Abstract
Rationale and objectives
Previous work has shown that wheel running reduced the maintenance of cocaine self-administration in rats. In the present study, the effect of wheel running on extinction and reinstatement of cocaine seeking was examined. Female rats were trained to run in a wheel during 6-h sessions, and they were then catheterized and placed in an operant conditioning chamber where they did not have access to the wheel but were allowed to self-administer iv cocaine. Subsequently, rats were divided into four groups and were tested on the extinction and reinstatement of cocaine seeking while they had varying access to a wheel in an adjoining compartment. The four groups were assigned to the following wheel access conditions: (1) wheel running during extinction and reinstatement (WER), (2) wheel running during extinction and a locked wheel during reinstatement (WE), (3) locked wheel during extinction and wheel running during reinstatement (WR), and (4) locked wheel during extinction and reinstatement (WL). WE and WR were retested later to examine the effect of one session of wheel access on cocaine-primed reinstatement.
Results
There were no group differences in wheel revolutions, in rate of acquisition of cocaine self-administration, or in responding during maintenance when there was no wheel access. However, during extinction, WE and WER responded less than WR and WL. WR and WER had lower cocaine-primed reinstatement than WE and WL. One session of wheel exposure in WE also suppressed cocaine-primed reinstatement.
Conclusions
Wheel running immediately and effectively reduced cocaine-seeking behavior, but concurrent access to running was necessary. Thus, exercise is a useful and self-sustaining intervention to reduce cocaine-seeking behavior.
Keywords: Alternative reinforcement, Cocaine, Environmental enrichment, Extinction, Rats, Reinstatement, Self-administration, Wheel running
Introduction
Environmental enrichment with dietary (Carroll et al. 2001), social (Bardo et al. 2001; Schenk et al. 1987), and novel (Bardo et al. 2001; Cosgrove et al. 2002; Klebaur et al. 2001; Smith et al. 2008) stimuli, as well as opportunities for physical activity (Cosgrove et al. 2002; Smith et al. 2008), reduce amphetamine and cocaine self-administration in animals, possibly by a reward substitution mechanism at the behavioral and/or neurochemical level (Bossert et al. 2005; Kalivas and McFarland 2003; Shalev et al. 2002; Solinas et al. 2008). Most of these studies were conducted during a short-access maintenance phase under fixed-ratio (FR) or progressive-ratio (PR) schedules. Except for the use of preferred dietary substances (see Carroll et al. 2001), little is known about how environmental enrichment affects critical transition phases of drug abuse such as acquisition, escalation, extinction, and reinstatement (see review by Carroll et al. 2009a). In one study, Solinas et al. (2008) showed that housing mice in an enriched environment after developing a cocaine-induced conditioned place preference (CPP) prevented cocaine-primed reinstatement of the CPP and reduced activation of corresponding brain circuitry.
In animal studies, access to exercise in a running wheel is a positive reinforcer. For example, rats lever-pressed to gain access to a running wheel (Belke et al. 2005, Belke and Wagner 2005), and they escalated running during unlimited wheel access (Lattanzio and Eikelboom 2003). Conditioned rewarding effects of the wheel were reflected in a study showing CPP for an environment associated with wheel running (Belke and Wagner 2005; Lett et al. 2000). Initial studies indicated that access to a running wheel reduced the maintenance levels of low dose (0.2 mg/kg) cocaine self-administration in female (but not male) rats under a FR 1 schedule (Cosgrove et al. 2002) and for low (0.3 mg/kg) and high (10 mg/kg) doses of cocaine under a PR schedule in female rats (Smith et al. 2008). The present experiment extended these findings of reduced cocaine-seeking behavior due to wheel running to the extinction and reinstatement phases of drug abuse.
Behavioral studies of exercise, drug self-administration, and other rewarding activities have revealed commonalities, interactions, and substitutions among the various behaviors (see Carroll et al. 2009a for a review). That an increasing number of findings from animal research indicated that exercise and other nondrug rewards interfere with drug self-administration suggests common neurobiological mechanisms. For example, brief exposure to exercise increases central dopamine concentrations (Meeusen 2005; Petzinger et al. 2007), and regular exercise increases dopamine concentration and dopamine-binding proteins (Fisher et al. 2004). These changes are similar to the effects of drugs such as cocaine on the mesolimbic and mesocortical pathways (Caine and Koob 1994; Wise et al. 1995; Pich et al. 1997). Recent imaging in human runners also indicates that release of endogenous opioids occurs in frontolimbic brain regions after sustained physical exercise and was correlated with perceived euphoria (Boecker et al. 2008). Since exercise, preferred foods, and other forms of environmental enrichment activate reward mechanisms similar to those activated by drugs of abuse, it is useful to consider physical activity as a substitute for the rewarding effects of cocaine and other drugs as well as a prevention for reinstatement or relapse of drug seeking after a drug-free period.
The purpose of this study was to examine the effect of access to a running wheel during extinction and/or cocaine-primed reinstatement in rats previously trained to self-administer iv cocaine. Cocaine-primed reinstatement is considered to be an animal model of relapse, and understanding and preventing relapse is a major challenge to the successful treatment of drug abuse. Initial results with dietary substances indicated that environmental enrichment interfered with reinstatement of drug-seeking behavior (Comer et al. 1995; Solinas et al. 2008). In the present study, rats were trained to self-administer iv cocaine (0.4 mg/kg) and then were subsequently divided into four groups according to the following 2×2 design in which access to wheel running or a locked wheel was given during extinction and/or reinstatement: (1) access to wheel running during extinction from cocaine self-administration and reinstatement of cocaine seeking (WER), (2) access to wheel running during extinction and a locked wheel (did not rotate) during reinstatement (WE), (3) access to the locked wheel during extinction and to wheel running during reinstatement (WR), and (4) access to a locked wheel during extinction and reinstatement (WL; i.e., no opportunity to run). It was hypothesized that the groups with access to running during extinction (WER, WE) and reinstatement (WR, WER) would show reduced responding during these phases compared with the group with locked wheel access (WL).
Materials and methods
Subjects
Thirty-seven female 90-day-old Wistar rats weighing 250–300 g at the start of the experiment served as subjects (Harlan Sprague-Dawley, Madison, WI, USA). Females were used, as they readily acquire wheel running (Jones et al. 1990), they run more than males (Boakes et al. 1999; Cosgrove et al. 2002; Eikelboom and Mills 1988; Lambert and Kinsley 1993), and they are more sensitive than males to the attenuating effects of wheel running on cocaine self-administration (Cosgrove et al. 2002). Estrous cycle was not monitored and was allowed to vary randomly in order to generalize findings across all cycle phases. In addition, the animals were housed in their operant chambers during the experiment, and testing for cycle phase may have disrupted the cycle as well as the cocaine and wheel-reinforced behavior. After arrival, rats were pair-housed in plastic cages and allowed at least 3 days to acclimate before testing. They had free access to pellet food (Rodent Diet 2018, Harlan Laboratories, Madison, WI, USA) and water. Subsequently, rats were removed from the plastic cages and placed in individual operant chambers where they remained for the duration of the study. While in the chambers, rats continued to have free access to water and they were fed 16 g of ground food (LabDiet 5001, Purina Laboratory Chow, Minneapolis, MN, USA) at 3:00 p.m. daily to maintain them at 85% of their free-feeding body weight. All rodent holding rooms were maintained at 24°C and at 40–50% humidity under a light/dark cycle of 12:12 h with room lights on at 6:00 a.m. The experimental protocol (0708A15263) was approved by the University of Minnesota Institutional Animal Care and Use Committee. The experiment was conducted in accordance with the Principles of Laboratory Animal Care (National Research Council 2003), and all laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
Octagonally shaped operant conditioning chambers consisting of alternating stainless steel and Plexiglas walls were used for housing and testing in the present study. Custom-made, sound-attenuating, wooden boxes equipped with a ventilation fan enclosed each operant conditioning chamber. Operant chambers contained slots that allowed for the insertion of stainless steel panels, a drinking bottle holder, a food receptacle, two response levers, two sets of stimulus lights, and a house light. The stimulus lights consisted of three multicolored light-emitting diode lights (red, yellow, and green) located above each response lever, and a single white house light (4.76 W) positioned in the upper corner illuminated each operant chamber. A guillotine-style door, when opened, allowed access to a free-spinning 35.6-cm-diameter running wheel (Med Associates Inc., St. Albans, VT, USA) that was fitted on the left side of each operant chamber. A microswitch recorded quarter-wheel turns and a lock could be put in place to allow entry but prevent rotation.
During self-administration sessions, a syringe pump (model PHM-100, MedAssociates Inc., St. Albans, VT, USA) was used to deliver response-contingent iv infusions and was located on the outside of the wooden sound-attenuating enclosure. During self-administration sessions, responding on the left lever delivered cocaine infusions through a segment of Tygon tubing (1.52 mm o.d., 0.51 mm i.d., Fisher Scientific, Springfield, NJ, USA) that extended from the 35-ml syringe to a plastic swivel (050–022, Alice King Chatham, Hawthorne, CA, USA) secured to the top center of the operant chamber. An additional segment of tubing, protected by a metal spring-covered tether (C313CS, PlasticsOne, Roanoke, VA, USA), was connected to the opposite end of the swivel and extended into the operant conditioning chamber. Inside the chamber it attached to an infusion harness (Instech, Laboratories, Plymouth Meeting, PA, USA) that was fitted to each rat following catheter implantation. The swivel and tether allowed free movement within the operant chamber and easy access to the adjoining running wheel. Data collection and programming were conducted using PC computers with a Med-PC interface (MedAssociates, Inc., St. Albans, VT, USA).
Drugs
Cocaine HCl was supplied by the National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA), dissolved in 0.9% NaCl at a concentration of 1.6 mg cocaine HCl/1 ml saline, and refrigerated. Heparin (1 ml/200 ml saline) was added to the cocaine solution to prevent catheter occlusion from thrombin accumulation. The flow rate of each cocaine infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g of body weight) was adjusted to provide a 0.4-mg/kg cocaine dose throughout self-administration testing.
Procedure
Wheel training
Table 1 shows the six phases of the procedure. In the first phase, rats were fitted with the infusion harness and tether and fed 16 g of ground food daily. They were allowed access to the wheel during 6-h sessions (9:00 a.m. to 3:00 p.m.) for approximately 8 days to acclimate subjects to running when connected to the harness/tether system. During training, the house light was illuminated and the door to the wheel was opened. Response levers were present during wheel training. Acquisition of wheel running was defined as 400 full wheel revolutions in a single session or 100 revolutions per session for three consecutive sessions. If an animal failed to reach either criterion within 2 weeks, it was not included in the study.
Table 1.
Experimental procedure
| Phase (days) | Wheel training (∼8) | Surgery/recovery (∼3) | S-A training (∼5) | Maintenance (10) | Extinction (14) | Between-subjects reinstatement S S C S C S C (7) |
Within-subjects reinstatement S C S C+W S C (6) |
|---|---|---|---|---|---|---|---|
| Group | Dose | 0.4 mg/kg, iv | Saline, iv | 5, 10, 15 mg/kg, ip | 15 mg/kg, ip | ||
| WER | Wheel | No Wheel | Wheel | – | |||
| WE | Wheel | No Wheel | Wheel | Wheel locked | Wheel (C + W) | ||
| WR | Wheel | No Wheel | Wheel locked | – | |||
| WL | Wheel | No Wheel | Wheel Locked | Wheel | Wheel (C + W) |
Surgical procedure
One to 3 days after achieving wheel-running behavior, rats were surgically implanted with an indwelling catheter in the right jugular vein following the procedure outlined by Carroll and Boe (1982). Briefly, rats were anesthetized with a combination of ketamine (60 mg/kg, ip) and xylazine (10 mg/kg, ip) and administered doxapram (5 mg/kg, ip) and atropine (0.4 mg/ml, 0.15 ml, sc) to facilitate respiration. An incision was made lateral to the trachea, the right jugular was exposed, and a small incision was made perpendicular to the vein. The beveled end of a polyurethane catheter (MRE-040, Braintree Scientific, Inc., Braintree, MA, USA) was inserted and then secured to the vein with silk sutures. The free end of the catheter was guided subcutaneously to the midscapular region of the neck where it exited via a small incision and attached to a metal cannula (C3236, PlasticsOne, Roanoke, VA, USA) that was embedded in the infusion harness.
Following the surgical procedure, doors to the wheels remained closed, and rats were allowed a 3-day recovery period during which antibiotic (gentamicin) and analgesic (buprenorphine) medications were administered. Each rat was fitted with an infusion harness and tether that was worn throughout the remainder of the study. After the recovery period, catheters were flushed with a heparinized saline solution at 8:00 a.m. daily to prevent catheter blockage. Every 7 days at 3:00 p.m. body weights were recorded, and catheter patency was checked by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline (KMS). If a loss of the righting reflex was not manifest upon a KMS catheter patency check, a second catheter was implanted in the left jugular vein following the methods described above, and the experiment resumed in 3 days.
Cocaine self-administration training and maintenance
In the third phase, rats were trained to lever press for iv infusions of cocaine (0.4 mg/kg) under a FR 1 schedule of reinforcement during daily 6-h sessions (9:00 a.m. to 3:00 p.m.) that were signaled by illumination of the house light. During sessions, a response on the active/drug-paired lever started the infusion pump and illuminated the stimulus lights located directly above the lever for the duration of the infusion. Responses on the active lever during the length of the infusion (2.5–3.0 s) were recorded but had no programmed consequences. Responses on the inactive/activity lever illuminated the stimulus lights above that lever but did not activate the pump. Initially, three experimenter-delivered priming infusions of 0.4 mg/kg cocaine were administered at the beginning of each training session (9:00 a.m.) followed by the placement of a small amount of peanut butter on the active/drug-paired lever. This training procedure continued until rats earned at least 60 infusions during a single session. Once rats achieved acquisition of cocaine self-administration, they were allowed to self-administer for ten additional 6-h sessions (maintenance). The door leading to the wheel remained closed throughout self-administration training and maintenance (no wheel access).
Extinction
Following day 10 of maintenance, the cocaine solution was replaced with saline, and responding produced a saline infusion (FR 1) along with the stimuli described above for maintenance. Based on total cocaine intake during maintenance, rats were separated into four groups with the following conditions: (1) access to a running wheel during extinction and reinstatement (WER), (2) access to a wheel during extinction and a locked wheel (unable to rotate) during reinstatement (WE), (3) access to the locked wheel during extinction and to wheel running during reinstatement (WR), and (4) access to a locked wheel during extinction and reinstatement (WL). Groups were compared during the extinction phase for 14, 6-h extinction sessions.
Reinstatement
In the reinstatement phase, the stimulus lights, house light, and pump were unplugged to ensure that reinstatement responding was due to ip priming injections and not to drug-associated cues (Larson and Carroll 2007). Initially, groups were compared in a between-subjects design, and subsequently, the WE and WR groups were tested in a within-subjects design to evaluate the effect of brief wheel exposure or removal during single reinstatement sessions.
Between subjects
Each of the four groups were administered saline (S)- or cocaine (C)-priming injections at session onset (9:00 a.m.), and their subsequent cocaine-seeking behavior was tested for the next 6 h (9:00 a.m. to 3:00 p.m.) for seven consecutive days. During the first two reinstatement sessions, rats were treated with a single S injection, and for the next 5 days, they were treated with a single C or S injection on alternating days (i.e., S S C S C S C). Response-contingent drug-paired cues and the house light were deactivated during this time. On the C days, rats received priming injections of one of three randomly assigned doses of C (5, 10, and 15 mg/kg). Doors to the adjoining wheel remained open during reinstatement testing, but only groups WR and WER could run; the WE and WL groups could enter the wheel, but it did not rotate.
Within subjects
For the rats in groups WE and WR, three S and three 15 mg/kg C-priming injections (alternating) were administered over 6 days. On the second C-priming day, rats in group WE were allowed access to wheel running (C+W) during the 6-h reinstatement session to determine whether acute exposure to the wheel produced the same effect as chronic exposure in the between-subjects condition for group WER. Similarly, group WR was given cocaine-priming injections on the second and sixth day to determine whether reinstatement would occur without wheel access. Thus, in the within-subjects condition, groups WE and WR received the following priming sequence over six consecutive days: S, C, S, C + W, S, C.
Data analysis
Responses, infusions, and full wheel revolutions during extinction and responses and wheel revolutions during reinstatement were the primary dependent measures. These measures were analyzed with two-factor mixed analyses of variance (ANOVA) with group as the between-subjects factor and blocks of days as the repeated measure. Data were grouped in 2-day blocks to reduce daily variability and the number of post-hoc contrasts. Separate two-factor ANOVAs were used to compare infusion lever and active lever presses during reinstatement with group as the between-subjects factor and the priming injection (e.g., S vs C) as the repeated measure. Infusion lever and inactive lever presses during reinstatement were also examined using a three-factor ANOVA with group and lever type (e.g., active vs inactive) as a between-subjects factor and dose as the repeated measure. A mixed ANOVA was used to compare priming conditions by group for groups WE and WR in the within-subject study. Post-hoc tests were performed with Fisher's least significant difference protected t tests, and results were considered significant if p<0.05. Statistical analyses were performed using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA).
Results
All rats consumed the daily 16-g food allotment and did not differ in mean water intake or body weight during the study. Groups also did not differ in number of wheel training days, wheel revolutions during training, or days to acquire cocaine self-administration (Table 2).
Table 2.
Summary of group data
| Group | Number | Mean H2O consumption (ml ± SEM) | Mean weight (g ± SEM) | Wheel training days (± SEM) | Training revolutions (± SEM) | Mean days to acquire (± SEM) |
|---|---|---|---|---|---|---|
| WER | 9 | 35.59 (±2.23) | 271.82 (±3.51) | 8.11 (±2.04) | 242.54 (±40.34) | 5.22 (±0.70) |
| WE | 9 | 35.22 (±2.35) | 269.46 (±7.58) | 3.89 (±1.14) | 298.07 (±44.17) | 4.00 (±0.17) |
| WR | 9 | 34.13 (±2.93) | 284.04 (±4.78) | 6.00 (±1.44) | 295.75 (±95.58) | 5.00 (±1.16) |
| WL | 10 | 31.76 (±2.78) | 271.06 (±4.63) | 4.44 (±0.75) | 234.01 (±47.60) | 4.44 (±0.53) |
Maintenance
Figure 1 shows mean responses (a) and infusions (b), respectively, across the 10-day maintenance period. Separately, responses and infusions were averaged across five 2-day blocks, and there was not a significant main effect of group for either responses or infusions. However, there was a significant main effect of session block for both responses (F4,184=4.81, p<0.01) and infusions (F4,184=7.61, p<0.0001). Data were collapsed and analyzed across groups, and a significant effect of session block was found when averaging responses (F4,184=4.86, p<0.01) and infusions (F4,184=7.53, p<0.0001) over five 2-day blocks. Post-hoc comparisons showed a significant increase in both responses and infusions during sessions 7 to 8 (p<0.05) and 9 to 10 (p<0.01) compared with those during sessions 1 to 2. There were no significant differences in inactive lever presses (data not shown) among groups or across blocks.
Fig. 1.
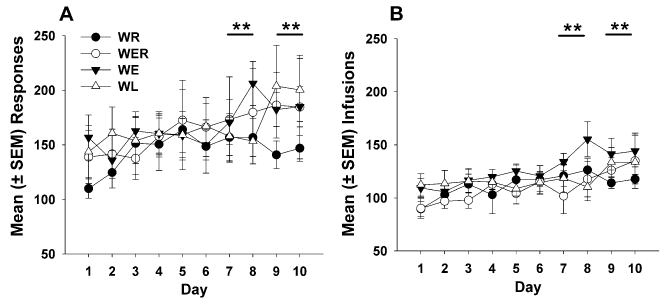
Mean (± SEM) responses (a) and iv 0.4 mg/kg cocaine infusions (b) in groups WR, WER, WE, and WL during daily 6-h self-administration sessions over the 10-day maintenance period. Session blocks 7 to 8 and 9 to 10 (**p<0.01) had significantly more responses (a) and infusions (b) than session blocks 1 to 2 when group data were collapsed and analyzed across five two-session blocks
Extinction
Extinction responses (a) and infusions (b) are shown in Fig. 2. When data were averaged in 2-day blocks, a significant reduction in responding occurred on both active and inactive levers and in saline infusions in rats with wheel access (WER and WE combined) compared with rats without wheel access (WL and WR combined). Since WER and WE did not have significantly different responses or infusions during maintenance (see above) or extinction (data not shown), and both groups underwent the same condition during extinction (i.e., wheel access), their data were collapsed and compared to WL and WR, whose data were similarly combined. There was a significant main effect of group (F1,258=9.64, p<0.01) and session block (F6,258=42.83, p<0.0001) and a group × session block interaction (F6, 258=9.82, p<0.0001) for responses. Post-hoc tests revealed significantly higher responding on extinction sessions 1 to 2 and 3 to 4 (p<0.01) and sessions 5 to 6 and 7 to 8 (p<0.05) for WL and WR combined vs WER and WE combined (Fig. 2a). A similar analysis was conducted for infusions revealing a significant main effect of group (F1,258=9.24, p<0.01) and session block (F6,258=41.92, p<0.0001) and a significant interaction (F6,258=7.85, p<0.0001). Post-hoc comparisons indicated more saline infusions for WL and WR vs WER and WE during sessions 1 to 2, 3 to 4, and 5 to 6 (p<0.01; Fig. 2b). Inactive lever presses were compared revealing significant main effects of group (F1,258=24.05, p<0.0001) and session block (F6,258= 10.29, p<0.0001) and a significant group × session block interaction (F6,258=3.68, p<0.01; data not shown). Post-hoc tests revealed that groups with wheel access (WER and WE) made significantly fewer responses on the inactive lever during sessions 1 to 2, 3 to 4, 5 to 6, 7 to 8, and 11 to 12 (p<0.01) and sessions 9 to 10 (p<0.05) than groups with locked wheel access (WL and WR). To assess whether groups WL and WR combined (locked wheel access) showed preference for the previously drug-paired lever during extinction, active and inactive lever responses were compared (F6,265= 15.16, p<0.0001), with post hoc tests indicating that significantly more responses were made on the active lever during sessions 1 to 2, 3 to 4, 5 to 6, and 7 to 8 (p<0.01).
Fig. 2.
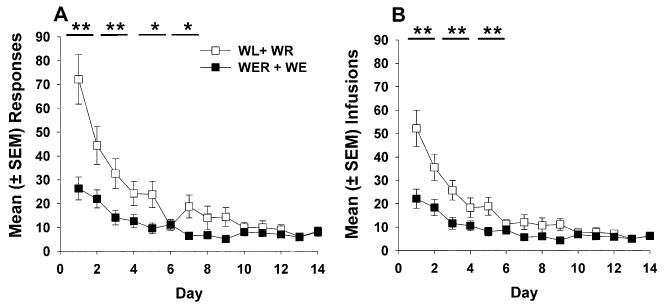
Mean (± SEM) responses (a) and iv saline infusions (b) during daily 6-h self-administration sessions over the 14-day extinction period. Groups WL and WR were combined and then, separately, WER and WE were combined, as they showed no significant differences. Following introduction of the wheel on day 1 of extinction, combined Groups WL+WR exceeded combined Groups WER+WE in responses (a) and infusions (b) on session blocks 1 to 2 (**p<0.01), 3 to 4 (**p<0.01), and 7 to 8, (*p<0.05). The combined groups also differed on responses on sessions 5 to 6 and 7 to 8 (p<0.05) and on infusions on session 5 to 6 (p<0.01)
Wheel revolutions during extinction were averaged in seven 2-day blocks to reduce variability across days, and they were compared between groups WER and WE (Fig. 3). There was a significant main effect of session block (F6, 125=5.21, p<0.01), but there was no effect of group or a group × session block interaction. This indicates that a similar increase in revolutions occurred over the 14 days in both groups. To test the hypothesis that removal of cocaine increased extinction responding on the first day, wheel revolutions were compared with a paired t test on the last day of training and the first day of extinction in groups WER and WE combined, as the groups did not differ, and revolutions were significantly higher on the first day of extinction (t17=2.61, p<0.05). A comparison of revolutions during training (Table 2) and on the first day of extinction (Fig. 3) for these groups showed that revolutions were nearly twice as high during extinction than training in groups that had wheel access (WE and WER).
Fig. 3.
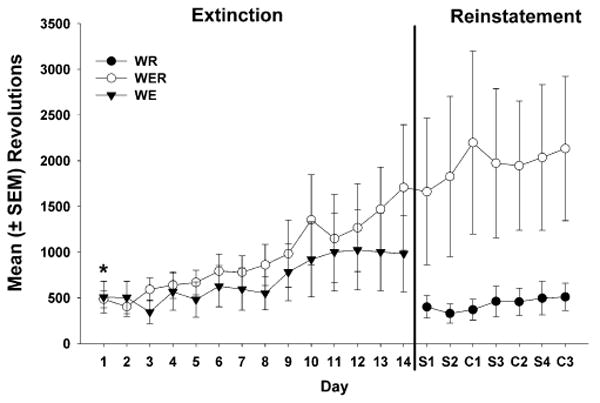
Mean (± SEM) wheel revolutions over the 14-day extinction period for groups WR (filled circles), WER (open circles), and WE (filled triangles) and during the reinstatement period for each saline (S) or cocaine (C) priming injection for groups WR and WER. During analysis, groups WE and WER were combined, as they did not significantly differ during cocaine extinction, and when wheel revolutions were compared on the first day of extinction vs the last day of training (see Table 2), they were significantly higher during extinction (*p<0.05)
Reinstatement
Between subjects
Figure 4a shows responding on the previously active lever following ip priming injections of S or C during the reinstatement period. Reinstatement began with two S-priming injections, and responding following the first priming injection was analyzed to determine the effect of discontinuing unlocked wheel access for group WE after extinction. Group differences were revealed (F3,39= 11.85, p<0.0001), and post hoc tests showed significantly greater responding for group WE (striped bar) on the first S day vs WR, WER, and WL (p's< 0.01). No significant differences were found in inactive lever responding for the first S-priming injection.
Fig. 4.
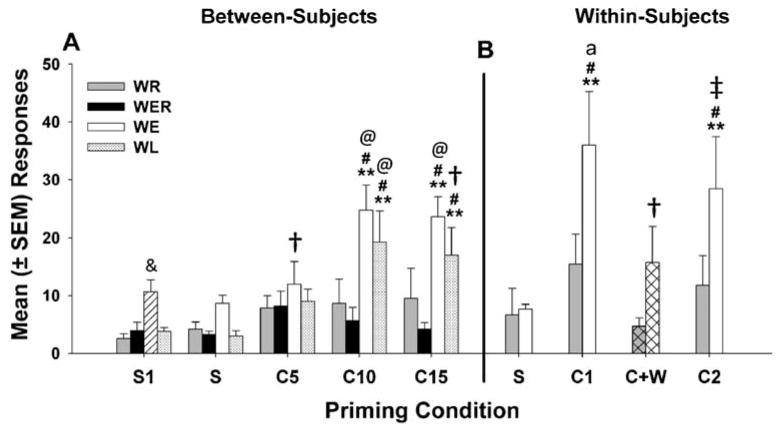
Mean (± SEM) responses on the previously active lever (a) following priming injections during reinstatement testing in the between-subjects design with ip saline (S) or 5-, 10-, and 15-mg/kg ip injections of cocaine (C), #p<0.01 vs WER, **p<0.01 vs S, @p<0.01 vs WR, +p<0.05 vs WR. The striped bar indicates the first day after termination of wheel running in WE, and &p<0.01 vs all other groups. Mean (± SEM) responses on the previously active lever (b) following priming injections of saline (S) or two 15-mg/kg cocaine (C1, C2) during a within-subjects reinstatement procedure in groups WE and WR (cocaine and wheel access-cross-hatched bar), **p<0.01 vs S, #p<0.01 vs WR, †p<0.05 vs WR, † = 0.05 vs WR, ‡p<0.05 vs C + W, a = p<0.01 vs C +W
Responses averaged over the remaining three S-priming days were compared to responses on the 5-, 10-, and 15-mg/kg dose C-priming days (Fig. 4a), and there were significant main effects of priming condition (F3,147= 15.02, p<0.0001), group (F3,147=6.58, p<0.01), and a significant priming condition × group interaction (F9,147=2.81, p<0.01). Post-hoc analyses indicated that the 10- and 15-mg/kg C-priming injections resulted in significantly more responding than S-priming injections for both groups WL and WE (p's<0.01). In contrast, responding on S- and C-priming days did not differ for WR and WER. Groups WE and WL did not significantly differ in responding after 10- and 15-mg/kg C-priming injections, but both groups responded significantly more than WER (p's<0.01) at these doses. Compared to WR, WE and WL responded significantly more following the 10 (p's<0.01)- and 15 (p<0.01 vs WE; p<0.05 vs WL)-mg/kg priming injections, while WE responded more after the 5 (p<0.05)-mg/kg priming dose as well. No significant group × priming condition interaction was found for inactive lever responses (not shown), although there were main effects of group (F3,147=4.96, p<0.01) and priming condition (F3,147=3.95, p<0.05) similar to responding on the previously active lever.
Inactive and previously-active lever responses during reinstatement were compared in a three-factor mixed ANOVA. This analysis revealed significant main effects of group (F3,295=12.19, p<0.0001), lever type (e.g., previously active > inactive; F1,295=18.73, p<0.0001), and priming condition (F3,295= 15.55, p<0.0001) as well as significant group × priming condition (F9,295=3.09, p< 0.01) and lever type × priming condition (F3,295=4.04, p< 0.01) interactions (not shown). However, no significant group × lever type × priming condition interaction was found. Finally, when comparing wheel revolutions for WER and WR over the 7-day reinstatement period, there was a near-significant effect of group (F1,71=3.67, p=0.0735), probably due to heterogeneity of variance. This suggested that running escalated over time in the WER group. Otherwise, there were no significant effects of interactions (see Fig. 3).
Within subjects
Figure 4b shows results for six rats in WE and all nine rats in WR for which a subsequent within-subject reinstatement procedure was implemented after the between-groups data were collected. The mean of three S-priming sessions was compared to corresponding C-priming sessions (C) and to the C + W (C priming + wheel running access) session in a two-factor ANOVA. There was a near-significant effect of group (F1,59=3.78, p=0.0738), a significant effect of priming condition (F3,59=12.05, p<0.0001), and a significant group × priming condition interaction (F3,59=2.92, p<0.05). WR did not respond differently for S than on any of the C-priming days. In WE, responding did not differ between C + W and S, but there was significantly higher responding for C1 and C2 vs S (p<0.01) and vs C + W (p<0.01 vs C1; p<0.05 vs C2). Comparing WE and WR, WR responded significantly more following C1 and C2 (p<0.01) and C + W (p<0.05). Analysis of inactive lever responding did not reveal significant differences (data not shown).
Discussion
In the present study, there were no differences among the four experimental groups in initial exposure to the running wheel (revolutions), in the rate of acquisition of cocaine self-administration, or in the level of cocaine maintenance responding. However, during the subsequent extinction phase, wheel running significantly reduced extinction responding in WER and WE compared to WL and WR. This finding suggests that wheel exposure reduced elevated drug seeking during this critical phase. Previous studies have shown that access to another type of reinforcer, sweet substances, decreased the extinction of cocaine (Liu and Grigson 2005)- and amphetamine (Ping and Kruzich 2008)-seeking behavior in rats. Similarly, extinction of behavior maintained by a sucrose reinforcer was reduced by an enriched environment that included more space, greater social contact, novel objects, and opportunity for exercise (Grimm et al. 2008; Stairs et al. 2006).
In the subsequent reinstatement phase, rats without wheel running access (groups WE and WL) reliably reinstated responding on the previously active lever. This was in contrast to groups WER and WR that were allowed to run during reinstatement and consequently showed a suppression of cocaine-primed reinstatement. That group WE did not have suppressed reinstatement indicated that recent running access during extinction did not carry-forward into the reinstatement phase. A consistent finding was that the subsequent within-subjects manipulation in group WE revealed that wheel access effectively reduced reinstatement when it was concurrently available during only one cocaine-priming session; however, there was not a carry-forward suppressive effect after wheel running access ended and the second C-priming condition occurred. The within-subjects reinstatement study also revealed significantly lower drug seeking in the WR group vs WE. This could have been due to the more recent exposure to the wheel in the WR group vs more distant exposure in WE or that exposure during the between-subjects reinstatement more readily generalized to subsequent within-subjects reinstatement testing in the WR vs the WE group.
The lack of an enduring suppressant effect of wheel running on reinstatement of cocaine seeking was consistent with the effects of recent but not previous environmental enrichment on sucrose seeking during extinction and reinstatement in rats (Grimm et al. 2008). However, these and the present results were not consistent with findings of earlier studies examining the effects of previous and chronic wheel running on the rewarding effects of drugs of abuse (Chen et al. 2008; Smith et al. 2008). Several methodological and procedural differences between the present and previous studies may account for the discrepant findings. The most important difference concerns the duration of wheel access between studies. In previous work, rats were allowed to run on the wheel for extended periods of time (Chen et al. 2008; Smith et al. 2008).
In rats, wheel running upregulates the opioid neuropeptide dynorphin (Werme et al. 2000), dopamine neurons (Ahmad et al. 2009), delta FosB (Werme et al. 2002), and brain-derived neurotrophic factor (Khabour et al. 2009; Macias et al. 2007; Neeper et al. 1996; Widenfalk et al. 1999) in areas of the brain associated with reward only after extended periods of free access to a running wheel. Additionally, increases in these neurosubstrates, through pharmacological manipulations, led to alterations in cocaine-primed reinstatement in rats (Berglind et al. 2007; Gyertyan et al. 2007; Mantsch et al. 2004; Micheli et al. 2007; Schmidt and Pierce 2006). Although these neural correlates were not analyzed in the present study, it may be assumed that the duration of exposure to wheel running may not have been sufficient to increase these measures to the point where they would produce enduring decreases in subsequent cocaine seeking.
The present investigation is consistent with previous results demonstrating that wheel running decreased concurrent cocaine (Cosgrove et al. 2002), amphetamine (Kanarek et al. 1995), and alcohol (McMillan et al. 1995) self-administration as well as MDMA-induced CPP (Chen et al. 2008) in rats. It also supported findings demonstrating suppression of acquisition of cocaine (Carroll and Lac 1992) and phencyclidine self-administration (Campbell et al. 1998) in rats and monkeys, respectively, using sweetened liquids as alternative reinforcers. While both sweetened liquids (Carroll and Lac 1992; Campbell et al. 1998; Carroll and Campbell 2000; Cosgrove and Carroll 2003; Rodefer and Carroll 1997; Carroll 1985; Kanarek and Marks-Kaufman 1988; Ping and Kruzich 2008) and food (Carroll and Lac 1992; Carroll 1996; Carroll et al. 2001) have been highly effective at interfering with drug self-administration, dietary substances can have adverse consequences if consumed in excess (Avena 2007; Avena et al. 2008; Corwin and Wojnicki 2006). The present results and recent work (Cosgrove et al. 2002) to the extinction and reinstatement phases of drug abuse, and indicate that exercise may be an effective and healthier nondrug alternative.
The present results extended the generality of wheel running, as a nondrug alternative reward. Another explanation for the attenuating effects of wheel running on extinction in the WER and WE groups is that cocaine seeking and wheel running were mutually exclusive, and time spent doing one resulted in less time for the other. However, analysis of the time course of wheel- and infusion-maintained behavior indicated that much of the 6-h session was spent on neither activity. For example, the average total during extinction was 50 responses or less, occupying only a small amount of time during the total 6-h session. Thus, the behaviors did not compete for limited time, and this was consistent with previous findings (Cosgrove et al. 2002).
The present findings suggest that rather than the actual behaviors competing, the reinforcing value of the wheel and cocaine reward competed. This interaction was also revealed by the increase in wheel running in group WER and WE from precocaine wheel training (Table 2) to extinction (Fig. 3) when cocaine access had been removed. Another indication of a possible substitution effect of wheel running with cocaine reward was the resurgence of cocaine seeking (e.g., lever responding) in the WE group on the first day of reinstatement when wheel running was prohibited. That this resurgence did not occur in the group that was reintroduced to wheel running (WR), the group that continued running in the reinstatement phase (WER) or in the locked wheel (WL) group suggests a reward interaction (wheel × cocaine) effect. When considering nondrug alternatives as a means of reducing drug-seeking behavior, it is important to consider that they may substitute for the drug and trigger reinstatement of behavior that was previously rewarded by the drug. Thus, extending access to the nondrug alternative (e.g., wheel) during extinction is a preventive strategy that could be employed.
Behavioral analysis of resurgence, or the elevation in previously reinforced behavior following the removal of an alternative reinforcer, has been documented (Leitenberg et al. 1970; Mulick et al. 1976; Carroll 1985; Carroll et al. 1989). For example, a study by Podlesnik et al. (2006) reported that rats that previously self-administered alcohol showed a resurgence in lever pressing following the removal of a food alternative reinforcer. Others have demonstrated a resurgence of lever pressing for a primary reinforcer following the removal of a secondary reinforcer (Epstein 1983; Leitenberg et al. 1970; Mulick et al. 1976; Wilson and Hayes 1996). The magnitude of resurgence is also dependent on extinction experience, with higher levels of resurgence occurring when there is less of an opportunity to extinguish (Cleland et al. 2000). Thus, the resurgence in responding for the primary reinforcer (drug) in the WE group may have resulted from a lack of extinction experience (Marlatt 1990) due to a greater amount of time being dedicated to wheel running during extinction testing.
Another explanation of the present findings may involve the attenuation of drug-related withdrawal effects by wheel running. Drug withdrawal effects are a primary contributor to elevated extinction responding in animal models (Carroll et al. 2009b; Kelamangalath and Wagner 2009; O'Dell et al. 2007), and reduction of withdrawal effects is integral to the implementation of effective treatment strategies in cocaine addiction (Ahmadi et al. 2006; Kampman et al. 2001a, b). Results from animal and human studies (Alaei et al. 2006; Daniel et al. 2007; Taylor and Katomeri 2007; Taylor et al. 2007; Ussher et al. 2001) indicate that withdrawal signs and symptoms are reduced following bouts of exercise. This effect may be attributed, in part, to exercise-induced increases of monoamines in brain areas associated with reward. For example, cocaine withdrawal is associated with decreases in dopamine and serotonin in the striatum (Parsons et al. 1995; Rossetti et al. 1992), and continued drug use may be related to avoidance of these negative effects (Koob 2009). Physical activity, on the other hand, increases several monamines, including dopamine, within the striatum of rats and humans (Dishman 1997; Fulk et al. 2004; Hattori et al. 1994; Meeusen 2005; Petzinger et al. 2007), and it decreases stress, anxiety, and depression (Dishman 1997; Fulk et al. 2004; Sarbadhikari and Saha 2006). Recent work extended these findings to show that voluntary exercise and sucrose consumption enhanced cannabinoid CB1 receptor sensitivity in the striatum (Di Chiara et al. 2009). Higher vs lower levels of physical activity were also associated with higher self-reported measures of well-being among adolescents (Ussher et al. 2007). Thus, extinction responding in the present study may be described as an attempt to reestablish homeostatic equilibrium following a withdrawal-induced disruption in neural mechanisms that regulate hedonic motivation. Wheel running may have helped to restore this equilibrium thereby decreasing extinction responding.
The present findings offer useful applications for preventing and reducing drug abuse in humans. As mentioned previously, physical activity decreases nicotine craving and withdrawal symptoms (Ussher et al. 2006, 2009; Taylor et al. 2007). A recent investigation using physically active and inactive twin cohorts showed that the physically active cohort was less likely to smoke cigarettes than the inactive cohort (Kujala et al. 2007), and the presence of regular physical activity during adolescence decreased the risk of smoking in early adulthood (Charilaou et al. 2009; Korhonen et al. 2009). Adolescents participating in drug intervention programs that include physical exercise demonstrate lower risk factors for drug abuse and decreased drug use (Collingwood et al. 1991, 2000). Physical activity has also been useful in combating alcohol abuse in inpatient treatment programs (Palmer et al. 1988; Sinyor et al. 1982) and in improving the probability of abstinence up to 3 months after program release (Sinyor et al. 1982).
Exercise has also been used as a supplement to contingency management (CM), a type of substance abuse treatment that rewards periodically verified abstinence with nondrug alternative reinforcers (e.g., money or vouchers for retail items). Patients who participated in physical activity in addition to CM intervention had higher drug abstinence rates than those who had CM alone (Weinstock et al. 2008). While CM is highly effective in maintaining abstinence (Pendergast et al. 2006; Lussier et al. 2006), it can be costly (Olmstead and Petry 2009; Sindelar et al. 2007; Higgins 1997). In contrast, exercise, by itself or in addition to CM, is an inexpensive and self-maintained behavior that may potentially have greater long-term consequences.
In summary, the present results indicated that access to wheel running decreased the extinction and reinstatement of cocaine-seeking behavior. Reinstatement was reduced by chronic, concurrent wheel running in a between-subjects study and by a single concurrent exposure during a within-subjects procedure. A comparison of four groups with differing wheel-running exposure indicated that concurrent wheel running was a key factor, and recent wheel access did not have a carry-forward effect on cocaine-primed reinstatement. The present findings, along with previous reports, suggest that access to wheel running reduces drug-seeking behavior during maintenance, extinction, and reinstatement.
Acknowledgments
This research was supported by the National Institute on Drug Abuse, R01 DA 003240-25, K05 015267-07 (MEC), and F31 DA 023301-02 (JJA). The authors would like to thank Nathan Holtz, Emily Kidd, Brandon Knight, Kinner Patel, Amy Saykao, Matthew Starr, Rachael Turner, Troy Velie, and Jeremy Williams for their technical assistance.
References
- Ahmad SO, Park JH, Stenho-Bittel L, Lau YS. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and probenecid-treated mice. Neurosci Lett. 2009;450:102–105. doi: 10.1016/j.neulet.2008.11.065. [DOI] [PubMed] [Google Scholar]
- Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. Am J Addict. 2006;15:434–439. doi: 10.1080/10550490600998476. [DOI] [PubMed] [Google Scholar]
- Alaei H, Borjeian L, Azizi M, Orian S, Pourshanazari A, Hanninen O. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536:138–141. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–491. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Belke TW, Oldford AC, Forgie MY, Beye JA. Responding for sucrose and wheel-running reinforcement: effect of D-amphetamine. Behav Pharmacol. 2005;16:219–225. doi: 10.1097/01.fbp.0000170912.61039.bd. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113:1080–1089. [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhowfer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner's high: opioidergic mechanisms in the human brain. Cerebral Cortex. 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav. 1994;61:213–221. doi: 10.1901/jeab.1994.61-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Thompson SS, Carroll ME. Acquisition of oral phencyclidine (PCP) self-administration in rhesus monkeys: effects of dose and an alternative nondrug reinforcer. Psychopharmacology. 1998;137:132–138. doi: 10.1007/s002130050602. [DOI] [PubMed] [Google Scholar]
- Carroll ME. Concurrent phencyclidine and saccharin access: presentation of an alternative reinforcer reduces drug intake. J Exp Anal Behav. 1985;43:131–144. doi: 10.1901/jeab.1985.43-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. Reducing drug abuse by enriching the environment with alternative nondrug reinforcers. In: Green L, Kagel J, editors. Advances in behavioral economics. Vol. 3. Ablex; Norwood: 1996. pp. 37–68. [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharm Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC. A behavioral economic analysis of the reinforcing effects of drugs: transition states of addiction. In: Bickel WK, Vuchinich R, editors. Reframing health behavior change with behavioral economics. Lawrence Erlbaum Associates; Mahwah: 2000. pp. 63–87. [Google Scholar]
- Carroll ME, Lac ST. The effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology. 1992;106:439–446. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology. 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Bickel WK, Higgins ST. Nondrug incentives to treat drug abuse: laboratory and clinical developments. In: Carroll ME, Overmier JB, editors. Animal research and human psychological health: advancing human welfare through behavioral science. American Psychological Association; Washington: 2001. pp. 139–154. [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009a;104:S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Mach JL, LaNasa RM, Newman JL. Impulsivity as a measure of withdrawal of orally-delivered PCP and nondrug rewards in male and female monkeys. Psychopharmacology. 2009b;207:85–98. doi: 10.1007/s00213-009-1636-y. [DOI] [PubMed] [Google Scholar]
- Charilaou M, Karekla M, Constantinou M, Price S. Relationship between physical activity and type of smoking behavior among adolescents and young adults in Cyprus. Nicotine Tob Res. 2009;11:969–976. doi: 10.1093/ntr/ntp096. [DOI] [PubMed] [Google Scholar]
- Chen HI, Kuo YM, Liao CH, Jen CJ, Huang AM, Cherng CG, Su SW, Yu L. Long-term compulsive exercise reduces the rewarding efficacy of 3, 4-methylenedioxymethamphetamine. Behav Brain Res. 2008;187:185–189. doi: 10.1016/j.bbr.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Cleland BS, Foster TM, Temple W. Resurgence: the role of extinction. Behav Processes. 2000;52:117–129. doi: 10.1016/s0376-6357(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. J Drug Educ. 1991;21:73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Sunderlin J, Reynolds R, Kohl HW., 3rd Physical training as a substance abuse prevention intervention for youth. J Drug Educ. 2000;30:435–451. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Curtis LK, Carroll ME. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology. 1995;121:150–157. doi: 10.1007/BF02245624. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci. 2006;Chapter 9(Unit9 23B) doi: 10.1002/0471142301.ns0923bs36. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Differential effects of a nondrug reinforcer, saccharin, on oral self-administration of phencyclidine (PCP) in male and female rhesus monkeys. Psychopharmacology. 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharm Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Daniel JZ, Cropley M, Fife-Schaw C. Acute exercise effects on smoking withdrawal symptoms and desire to smoke are not related to expectation. Psychopharmacology (Berl) 2007;195:125–129. doi: 10.1007/s00213-007-0889-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sachetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, Usiello A, Centonze D. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2009;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Epstein R. Resurgence of previously reinforced behavior during extinction. Behav Anal Lett. 1983;3:391–397. [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of surose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyan I, Kiss B, Gal K, Laszlovszky I, Horvath A, Gemesi LI, Saghy K, Pasztor G, Zajer M, Kapas M, Csongor EA, Domany G, Tihanyi K, Szombathelyi Z. Effects of RGH-237 [N-{4-[4-(3-aminocarbonyl-phenyl)-piperazin-1-yl]-butyl}-4-bromobenzamide], an orally active, selective dopamine D(3) receptor partial agonist in animal models of cocaine abuse. J Pharmacol Exp Ther. 2007;320:1268–1278. doi: 10.1124/jpet.106.107920. [DOI] [PubMed] [Google Scholar]
- Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res Bull. 1994;35:41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav. 1997;57:419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Jones LC, Bellingham WP, Ward LC. Sex differences in voluntary locomotor activity of food-restricted and ad libitum-fed rats. Implications for the maintenance of a body weight set-point. Comp Biochem Physiol. 1990;96:287–290. doi: 10.1016/0300-9629(90)90694-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O'Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav. 2001a;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O'Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001b;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R. Animal models of appetitive behavior: interaction of nutritional factors and drug seeking behavior. Curr Concepts Nutr. 1988;16:1–25. [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R, D'Anci KE, Przypek J. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav. 1995;51:725–729. doi: 10.1016/0091-3057(95)00022-o. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Wagner JJ. Effects of abstinence or extinction on cocaine seeking as a function of withdrawal duration. Behav Pharmacol. 2009;20:195–203. doi: 10.1097/FBP.0b013e32832a8f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2009 doi: 10.1002/hipo.20657. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Phillips SB, Kelly TH, Bardo MT. Exposure to novel environmental stimuli decreases amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2001;9:372–379. [PubMed] [Google Scholar]
- Koob GF. New dimensions in human laboratory models of addiction. Addict Biol. 2009;14:1–8. doi: 10.1111/j.1369-1600.2008.00127.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin Res Hum Genet. 2009;12:261–268. doi: 10.1375/twin.12.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Rose RJ. Physical activity in adolescence and smoking in young adulthood: a prospective twin cohort study. Addiction. 2007;102:1151–1157. doi: 10.1111/j.1360-0443.2007.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG, Kinsley CH. Sex differences and gonadal hormones influence susceptibility to the activity-stress paradigm. Physiol Behav. 1993;53:1085–1090. doi: 10.1016/0031-9384(93)90363-k. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaineseeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Lattanzio SB, Eikelboom R. Wheel access duration in rats: I. Effects on feeding and running. Behav Neurosci. 2003;117:496–504. doi: 10.1037/0735-7044.117.3.496. [DOI] [PubMed] [Google Scholar]
- Leitenberg H, Rawson RA, Bath K. Reinforcement of competing behavior during extinction. Science. 1970;169:301–303. doi: 10.1126/science.169.3942.301. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Liu C, Grigson PS. Brief access to sweets protect against relapse to cocaine-seeking. Brain Res. 2005;1049:128–131. doi: 10.1016/j.brainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Macias M, Dwornik A, Ziemlinska E, Fehr S, Schachner M, Czarkowska-Bauch J, Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. Eur J Neurosci. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- Meeusen R. Exercise and the brain: insight in new therapeutic modalities. Ann Transplant. 2005;10:49–51. [PubMed] [Google Scholar]
- Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Fabio RD, Donati D, Gentile G, Gribble A, Hamprecht D, Tedesco G, Terreni S, Tarsi L, Lightfoot A, Stemp G, Macdonald G, Smith A, Pecoraro M, Petrone M, Perini O, Piner J, Rossi T, Worby A, Pilla M, Valerio E, Griffante C, Mugnaini M, Wood M, Scott C, Andreoli M, Lacroix L, Schwarz A, Gozzi A, Bifone A, Ashby CR, Jr, Hagan JJ, Heidbreder C. 1, 2, 4-Triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- Mulick JA, Leitenberg H, Rawson RA. Alternative response training, differential reinforcement of other behavior, and extinction in squirrel monkeys (Saimiri sciureus) J Exp Anal Behav. 1976;25:311–320. doi: 10.1901/jeab.1976.25-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of mammals in neuroscience and behavioral research. National Academies; Washington: 2003. [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine-or opioid-dependent outpatients. Drug Alcohol Depend. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Vacc N, Epstein J. Adult inpatient alcoholics: physical exercise as a treatment intervention. J Stud Alcohol. 1988;49:418–421. doi: 10.15288/jsa.1988.49.418. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Pendergast DR, Lindholm P, Wylegala J, Warkander D, Lundgren CE. Effects of respiratory muscle training on respiratory CO2 sensitivity in SCUBA divers. Undersea Hyperb Med. 2006;33:447–453. [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Ping A, Kruzich PJ. Concurrent access to sucrose pellets decreases methamphetamine-seeking behavior in Lewis rats. Pharmacol Biochem Behav. 2008;90:492–496. doi: 10.1016/j.pbb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez STA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. A comparison of progressive ratio schedules vs behavioral economic measures: effect of an alternative reinforcer on the reinforcing efficacy of phencyclidine. Psychopharmacology. 1997;132:95–103. doi: 10.1007/s002130050325. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann N Y Acad Sci. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- Sarbadhikari SN, Saha AK. Moderate exercise and chronic stress produce counteractive effects on different areas of the brain by acting through various neurotransmitter receptor subtypes: a hypothesis. Theor Biol Med Model. 2006;3:33. doi: 10.1186/1742-4682-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Systemic administration of a dopamine, but not a serotonin or norepinephrine, transporter inhibitor reinstates cocaine seeking in the rat. Behav Brain Res. 2006;175:189–194. doi: 10.1016/j.bbr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102:1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. J Stud Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res. 2007;9:1183–1190. doi: 10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology (Berl) 2001;158:66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Doshi R, Sampuran AK. Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Hum Psychopharmacol. 2006;21:39–46. doi: 10.1002/hup.744. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Owen CG, Cook DG, Whincup PH. The relationship between physical activity, sedentary behaviour and psychological wellbeing among adolescents. Soc Psychiatry Psychiatri Epidemiol. 2007;42:851–856. doi: 10.1007/s00127-007-0232-x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Cropley M, Playle S, Mohidin R, West R. Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction. 2009;104:1251–1257. doi: 10.1111/j.1360-0443.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict Behav. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34:125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Hayes SC. Resurgence of derived stimulus relations. J Exp Anal Behav. 1996;66:267–281. doi: 10.1901/jeab.1996.66-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]


