TNF-α, and their specific inhibitors in chronic renal failure and maintenance dialysis: relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–92. [PubMed] [Google Scholar]
- 37.Herbelin A, Nguyen AT, Zingraff J, Urena P, Descamps-Latscha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor α. Kidney Int. 1990;37:116–25. doi: 10.1038/ki.1990.16. [DOI] [PubMed] [Google Scholar]
- 38.Haffner SM. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol. 2003;92:18J–26J. doi: 10.1016/s0002-9149(03)00612-x. [DOI] [PubMed] [Google Scholar]
- 39.Martos-Moreno GA, Burgos-Ramos E, Canelles S, Argente J, Barrios V. Analysis of insulin and cytokines during development using a multiplexed immunoassay: implications in pediatrics. An Pediatr (Barc) 2011;74:356–62. doi: 10.1016/j.anpedi.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Gerber M, Boettner A, Seidel B, Lammert A, Bar J, Schuster E, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–9. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- 41.Celi F, Bini V, Papi F, Santilli E, Castellani MS, Ferretti A, et al. Circulating adipocytokines in non-diabetic and type 1 diabetic children: relationship to insulin therapy, glycaemic control and pubertal development. Diabetes Med. 2006;23:660–5. doi: 10.1111/j.1464-5491.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 42.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]