Graphical abstract
Highlights
► Reduced ocular perfusion pressure is a risk factor for the prevalence, incidence and progression of glaucoma. ► The death of retinal ganglion cells appears to involve primary and secondary insults. ► Reduced OPP may enhance both primary and secondary insults. ► Abnormal autoregulation and neurovascular coupling may lead to ganglion cell death.
Abstract
Glaucoma is a progressive optic neuropathy of unknown origin. It has been hypothesized that a vascular component is involved in glaucoma pathophysiology. This hypothesis has gained support from studies showing that reduced ocular perfusion pressure is a risk factor for the disease. The exact nature of the involvement is, however, still a matter of debate. Based on recent evidence we propose a model including primary and secondary insults in glaucoma. The primary insult appears to happen at the optic nerve head. Increased intraocular pressure and ischemia at the post-laminar optic nerve head affects retinal ganglion cell axons. Modulating factors are the biomechanical properties of the tissues and cerebrospinal fluid pressure. After this primary insult retinal ganglion cells function at a reduced energy level and are sensitive to secondary insults. These secondary insults may happen if ocular perfusion pressure falls below the lower limit of autoregulation or if neurovascular coupling fails. Evidence for both faulty autoregulation and reduced hyperemic response to neuronal stimulation has been provided in glaucoma patients. The mechanisms appear to involve vascular endothelial dysfunction and impaired astrocyte-vessel signaling. A more detailed understanding of these pathways is required to direct neuroprotective strategies via the neurovascular pathway.
Current Opinion in Pharmacology 2013, 13:36–42
This review comes from a themed issue on Neurosciences
Edited by Carlo Nucci, Nicholas G Strouthidis and Peng Tee Khaw
For a complete overview see the Issue and the Editorial
Available online 23rd September 2012
1471-4892/$ – see front matter, © 2012 Elsevier Ltd. All rights reserved.
Introduction
Glaucoma is a family of multifactorial optical neuropathies characterized by loss of retinal ganglion cells (RGCs) leading to typical optic nerve head (ONH) damage and distinctive visual field defects. Although the pathogenesis of the disease is unknown, it is well established that the main risk factor for glaucoma is elevated intraocular pressure (IOP). Reducing IOP is effective in slowing down the progression of the disease but some patients still progress despite adequately controlled IOP.
Several studies implicated vascular risk factors in the pathogenesis of glaucoma, blood pressure (BP) and ocular perfusion pressure (OPP) being the most studied. This vascular hypothesis is based on the premise that abnormal perfusion and the subsequent ischemia of the ONH play a major role in the glaucomatous damage. The OPP is the difference between arterial and venous BP. In the eye the venous pressure almost equals IOP. As such the OPP can be estimated as the difference between the arterial pressure and IOP. The relationship between BP and glaucoma is complex and controversial. On the one hand some studies indicate that systemic hypertension is a risk factor for glaucoma [1–3]. On the other hand some studies indicate that low systemic BP is a risk factor for development and progression of glaucoma. A direct and clear relationship between BP level and glaucomatous damage has, however, not been established [4]. The good irrigation of the ocular tissues is ensured by an adequate OPP depending on a complex regulation process that balances BP and the IOP. As such dealing with the concept of OPP and BP at the same time is more pertinent.
Large epidemiological studies have shown that low OPP is a risk factor for the prevalence, incidence and progression of glaucoma. In the Barbados Eye Study [5] low systolic BP doubled the risk for glaucoma incidence. Subjects with the lowest 20% of diastolic perfusion pressure were 3.3 times more likely to develop glaucoma. In the Proyecto VER Study [6] it was established that patients with a diastolic perfusion pressure as low as 45 mmHg had a three times greater risk of developing glaucoma compared to those with a diastolic perfusion pressure of 65 mmHg. This is in keeping with data from the Egna–Neumarkt Study [1] showing that the prevalence of the disease in patients with diastolic perfusion pressure less than 50 mmHg increases 4.5 fold compared to patients with diastolic perfusion pressure of 65 mmHg.
Data on glaucoma progression are available from The Early Manifest Glaucoma Trial (EMGT). Patients with low systolic perfusion pressure at baseline progressed faster than their counterparts. Low systolic perfusion pressure was a predictor of progression with an almost 50% higher risk [7]. More in depth reviews on the data linking OPP to glaucoma prevalence, incidence and progression have been provided [4,8,9•]. This review, however, will formulate some hypotheses as to why OPP is a risk factor.
Why is low OPP a risk factor for glaucoma?
Vascular factors have been identified in many chronic neurodegenerative disorders including Alzheimer's disease and amyotrophic lateral sclerosis [10••]. In glaucoma the hypothesis of a vascular involvement in the disease process has been formulated a long time ago. The evidence that low OPP is a risk factor for the disease has further supported this concept. Calculation of OPP as presented above is only an estimate of the true OPP. Indeed it assumes that mean arterial pressure (MAP) as measured at the brachial artery is a good measure of MAP at the level of the ophthalmic artery. To which degree this assumption is fulfilled, however, is not clear. In addition, IOP does not equal venous pressure in the eye. Obviously IOP is always slightly lower than pressure in retinal or choroidal veins [11,12]. In addition, there is evidence that the difference between venous pressure and IOP is increased in patients with glaucoma. This is related to the phenomenon of spontaneous venous pulsations in the central retinal vein, which appear to be a risk factor for glaucoma [13]. Hence our current way of estimating OPP involves both systematic and statistical errors. As such it is likely that the relationship between true OPP and glaucomatous damage is much stronger than indicated above.
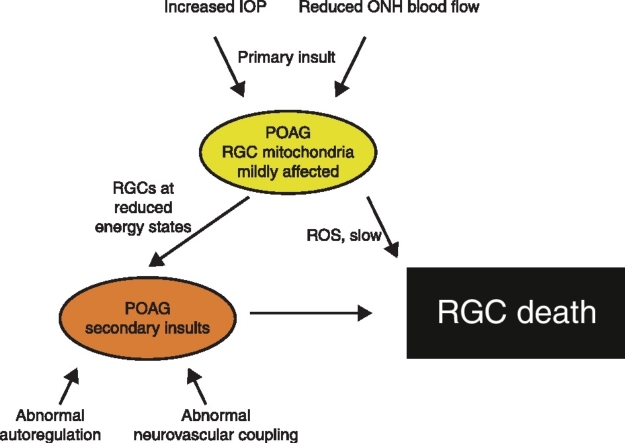
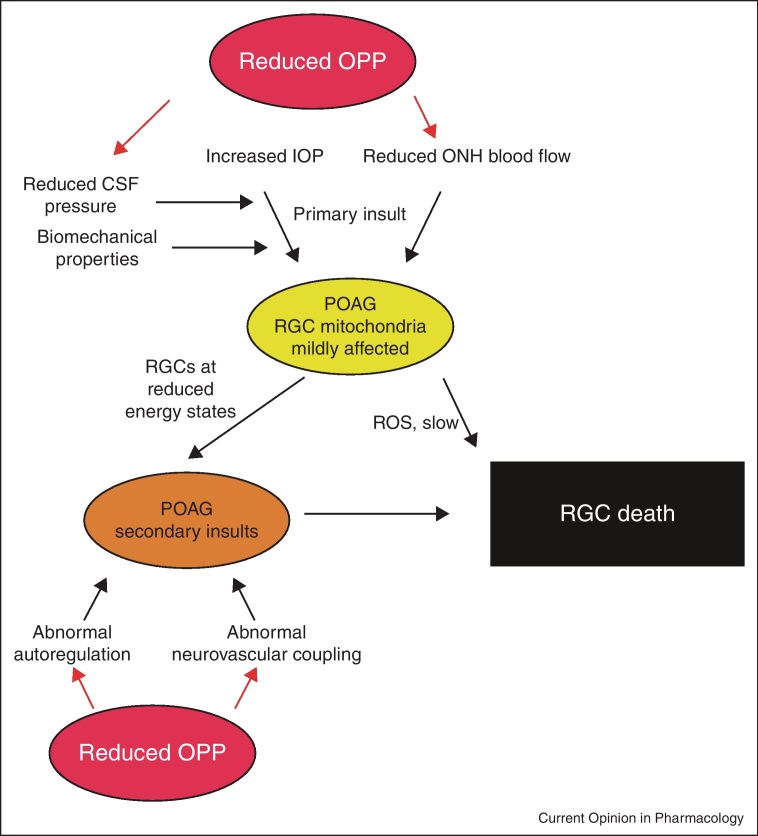
Whilst it is well-established that reduced OPP (as currently estimated) is a risk factor for glaucoma there is considerable controversy as to why this is the case. In the following we present a theory describing pathways that may depend on OPP and contribute to glaucomatous damage. As indicated in Figure 1 we assume that loss of RGCs is the consequence of primary and secondary insults as suggested previously [14•]. The site of primary insult to RGC axons in glaucoma is most likely within the ONH, more specifically at the lamina cribrosa. Increased IOP may be responsible for this loss of RGC axons modulated by the biomechanical properties of the ocular tissues [15,16,17••] and the level of cerebrospinal fluid pressure (CSF) [18–20]. As such, low CSF pressure may be one of the factors by which OPP modulates the risk of glaucoma because changes in either of them lead to a change in the trans-lamina cribrosa pressure gradient.
Figure 1.
A model of primary and secondary insults in glaucoma and the involvement of low ocular perfusion pressure (OPP). The primary insult occurs at the optic nerve head owing to increased intraocular pressure (IOP) and optic nerve head (ONH) ischemia. Reduced cerebrospinal fluid (CSF) pressure and the biomechanical properties of ocular tissues modify susceptibility. In primary open angle glaucoma (POAG) retinal ganglion cells (RGCs) then function at reduced energy states with affected mitochondria. Oxidative stress due to reactive oxygen species (ROS) and secondary insults such as abnormal autoregulation of abnormal neurovascular coupling can lead to RGC death.
Another factor in the primary glaucomatous insult that may be enhanced by low OPP is ONH ischemia associated with reduced flow of nutrients to the RGC axons. Some investigators assume that this ischemia at the ONH is primary and at least in some cases associated with systemic disease [21]. Another possibility is that IOP-related strain within the peri-papillary sclera affects perfusion through the scleral branches of the short posterior ciliary arteries [17••]. The vasculature of the ONH is complex and the post-laminar regions of the ONH are supplied by branches of the posterior ciliary arteries [22]. Owing to its deep anatomical location little is known about blood flow (BF) and its regulation in this part of the ONH. Most quantitative data about ONH BF regulation stem from the anterior ONH regions that are supplied by the central retinal artery [23•].
Once a primary insult has occurred at the level of the ONH RGCs appear to function at reduced energy levels with affected mitochondria. This is supported by numerous experimental data [14•,24] including electrophysiological studies in the primate revealing that affected RGCs retain some of their functionality [25]. As such neuroprotective strategies may be implemented to rescue injured RGCs independently of the primary insult. Indeed targeting mitochondria may offer a wide range of strategies for RGC survival including the dynamic processes of mitochondrial fission and fusion, the electron transport chain components, ion channels and defense strategies against oxidative stress.
Oxidative stress associated with extensive production of reactive oxygen species (ROS) such as free radicals, hydrogen peroxide, or singlet oxygen is another factor that may be enhanced by ischemia associated with low OPP. Free radicals are molecules containing unpaired electrons in their outer orbits. Singlet oxygen and hydrogen peroxide do not have unpaired electrons but are unstable and in a reactive state. ROS are constantly produced in cells. Oxidative stress occurs when the balance between production of ROS and endogenous antioxidative defense systems is disturbed either owing to increased ROS activity or owing to reduced antioxidative capacity. Ischemia and ischemia/reperfusion are classic triggers for ROS generation and oxidative stress. Oxidative stress offers a potential target for neuroprotection in glaucoma. Strategies may be wide and include inhibition of ROS formation, administration and/or supplementation with antioxidants or with agents that increase the reducing power necessary for ROS detoxification or stimulation of gene expression for increasing mitochondrial antioxidant defenses [26].
Another pathway in which reduced OPP may contribute is related to secondary insults associated with abnormal autoregulation or a breakdown in neurovascular coupling. Here it is assumed that RGCs that function at low energy states are susceptible to periods of ischemia or reduced nutritional support. Such periods can happen if OPP falls below the lower autoregulatory limit or if the functional hyperemic reaction to visual stimulation is dysfunctional in patients with glaucoma. These two possibilities are discussed in the next sections.
Autoregulation
Autoregulation is the ability of a vascular bed to maintain its BF despite changes in perfusion pressure. We have recently provided an in-depth review on the relation between OPP and ocular BF [27••] and as such only some of the aspects that are relevant for the present topic will be covered. Nowadays there is evidence that retinal, ONH and choroidal BF show some regulatory capacity in response to changes in OPP [22,27••,28]. Traditionally it is assumed that at the lower limit of autoregulation vessels are fully dilated. This is, however, not the case because the hyperemic vasodilator response to flicker stimulation is fully preserved even below the lower limit of autoregulation [29].
The mechanisms of autoregulation are complex and not fully understood. In the retina and the ONH it appears that autoregulation is strongly dependent on myogenic and metabolic mechanisms. In the choroid the rich parasympathetic, sympathetic and sensory innervation as well as intrinsic choroidal neurons plays a key role in BF regulation in face of changes in OPP [22].
In glaucoma abnormal autoregulation of ocular BF was observed in a large variety of studies. This includes experiments in which IOP was modified and studies in which the response to posture changes was assessed [27••]. Evidence for altered autoregulatory capacity in glaucoma also arises from group correlations between ocular BF and OPP [30–32].
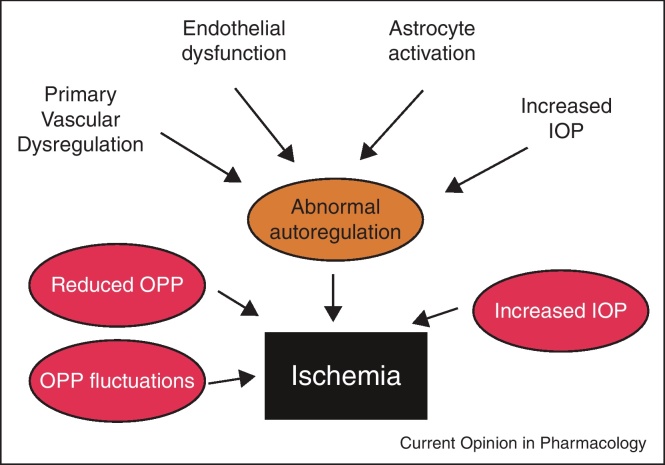
The reason for abnormal autoregulation in glaucoma patients is not fully understood (Figure 2). Obviously reduced OPP as well as increased OPP variability may lead to a fall of BF when the lower limit of autoregulation is reached [27••]. Increased variability of OPP and nocturnal BP dipping have indeed been identified as risk factors for glaucoma [33,34,35••].
Figure 2.
Abnormal autoregulation in glaucoma and factors that may contribute. Primary vascular dysregulation, endothelial dysfunction, astrocyte activation and increased intraocular pressure induce compromised autoregulation leading to abnormal pressure-flow relationship. Periods of ischemia are then more likely to occur when ocular perfusion pressure (OPP) is reduced, when OPP fluctuates and when intraocular pressure (IOP) is increased.
Evidence has accumulated that, at least in the choroid, BF regulates better when MAP is modified than when IOP is modulated. This means that at the same level of OPP BF may also depend on the absolute value of MAP and IOP. This phenomenon has already been described in the landmark paper by Kiel and Shepherd. The authors have developed a rabbit model in which MAP can be modulated by placing an occluder around the thoracic vena cava. This allows for measurement of choroidal BF while OPP is modified although IOP is held constant. Interestingly BF regulated better when IOP was kept constant at levels of 5 mmHg than at levels of 25 mmHg [36]. In recent years we have performed several studies indicating that this is also the case in humans [37•,38,39]. As such any reduction in IOP is associated with an improvement in BF regulation.
Alterations in autoregulation in glaucoma may also arise from a phenomenon called primary vascular dysregulation. This term was introduced by Flammer describing otherwise healthy subjects that show abnormal regulation in response to temperature changes and mechanical or emotional stress [40]. The basis for this dysregulation is not clear, but may be related to vascular endothelial dysfunction. Several observations indicate that endothelial dysfunction is associated with glaucoma [41,42•]. In addition, both endothelin and nitric oxide (NO) are key regulators of ONH and choroidal BF at baseline and during isometric exercise [43,44].
In the ONH there is evidence that glial cells play a role in autoregulatory processes. This may be related to loss of autoregulation in glaucoma, because astrocytes are considered to play a key role in tissue remodeling of the ONH [45]. It is, however, unclear how early this activation of astrocytes occurs although there is evidence that release of substances such as glutamate and tumor necrosis factor α from astrocytes is involved in RGC death [14•]. In the ONH astrocytes are involved in autoregulation during an increase in IOP, because the gliotoxic agent L-2-aminoadipic acid modifies the BF response during the decrease in OPP [46••].
Neurovascular coupling
In the brain and the retina BF increases when neurons get active, a response called functional hyperemia [47••]. This phenomenon called neurovascular coupling has attracted much interest because an abnormal BF response to neuronal stimulation causes cell death caused by inadequate nutrient supply. Our understanding of the mechanisms that underlie neurovascular coupling has increased significantly in the recent years. Astrocytes play a key role in mediating the vasodilator response associated with neural activity. Briefly, synaptically released glutamate activates N-methyl-d-aspartate receptors and metabotropic glutamate receptors in neurons and astrocytes, respectively [47••]. This leads to an increase in intracellular Ca2+ activating arachidonic acid pathways associated with the synthesis of vasodilators such as prostaglandins and epoxyeicosatrienoic acids and vasoconstrictors such as 20-hydroxy-eicosatetraenoic acid.
In addition NO synthesized from NO synthase-1 in neurons may play a role in the vasodilator response. Indeed, NO synthase inhibition blunts the retinal hyperemic response to flicker stimulation in humans [48]. Generally it is, however, believed that NO has a modulatory rather than a mediating role in the human retinal neurovascular coupling, because the activity of the enzymes in the arachidonic acid pathways depend on the level of NO [49•]. As such the hyperemic response may also depend on endothelial NO related to flow-mediated mechanisms [50].
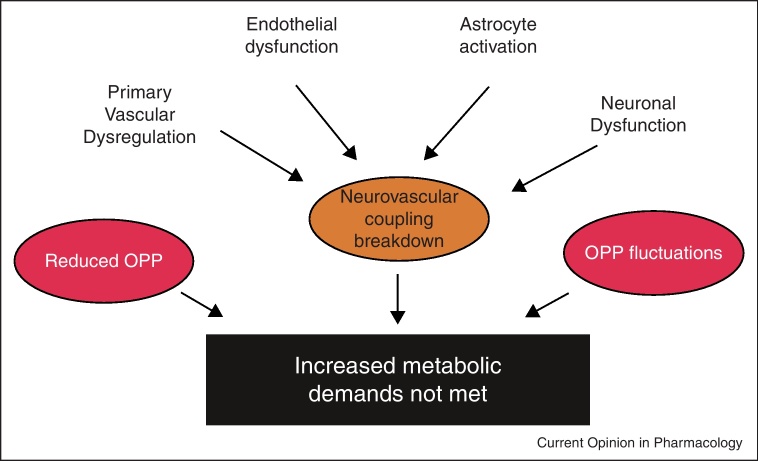
In glaucoma the response of retinal and ONH BF to flicker stimulation is reduced [51–53]. Factors contributing to the reduced hyperemic response in glaucoma are presented in Figure 3. Primary vascular dysregulation appears to be associated with abnormal retinal neurovascular coupling, because vasospastic subjects show a reduced response to flicker stimulation [54]. In keeping with this idea endothelial dysfunction may be also related to the altered responses seen in glaucoma [42•]. The level of IOP, however, does not appear to be directly related to the reduced hyperemic response, because short-term elevation does not influence flicker-induced vasodilatation [29]. Activated astrocytes in glaucoma are also potential sources of impaired vasodilator response to flicker stimulation, although this hypothesis remains unproven. Independently of the mechanisms contributing to reduced flicker responses in glaucoma it may well be that abnormal neurovascular coupling plays a role in the secondary insults to RGCs as shown in Figure 1.
Figure 3.
Abnormal neurovascular coupling in glaucoma and factors that may contribute. Primary vascular dysregulation, endothelial dysfunction, astrocyte activation and neuronal dysfunction induce compromised neurovascular coupling. This will lead to periods of reduced nutrient supply (including oxygen and glucose) during neuronal stimulation. Such periods are more likely to occur when ocular perfusion pressure (OPP) is reduced or when OPP fluctuates.
Conclusions and future directions
One of the reasons why our understanding of the relation between OPP and glaucoma is still limited lies in the difficulties to measure retinal and ONH BF [55–58]. Doppler optical coherence tomography may become a technique capable of measuring BF in a valid and reproducible way [59–61,62•]. This improvement in technology is associated with the hope of gaining more insight into ocular BF regulation.
Is it feasible to increase OPP as part of glaucoma treatment? Most probably this is not the case. On the one hand it is pharmacologically difficult to increase OPP. On the other hand one needs to be careful not to induce systemic hypertension with such approaches. An exception may be reducing anti-hypertensive treatment in patients with systemic hypertension in order to prevent very low OPPs [63]. When neuroprotective strategies are implemented it appears that it is not enough to focus on the pathways that are involved in programmed cell death of RGCs (Figure 4). Most probably neurovascular as well as neuroinflammatory pathways (not described in this review) need to be targeted as well [10••]. Generally the pathways leading to primary and secondary insults in glaucoma need to be better described to target the neurovascular component in glaucoma.
Figure 4.
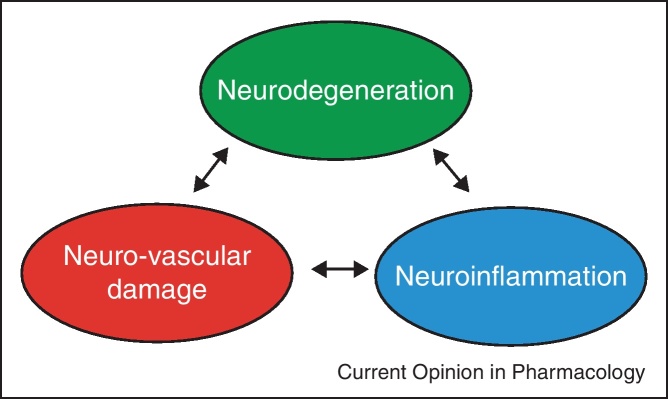
Neuroprotective strategies need not only to consider the neurodegenerative loss of RGCs, but also neurovascular and neuroinflammatory components.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Part of the experimental work mentioned in this article was supported by the following grants: Fonds zur Förderung der Wissenschaftlichen Forschung (FWF), Projects No. APP21570FW and APP21406FW, Die Österreichische Forschungsförderungsgesellschaft (FFG) project FA 607A0502, Christian Doppler Laboratory for Laser Development and their Application in Medicine.
References
- 1.Bonomi L., Marchini G., Marraffa M., Bernardi P., Morbio R., Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna–Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Lee A.J., Rochtchina E., Wang J.J. Open-angle glaucoma and systemic hypertension: the blue mountains eye study. J Glaucoma. 2004;13:319–326. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Orzalesi N., Rossetti L., Omboni S. Vascular risk factors in glaucoma: the results of a national survey. Graefes Arch Clin Exp Ophthalmol. 2007;245:795–802. doi: 10.1007/s00417-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 4.Leske M.C. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20:73–78. doi: 10.1097/ICU.0b013e32831eef82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leske M.C., Wu S.Y., Hennis A., Honkanen R., Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Quigley H.A., West S.K., Rodriguez J., Munoz B., Klein R., Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 7.Leske M.C., Heijl A., Hyman L., Bengtsson B., Dong L., Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Costa V.P., Arcieri E.S., Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93:1276–1282. doi: 10.1136/bjo.2008.149047. [DOI] [PubMed] [Google Scholar]
- 9•.He Z., Vingrys A.J., Armitage J.A., Bui B.V. The role of blood pressure in glaucoma. Clin Exp Optom. 2011;94:133–149. doi: 10.1111/j.1444-0938.2010.00564.x. [DOI] [PubMed] [Google Scholar]; A concise overview on the relation between blood pressure and glaucoma.
- 10••.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]; A summary of our current evidence for neurovascular involvement in neueodegenerative disease.
- 11.Glucksberg M.R., Dunn R. Direct measurement of retinal microvascular pressures in the live, anesthetized cat. Microvasc Res. 1993;45:158–165. doi: 10.1006/mvre.1993.1015. [DOI] [PubMed] [Google Scholar]
- 12.Maepea O. Pressures in the anterior ciliary arteries, choroidal veins and choriocapillaris. Exp Eye Res. 1992;54:731–736. doi: 10.1016/0014-4835(92)90028-q. [DOI] [PubMed] [Google Scholar]
- 13.Morgan W.H., Hazelton M.L., Balaratnasingamm C., Chan H., House P.H., Barry C.J., Cringle S.J., Yu D.Y. The association between retinal vein ophthalmodynamometric force change and optic disc excavation. Br J Ophthalmol. 2009;93:594–596. doi: 10.1136/bjo.2008.149963. [DOI] [PubMed] [Google Scholar]
- 14•.Osborne N.N. Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. doi: 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]; An in depth review of the role of mitochondrial damage in glaucomatous ganglion cell loss.
- 15.Hommer A., Fuchsjager-Mayrl G., Resch H., Vass C., Garhofer G., Schmetterer L. Estimation of ocular rigidity based on measurement of pulse amplitude using pneumotonometry and fundus pulse using laser interferometry in glaucoma. Invest Ophthalmol Vis Sci. 2008;49:4046–4050. doi: 10.1167/iovs.07-1342. [DOI] [PubMed] [Google Scholar]
- 16.Sigal I.A., Ethier C.R. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 17••.Burgoyne C.F. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93:120–132. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a concept of glaucoma pathogenesis with special reference to the biomechanical properties of ocular tissues.
- 18.Morgan W.H., Yu D.Y., Balaratnasingam C. The role of cerebrospinal fluid pressure in glaucoma pathophysiology: the dark side of the optic disc. J Glaucoma. 2008;17:408–413. doi: 10.1097/IJG.0b013e31815c5f7c. [DOI] [PubMed] [Google Scholar]
- 19.Berdahl J.P., Allingham R.R., Johnson D.H. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115:763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Ren R., Wang N., Zhang X., Cui T., Jonas J.B. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:1057–1063. doi: 10.1007/s00417-011-1657-1. [DOI] [PubMed] [Google Scholar]
- 21.Pache M., Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv Ophthalmol. 2006;51:179–212. doi: 10.1016/j.survophthal.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Riva C.E., Schmetterer L. Microcirculation of the ocular fundus. In: Tuma R.F., Durán W.N., Ley K., editors. Handbook of Physiology: Microcirculation. edn 2. Academic Press; 2008. pp. 735–765. [Google Scholar]
- 23•.Schmidl D., Boltz A., Kaya S., Werkmeister R., Dragostinoff N., Lasta M., Polska E., Garhofer G., Schmetterer L. Comparison of choroidal and optic nerve head blood flow regulation during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2012;53:4337–4346. doi: 10.1167/iovs.11-9055. [DOI] [PubMed] [Google Scholar]; A study indicating that there are considerable differences in choroidal and optic nerve head blood flow autoregulation during both an increase and a decrease in perfusion pressure.
- 24.Lee S., Van Bergen N.J., Kong G.Y., Chrysostomou V., Waugh H.S., O’Neill E.C., Crowston J.G., Trounce I.A. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011;93:204–212. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Morgan J.E. Retina ganglion cell degeneration in glaucoma: an opportunity missed? A review. Clin Experiment Ophthalmol. 2012;40:364–368. doi: 10.1111/j.1442-9071.2012.02789.x. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Pinzon M.A., Stetler R.A., Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Schmidl D., Garhofer G., Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow – relevance for glaucoma. Exp Eye Res. 2011;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]; A review of the current understanding of autoregulation in ocular vascular beds.
- 28.Schmidl D., Boltz A., Kaya S., Werkmeister R., Dragostinoff N., Lasta M., Polska E., Garhofer G., Schmetterer L. Comparison of choroidal and optic nerve head blood flow regulation during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2012;53:4337–4346. doi: 10.1167/iovs.11-9055. [DOI] [PubMed] [Google Scholar]
- 29.Garhofer G., Resch H., Weigert G., Lung S., Simader C., Schmetterer L. Short-term increase of intraocular pressure does not alter the response of retinal and optic nerve head blood flow to flicker stimulation. Invest Ophthalmol Vis Sci. 2005;46:1721–1725. doi: 10.1167/iovs.04-1347. [DOI] [PubMed] [Google Scholar]
- 30.Gherghel D., Orgul S., Gugleta K., Gekkieva M., Flammer J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol. 2000;130:597–605. doi: 10.1016/s0002-9394(00)00766-2. [DOI] [PubMed] [Google Scholar]
- 31.Fuchsjager-Mayrl G., Wally B., Georgopoulos M., Rainer G., Kircher K., Buehl W., Amoako-Mensah T., Eichler H.G., Vass C., Schmetterer L. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45:834–839. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 32.Garhofer G., Fuchsjager-Mayrl G., Vass C., Pemp B., Hommer A., Schmetterer L. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Invest Ophthalmol Vis Sci. 2010;51:6652–6657. doi: 10.1167/iovs.10-5490. [DOI] [PubMed] [Google Scholar]
- 33.Tokunaga T., Kashiwagi K., Tsumura T., Taguchi K., Tsukahara S. Association between nocturnal blood pressure reduction and progression of visual field defect in patients with primary open-angle glaucoma or normal-tension glaucoma. Jpn J Ophthalmol. 2004;48:380–385. doi: 10.1007/s10384-003-0071-6. [DOI] [PubMed] [Google Scholar]
- 34.Choi J., Kim K.H., Jeong J., Cho H.S., Lee C.H., Kook M.S. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2007;48:104–111. doi: 10.1167/iovs.06-0615. [DOI] [PubMed] [Google Scholar]
- 35••.Sung K.R., Lee S., Park S.B., Choi J., Kim S.T., Yun S.C., Kang S.Y., Cho J.W., Kook M.S. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci. 2009;50:5266–5274. doi: 10.1167/iovs.09-3716. [DOI] [PubMed] [Google Scholar]; A study showing that ocular perfusion pressure fluctuation is an important risk factor for glaucoma progression.
- 36.Kiel J.W., Shepherd A.P. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1992;33:2399–2410. [PubMed] [Google Scholar]
- 37•.Polska E., Simader C., Weigert G., Doelemeyer A., Kolodjaschna J., Scharmann O., Schmetterer L. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]; The first human data indicating that blood flow may not only depend on ocular perfusion pressure but also on the level of intraocular pressure and blood pressure.
- 38.Fuchsjager-Mayrl G., Georgopoulos M., Hommer A., Weigert G., Pemp B., Vass C., Garhofer G., Schmetterer L. Effect of dorzolamide and timolol on ocular pressure: blood flow relationship in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2010;51:1289–1296. doi: 10.1167/iovs.09-3827. [DOI] [PubMed] [Google Scholar]
- 39.Boltz A., Schmidl D., Weigert G., Lasta M., Pemp B., Resch H., Garhofer G., Fuchsjager-Mayrl G., Schmetterer L. Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Invest Ophthalmol Vis Sci. 2011;52:4410–4415. doi: 10.1167/iovs.11-7263. [DOI] [PubMed] [Google Scholar]
- 40.Flammer J., Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317–321. doi: 10.3129/i08-056. [DOI] [PubMed] [Google Scholar]
- 41.Polak K., Luksch A., Berisha F., Fuchsjaeger-Mayrl G., Dallinger S., Schmetterer L. Altered nitric oxide system in patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:494–498. doi: 10.1001/archopht.125.4.494. [DOI] [PubMed] [Google Scholar]
- 42•.Resch H., Garhofer G., Fuchsjager-Mayrl G., Hommer A., Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]; A review summarizing our current knowledge of endothelial dysfunction in glaucoma.
- 43.Luksch A., Polska E., Imhof A., Schering J., Fuchsjager-Mayrl G., Wolzt M., Schmetterer L. Role of NO in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci. 2003;44:734–739. doi: 10.1167/iovs.02-0177. [DOI] [PubMed] [Google Scholar]
- 44.Fuchsjager-Mayrl G., Luksch A., Malec M., Polska E., Wolzt M., Schmetterer L. Role of endothelin-1 in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci. 2003;44:728–733. doi: 10.1167/iovs.02-0372. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez M.R. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 46••.Shibata M., Sugiyama T., Kurimoto T., Oku H., Okuno T., Kobayashi T., Ikeda T. Involvement of glial cells in the autoregulation of optic nerve head blood flow in rabbits. Invest Ophthalmol Vis Sci. 2012;53:3726–3732. doi: 10.1167/iovs.11-9316. [DOI] [PubMed] [Google Scholar]; The first study indicating a role for astrocyte involvement in ocular blood flow autoregulation.
- 47••.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reviews our current understanding of cerebral neurovascular coupling.
- 48.Dorner G.T., Garhofer G., Kiss B., Polska E., Polak K., Riva C.E., Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- 49•.Kur J., Newman E.A., Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of ocular blood flow regulation with special emphasis on neurovascular mechanisms.
- 50.Pemp B., Weigert G., Karl K., Petzl U., Wolzt M., Schmetterer L., Garhofer G. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care. 2009;32:1536–1541. doi: 10.2337/dc08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garhofer G., Zawinka C., Resch H., Huemer K.H., Schmetterer L., Dorner G.T. Response of retinal vessel diameters to flicker stimulation in patients with early open angle glaucoma. J Glaucoma. 2004;13:340–344. doi: 10.1097/00061198-200408000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Riva C.E., Logean E., Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Gugleta K., Kochkorov A., Waldmann N., Polunina A., Katamay R., Flammer J., Orgul S. Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefes Arch Clin Exp Ophthalmol. 2012;250:589–594. doi: 10.1007/s00417-011-1842-2. [DOI] [PubMed] [Google Scholar]
- 54.Gugleta K., Zawinka C., Rickenbacher I., Kochkorov A., Katamay R., Flammer J., Orgul S. Analysis of retinal vasodilation after flicker light stimulation in relation to vasospastic propensity. Invest Ophthalmol Vis Sci. 2006;47:4034–4041. doi: 10.1167/iovs.06-0351. [DOI] [PubMed] [Google Scholar]
- 55.Sugiyama T., Araie M., Riva C.E., Schmetterer L., Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88:723–729. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 56.Riva C.E., Geiser M., Petrig B.L. Ocular blood flow assessment using continuous laser Doppler flowmetry. Acta Ophthalmol. 2010;88:622–629. doi: 10.1111/j.1755-3768.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 57.Garhofer G., Bek T., Boehm A.G., Gherghel D., Grunwald J., Jeppesen P., Kergoat H., Kotliar K., Lanzl I., Lovasik J.V. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010;88:717–722. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 58.Stalmans I., Vandewalle E., Anderson D.R., Costa V.P., Frenkel R.E., Garhofer G., Grunwald J., Gugleta K., Harris A., Hudson C. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011;89:e609–e630. doi: 10.1111/j.1755-3768.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- 59.Werkmeister R.M., Dragostinoff N., Pircher M., Gotzinger E., Hitzenberger C.K., Leitgeb R.A., Schmetterer L. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008;33:2967–2969. doi: 10.1364/ol.33.002967. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Lu A., Gil-Flamer J., Tan O., Izatt J.A., Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93:634–637. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumann B., Potsaid B., Kraus M.F., Liu J.J., Huang D., Hornegger J., Cable A.E., Duker J.S., Fujimoto J.G. Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed Opt Express. 2011;2:1539–1552. doi: 10.1364/BOE.2.001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Werkmeister R.M., Dragostinoff N., Palkovits S., Told R., Boltz A., Leitgeb R.A., Gröschl M., Garhöfer G., Schmetterer L. Measurement of absolute blood flow velocity and blood flow in the human retina by dual-beam bidirectional Doppler Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:6062–6071. doi: 10.1167/iovs.12-9514. [DOI] [PubMed] [Google Scholar]; A study showing that Doppler OCT is a valid method for measuring retinal blood flow.
- 63.Topouzis F., Coleman A.L., Harris A., Jonescu-Cuypers C., Yu F., Mavroudis L., Anastasopoulos E., Pappas T., Koskosas A., Wilson M.R. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: the Thessaloniki eye study. Am J Ophthalmol. 2006;142:60–67. doi: 10.1016/j.ajo.2006.02.055. [DOI] [PubMed] [Google Scholar]