Abstract
Objective
To determine overall and age -specific incidence rates of Alzheimer’s disease (AD) in a southern Indian province, Kerala.
Methods
A 10-year (2001–2011) prospective epidemiologic study of community residing subjects aged ≥55 years at enrollment. The catchment area included four urban and semi-urban regions of Trivandrum city in Kerala, India, was selected to provide a range of demographic and socioeconomic representation. Cognitive and functional ability screening were done at baseline and 24-month follow-up assessments. Consensus diagnostic procedures were done using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) criteria for the diagnosis of dementia and AD.
Results
Among the 1066 eligible participants who were cognitively normal at baseline, 104 developed dementia (98 with AD) over a follow-up period of 8.1 years. The incidence rates per 1000 person-years for AD was 11.67 (95% CI: 10.9–12.4) for those aged ≥55 years and higher for those aged ≥65 years (15.54, 95% CI: 14.6–16.5). In those aged ≥65 years, the world age standardized incidence rate was 21.61 per 100,000, and standardized against the age distribution for the year 2000 U.S. Census, the age-adjusted incidence rate was 9.19 (95% CI: 9.03–9.35) per 1000 person-years. Incidence rate of AD increased significantly and proportionately with increasing age.
Conclusion
These are the first AD incidence rates to be reported from southern India. The incidence rates appear to be much higher than that reported from rural north India, comparable with that reported from China, and marginally lower than that reported from the western world.
Keywords: Addenbrooke’s cognitive examination, Alzheimer’s disease, dementia, incidence, vascular dementia
Introduction
Alzheimer’s disease (AD) and other forms of dementia are a growing public health problem among the elderly in developing countries, whose aging population is increasing rapidly. It is estimated that by the year 2020, approximately 70% of the world’s population aged 60 and above will be living in developing countries, with 14.2% in India.[1] The estimates of rate at which new disease develops can only be measured accurately in prospective incidence studies. The reported incidence rates for AD has been lower in Asian countries than in the industrialized world.[2–6] Although dementia prevalence studies are available from Asian countries including India,[7–15] there is a dearth of incidence,[2–6] data, particularly from prospective long-term studies. We have previously reported the age-adjusted prevalence of dementia including AD to be 4.86% (AD 1.91%) in a community residing population in a southern Indian province of Kerala, as a part of the cognition in older adults in Trivandrum (COAT) study.[16] This study reports the incidence rates after a 10 years follow-up of the COAT cohort.
Methods
The COAT study was initiated in 2001 as a longitudinal study to examine cognition in older adults in the southern Indian province of Kerala. Informed consent was obtained prior to enrollment from all participants according to procedures approved by the Institutional Ethics Committee of Sree Chitra Tirunal Institute for Medical Sciences & Technology, a University Hospital in the city of Trivandrum. We first developed cognition[17] and the activities of daily living[18] screening and clinical evaluation instruments and derived population norms.[19] Subsequently we undertook a prevalence survey[16] and a follow-up incidence survey.
Study population
The prevalence cohort described previously[16] consisted of 2466 subjects aged 55 and above who consented to participate in the study, were from four(population = 41,920) of the eight wards, i.e, administrative districts of the city corporation of Trivandrum (population = 524,006) in the state of Kerala (population = 31,838,619). Individuals aged 60 years and above constituted 8.6% of this population[20] and the sample consisted of 5.9% of the population of the four wards. The four selected wards included a costal district (populated by the economically poorer sher-folk communities that scarcely utilize the federal health services and are a religious minority), a commercial district (progressive, well-educated, and economically well-off communities) and an inner-city district (moderately-educated families migrated decades or generations ago from rural Kerala or neighboring states). These four wards provided a good admixture and faithful representation of the socio-economically and culturally diverse population of Trivandrum. We chose 55 years as a cut-off age based on what had been done in some of the earlier studies reported from India,[15, 21] and to estimate presenile dementia. Subjects were selected according to their ages listed in the local census registry; subsequently, their ages were confirmed in person by reference to personal and historical sentinel events, as is the standard research practice in developing countries.[22] At study entry, 93 subjects had prevalent dementia, including 47 with AD. The follow-up study could only be conducted, on a proportion of the cohort, largely because of limitations of funds and many participants not consenting for a long -term follow-up. The incidence cohort thus consisted of 1066 previously non-demented subjects.
Study instruments (Cognition in Older Adults in Trivandrum study)
The initial stage of the study was devoted to developing cognitive and functional instruments appropriate to the study population. The cognitive screening instruments had to be reliable, valid, sensitive, and specific for dementia, culturally and linguistically appropriate for the population, and as comparable as possible, in content, format, and relative level of difficulty with the cognitive test battery used in the population for whom it was initially developed in English. Malayalam (the local language) instruments, both cognition and the activities of daily living evaluation tools were developed by means of a systematic, iterative process described in the earlier reports.[17,18] Once the tools were ready, population norms were derived.[18,19]
Screening
All subjects were screened (Phase I) at their homes, and a subset was identified for detailed clinical evaluation at our research center. Screening was first performed at baseline during the prevalence survey[16] and repeated thereafter every alternate year, including after approximately 10 years, in the incidence cohort. Trained medical social workers and qualified psychologists administered a standardized Malayalam Addenbrooke’s cognitive examination (mACE),[17] which is similar to the battery of the Consortium to Establish a Registry for AD (CERAD). Briefly, it consists of the global cognitive scale mini mental status examination (MMSE), tests for episodic memory (immediate and delayed recall of a seven-item address list), verbal fluency (initial letter P and categories of animals), confrontation naming (10 items), constructional praxis (copying line-drawing of wire-cube), the clock-drawing test, tests for language and remote memory. Interviewers also administered the instrumental activities of daily living for elderly (IADL-E)[18] to the family member. Thus, even for subjects cognitively un-testable on neuropsychological tests (e.g., as in very advanced dementia), we were able to obtain functional ability data from a reliable informant. As in the prevalence study,[16] all subjects who scored 20th percentile or below on the education-stratified population norms on either the mACE or the mMMSE,[19] or ≤16 on the Cognitive Disability Index on IADL-E were considered screen-positives and were administered detailed neuropsychological tests by neuropsychologists, which was followed by a clinical evaluation by a neurologist, when necessary, in accordance with the diagnostic evaluation protocol of the prevalence study. We have shown during the prevalence assessment that this two-phase protocol was robust and the dementia rate in the screen negatives was only 2%.[16]
Neuropsychological, Clinical evaluation and Diagnosis
Like in our prevalence study, all screen-positive subjects underwent this phase of the evaluations which included:
Inquiry into cognition and behavioral symptoms (over past month) using a standardized structured questionnaire to elicit all cognition and behavior symptoms (memory, language, attention, visuospatial orientation, and neuropsychiatric) by a clinician. When necessary, family members were also interviewed.
Clinical evaluation by a clinician using a brief structured standardized medical history, examination and a bed-side mental status examination.
Neuropsychological evaluation: Qualified psychologists administered a battery of neuropsychological tests. This included the forward and backward digit span, logical memory and memory for designs components of the Wechsler’s memory scale,[23] trail making forms A and B,[24] verbal fluency (category and initial letter), confrontation naming test,[25] hospital anxiety and depression scale,[26] and the modified 5-point Barthel Index.[27] After evaluating the aforementioned Phase II examination results, in subjects in whom the study team thought it necessary to have further evaluation for reaching a diagnosis, we did screening blood investigations (including hemogram, thyroid function tests, and vitamin B12 levels) and/or a neuroimaging (computed tomography [CT] scan or magnetic resonance imaging [MRI].
A diagnosis was made in a consensus conference including the neurologist, neuropsychiatrist, and psychologists based on the review of the clinical, neuropsychological, and investigation results. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria[28] was used for diagnosing dementia, National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) criteria[29] for possible or probable AD and Hachinski’s Ischemic Scale[30] for vascular dementia (VaD). A case of incident dementia was defined as an individual who fulfilled criteria for DSM-IV diagnosis of dementia with estimated disease onset occurring subsequent to study entry (baseline assessment).
Statistical methods
Incidence rates for AD were calculated by dividing the number of cases with onset of AD in each age group by the number of person-years of observation in that group. Since majority of incident cases of dementia were accounted by AD (94%), AD is reported as the primary outcome. Person-years were calculated from the time of study entry for each individual until the time of AD onset, or until the last date the subject was known to be nondemented (death, dropout, or most recent contact). The person-years method naturally accommodates both left truncation and right censoring. Incidence rates were also calculated within both gender categories and four age categories (55–59, 60–74, 75–79, 80–84, and ≥85 years); 95% confidence intervals (CI) around these rates were obtained based on Poisson distributions (appropriate for rare events). In order to be able to compare with some of the published incidence rates in the western world and US, we also calculated the age-adjusted incidence rate taking the US population of the year 2000 as the reference and the world age standardized rate taking the WHO world age standardized population 2000–2025. Using Cox regression analysis, we also modeled the association of age, education and gender on the incidence of dementia.
Results
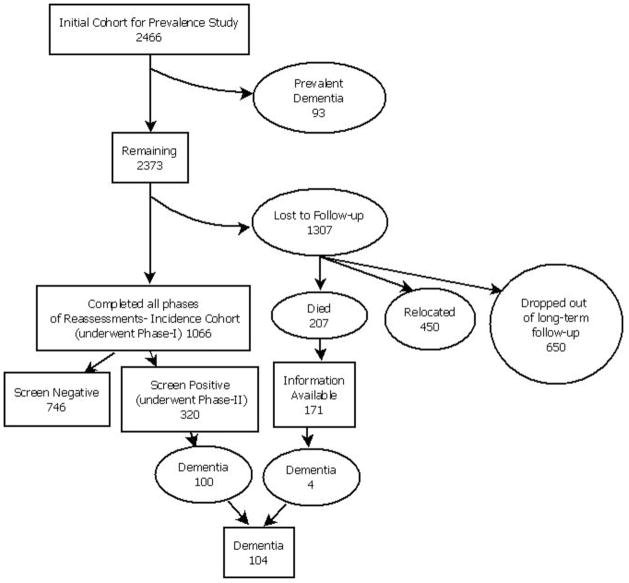
At baseline, 2466 subjects aged ≥55 years underwent cognitive and functional screening. Among them, 93 subjects found to be demented at study entry (prevalent cases) were excluded from the incidence calculations. Among the 2373 remaining subjects, 1066 completed all of the incidence phase screening and evaluation procedures thus forming the incidence cohort [Figure 1]. This included 171 subjects, who had died before follow -up screening, but information regarding their mental and functional status shortly before death was obtained from their family members; they were therefore included in the incidence study sample. The duration of follow-up of the incidence cohort ranged from 5 to 10 years with a mean of 8.1 years.
Figure 1.
An algorithimic chart of the cohort participating in the incident study
Demographic details of the incident cohort are shown in Table 1. The mean (SD) age of the incidence cohort was 67.8 (7.5) years and education was 9.2 (5.3) at study entry. Men comprised 43.1% of the cohort, had a mean (SD) age of 68.2 (7.5) years, and women had a mean (SD) age of 67.6 (7.6) years. The mean (SD) mACE and mMMSE score for the 1066 subjects who completed cognitive testing was 75.3 (18.4) for mACE and 25.7 (4.9) for mMMSE at baseline and 69.4 (19.1) for mAC E and 23. 1 (5.5) for mMMS E at follow-up.
Table 1.
Age and sex distribution of the study cohort
| Age group | Men (n) | Women (n) | Total (n) |
|---|---|---|---|
| 55–59 | 5 | 20 | 25 |
| 60–64 | 155 | 222 | 377 |
| 65–69 | 132 | 146 | 278 |
| 70–74 | 85 | 106 | 191 |
| 75–79 | 43 | 63 | 106 |
| 80–84 | 21 | 35 | 56 |
| ≥85 | 18 | 15 | 33 |
| ≥65 | 299 | 365 | 664 |
| ≥55 | 459 | 607 | 1066 |
Of the 1066 members of the incidence cohort, 320 (20%) who screened positive were selected for detailed neuropsychological testing and clinical evaluation. There were 104 incident cases of dementia: Probable or possible AD 98 subjects, 6 probable vascular dementia, and none with mixed dementia. Only 4 of those diagnosed dementia were from the 171 who had died before follow-up screening. The crude (i.e., unadjusted) overall incidence rate was 15.54 per 1000 person-years (95% CI: 14.6–16.5) for AD in those aged ≥65 years. Age-specific incidence rates, by gender, of AD in the COAT cohort are shown in Table 2. Age-specific incidence rates in COAT cohort were lower for men than women and expectedly showed a rising trend with increasing age, being highest for those aged ≥85 years. Standardized against the age distribution of the US population in the year 2000 US Census, the overall age-adjusted AD incidence rate among those aged ≥65 years was 9.19 (95% CI: 9.03–9.35) per 1000 person-years in Trivandrum. Against the WHO World Standard population of 2000–2005, the world age standardized incidence rates for age ≥65 years is 21.61 (95% CI: 20.5–22.7), for age ≥55 years is 27.99 (95% CI: 27.0–28.9) per 100,000 population.
Table 2.
Incidence rates (per 1000 person-years) for Alzheimer’s disease (AD)
| Age (years) | Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Person-years | * Rate (95% CI) | n | Person-years | * Rate (95% CI) | n | Person-years | * Rate (95% CI) | WASR# (95% CI) | |
| 55–59 | 0 | 0 | 0 (0–0) | 0 | 44 | 0 (0–0) | 0 | 44 | 0 (0–0) | 4.55 (−1.6–10.7) |
| 60–64 | 6 | 1280 | 4.69 (3.5–5.9) | 11 | 1936 | 5.68 (4.7–6.7) | 1 7 |
3216 | 5.29 (4.6–6.1) | 9.01 (8.0–10.0) |
| 65–69 | 6 | 1056 | 5.68 (4.3–7.1) | 22 | 1168 | 18.84 (16.6–21.1) | 2 8 |
2224 | 12.59 (11.2–13.9) | 15.55 (14.4–17.1) |
| 70–74 | 9 | 680 | 13.24 (10.7–15.8) | 20 | 848 | 23.58 (20.7–26.4) | 2 9 |
1528 | 18.98 (17.0–20.9) | 21.19 (19.1–23.2) |
| 75–79 | 1 | 344 | 2.91 (1.1–4.7) | 12 | 504 | 23.81 (20.1–27.5) | 1 3 |
848 | 15.33 (12.9–17.8) | 16.85 (14.3–19.4) |
| 80–84 | 0 | 168 | 0 (0–0) | 8 | 280 | 28.57 (23.3–33.9) | 8 | 448 | 17.86 (14.3–21.4) | 18.77 (15.2–22.4) |
| ≥85 | 2 | 144 | 13.89 (8.2–19.5) | 1 | 120 | 8.33 (3.4–13.3) | 3 | 264 | 11.36 (7.5–15.2) | 11.99 (8.1–15.9) |
| ≥55 | 2 4 |
3672 | 6.54 (5.8–7.3) | 74 | 4856 | 15.24 (14.2–16.3) | 9 8 |
8528 | 11.49 (10.8–12.2) | 27.99 (27.0–28.9) |
| ≥65 | 1 8 |
2392 | 7.53 (6.5–8.6) | 63 | 2920 | 21.58 (20.1–23.1) | 8 1 |
5312 | 15.25 (14.3–16.2) | 21.61 (20.5–22.7) |
Incidence rate per 1000 person-years, 95% CI - 95% confidence interval,
WASR - World age standardized rate against world standard population 2000–2025
On Cox regression analysis, incident dementia showed a significant risk of incident AD with increasing age (Hazard coefficient 1.09 (95% CI: 1.06–1.13), P < 0.001), female gender (Hazard coefficient 0.51 (95% CI: 0.31–0.82), P < 0.006) and lower education (Hazard coefficient 0.90 (95% CI: 0.86–0.95), P < 0.001),
Discussion
Our results indicate high incidence of AD in this cohort of Kerala population, higher than reported previously from India though lower than developed countries. Suggestions that the incidence of AD is lower in Asia than in Europe and North America[31] are based on very few Asian incidence studies, most of which are from eastern Asia, Japan,[32] China,[33] and Taiwan[3] with reporting rates from 5.1 to 8.9 per 1000 person-years among seniors aged ≥65 years. Incidence of AD among seniors aged ≥65 years in Trivandrum, standardized against the age distribution of the year 2000 US population, was 9.19 per 1000, lower than the 17.5 per 100 found by similar methods, in the Monongahela Valley of Pennsylvania[34] and lower than 1.15% in Shanghai[6] and 15.7% in Anhui,[2] in China. However, the Anhui study population excluded the illiterate, follow-up was for 1 year, and an algorithmic dementia diagnosis procedure was used. The incidence rates in our cohort, nevertheless, was much higher than 4.7 per 1000 found in rural Ballabgarh[4] in India, this study was based on a limited follow-up of 1 year. The Ballabgarh cohort was largely rural illiterate older adults. In contrast, COAT cohort is largely literate, urban, and semi-urban population with better Human Development Index (HDI) of 0.64 versus 0.54 in Ballabgarh and 0.5 in India, and longer life expectancy at birth in years 74 versus 66.2 in Ballabgarh and 63.5 in India.[20,35] Our result emphasizes that there are substantial regional differences in AD incidence within the heterogeneous population of India.
The strengths of our study are long follow-up for a decade and the tools used were culturally and linguistically appropriate tools developed and validated locally.[17–19] Fairly tight CI of incidence estimates (except in those ≥85 years where expectedly low numbers due to lower) lend confidence to our incidence estimates. All older adults in our study community lived with their families providing us with opportunities to interview relatives, which was particularly useful when subjects were cognitively untestable for any reason or even when recently deceased.
The limitation of our study is that we could not follow-up the entire cohort recruited at the beginning of the prevalence study, due to the prolonged period of follow-up, and limitations in funding support. Hence, our rates possibly underestimate incident AD in this community. It is possible that very mild dementias were underestimated in the semi-urban localities of the survey area because the daily functional demands on older adults in these semi-rural localities are limited because of their living with and being cared for by their families. However, overseas migration of the younger generations in search of better employment opportunities has resulted in many elders in Kerala living alone.
It is evident from our study that the incidence of AD in southern India is not as low as that found in northern India. Nevertheless, it is still lower than that of the average incidence rates of AD reported in Europe and North America. This could possibly suggest the presence of underlying protective factors, or the absence of certain risk factors. The frequency of the APOE4 allele, a well recognized risk factor for AD is reported to be low in the Indian population in general,[36] with regional differences. The frequency of the APOE4 allele is lower in the north[37,38] and central[39] Indian populations compared with the US population.[38] Notably, the education levels in our population significantly correlated with incident dementia.
Acknowledgments
Source of Support: Sir Ratan Tata Trust, Mumbai, India; Kerala State Council for Science Technology & Environment (Grant No. 5462/B5/2002/STED), Trivandrum, India; National Institute on Aging, USA (Grant Nos. R21AG029799 and R01AG039330-01).
The authors wish to thank Mrs. Meera Pattabhi and Mr. Radhamoni of the Trivandrum Chapter of the Alzheimer’s & Related Disorders Society of India (ARDSI), the office bearers of the various Resident’s Associations in the survey area for facilitating the survey, and the participants of the COAT study. This study was supported in parts by research grants to Dr. Mathuranath from Sir Ratan Tata Trust, Mumbai, India, Kerala State Council for Science Technology & Environment (Grant No. 5462/B5/2002/STED), Trivandrum, India, and National Institute on Aging (Grant Nos. R21AG029799 and R01AG039330-01), USA. Dr. Ranjith was supported by a fellowship from Lady Tata Trust, Mumbai.
Footnotes
Conflict of Interest: None declared.
References
- 1.World Health Organization. Fact Sheet No 135. Geneva: World Health Organization; 1998. Population Ageing – A Public Health Challenge. [Google Scholar]
- 2.Chen R, Hu Z, Wei L, Ma Y, Liu Z, Copeland JR. Incident dementia in a defined older Chinese population. PLoS One. 2011;6:e24817. doi: 10.1371/journal.pone.0024817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CK, Lai CL, Tai CT, Lin RT, Yen YY, Howng SL. Incidence and subtypes of dementia in southern Taiwan: Impact of socio-demographic factors. Neurology. 1998;50:1572–9. doi: 10.1212/wnl.50.6.1572. [DOI] [PubMed] [Google Scholar]
- 4.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, et al. Incidence of Alzheimer’s disease in a rural community in India: The Indo-US study. Neurology. 2001;57:985–9. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 5.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: The Hisayama study. Neurology. 1995;45:1161–8. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Katzman R, Yu E, Liu W, Xiao SF, Yan H. A preliminary analysis of incidence of dementia in Shanghai, China. Psychiatry Clin Neurosci. 1998;52:S291–4. doi: 10.1111/j.1440-1819.1998.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiu HF, Lam LC, Chi I, Leung T, Li SW, Law WT, et al. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology. 1998;50:1002–9. doi: 10.1212/wnl.50.4.1002. [DOI] [PubMed] [Google Scholar]
- 8.de Silva HA, Gunatilake SB, Smith AD. Prevalence of dementia in a semi-urban population in Sri Lanka: Report from a regional survey. Int J Geriatr Psychiatry. 2003;18:711–5. doi: 10.1002/gps.909. [DOI] [PubMed] [Google Scholar]
- 9.Jitapunkul S, Kunanusont C, Phoolcharoen W, Suriyawongpaisal P. Prevalence estimation of dementia among Thai elderly: A national survey. J Med Assoc Thai. 2001;84:461–7. [PubMed] [Google Scholar]
- 10.Liu HC, Fuh JL, Wang SJ, Liu CY, Larson EB, Lin KN, et al. Prevalence and subtypes of dementia in a rural Chinese population. Alzheimer Dis Assoc Disord. 1998;12:127–34. doi: 10.1097/00002093-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Guo XE, Zhou YQ, Xia JL. Prevalence of dementia in China. Dement Geriatr Cogn Disord. 2003;15:226–30. doi: 10.1159/000068784. [DOI] [PubMed] [Google Scholar]
- 12.Meguro K, Ishii H, Yamaguchi S, Ishizaki J, Shimada M, Sato M, et al. Prevalence of dementia and dementing diseases in Japan: The Tajiri project. Arch Neurol. 2002;59:1109–14. doi: 10.1001/archneur.59.7.1109. [DOI] [PubMed] [Google Scholar]
- 13.Shaji S, Bose S, Verghese A. Prevalence of dementia in an urban population in Kerala, India. Br J Psychiatry. 2005;186:136–40. doi: 10.1192/bjp.186.2.136. [DOI] [PubMed] [Google Scholar]
- 14.Shaji S. Prevalence of dementia. Br J Psychiatry. 2005;187:90. doi: 10.1192/bjp.187.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13:439–50. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- 16.Mathuranath PS, Cherian PJ, Mathew R, Kumar S, George A, Alexander A, et al. Dementia in Kerala, South India: Prevalence and influence of age, education and gender. Int J Geriatr Psychiatry. 2010;25:290–7. doi: 10.1002/gps.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathuranath PS, Hodges JR, Mathew R, Cherian PJ, George A, Bak TH. Adaptation of the ACE for a Malayalam speaking population in southern India. Int J Geriatr Psychiatry. 2004;19:1188–94. doi: 10.1002/gps.1239. [DOI] [PubMed] [Google Scholar]
- 18.Mathuranath PS, George A, Cherian PJ, Mathew R, Sarma PS. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17:461–74. doi: 10.1017/s1041610205001547. [DOI] [PubMed] [Google Scholar]
- 19.Mathuranath PS, Cherian JP, Mathew R, George A, Alexander A, Sarma SP. Mini mental state examination and the Addenbrooke’s cognitive examination: Effect of education and norms for a multicultural population. Neurol India. 2007;55:106–10. doi: 10.4103/0028-3886.32779. [DOI] [PubMed] [Google Scholar]
- 20.Director of Census Operations Kerala. Provisional Population Totals. Kerala: Director of Census Operations; 2001. [Google Scholar]
- 21.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer’s disease and other dementias in rural India: The Indo-US study. Neurology. 1998;51:1000–8. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 22.Chandra V, DeKosky ST, Pandav R, Johnston J, Belle SH, Ratcliff G, et al. Neurologic factors associated with cognitive impairment in a rural elderly population in India: The Indo-US Cross-National Dementia Epidemiology Study. J Geriatr Psychiatry Neurol. 1998;11:11–7. doi: 10.1177/089198879801100104. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale - Revised Manual. San Antonio, TX: Psychological Corporation; 1987. ??? [Google Scholar]
- 24.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 25.George A, Mathuranath PS. Primary progressive aphasia: A comparative study of progressive nonfluent aphasia and semantic dementia. Neurol India. 2005;53:162–5. doi: 10.4103/0028-3886.16398. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Hobart JC, Thompson AJ. The five item Barthel index. J Neurol Neurosurg Psychiatry. 2001;71:225–30. doi: 10.1136/jnnp.71.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 30.Hachinski VC, Bowler JV. Vascular dementia. Neurology. 1993;43:2159–60. doi: 10.1212/wnl.43.10.2159-a. [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF, Jolley D. The incidence of dementia: A meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 32.Fukunishi I, Hayabara T, Hosokawa K. Epidemiological surveys of senile dementia in Japan. Int J Soc Psychiatry. 1991;37:51–6. doi: 10.1177/002076409103700107. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Shen YC, Chen CH, Zhau YW, Li SR, Lu M. A three-year follow-up study of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand. 1991;83:99–104. doi: 10.1111/j.1600-0447.1991.tb07373.x. [DOI] [PubMed] [Google Scholar]
- 34.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: The MoVIES Project. Neurology. 2000;54:1109–16. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 35.Suryanarayana MH, Agrawal A, Prabhu KS. UNDP Report: Inequality adjusted Human Development Index for India’s States: UNDP India 2011. UNDP; New Delhi: 2011. [Google Scholar]
- 36.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 37.Singh PP, Singh M, Mastana SS. Genetic variation of apolipoproteins in North Indians. Hum Biol. 2002;74:673–82. doi: 10.1353/hub.2002.0057. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, et al. Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch Neurol. 2000;57:824–30. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- 39.Chandak GR, Sridevi MU, Vas CJ, Panikker DM, Singh L. Apolipoprotein E and presenilin-1 allelic variation and Alzheimer’s disease in India. Hum Biol. 2002;74:683–93. doi: 10.1353/hub.2002.0051. [DOI] [PubMed] [Google Scholar]