Highlights
► Determination of subcellular glutathione concentrations in different Arabidopsis leaf areas. ► Measurement of cell volumes and glutathione gold particle density by TEM. ► Glutathione concentrations of up to 15 mM were calculated for mitochondria. ► Main differences of glutathione contents between the leaf areas in vacuoles and mitochondria.
Keywords: Arabidopsis, Glutathione, Mitochondria, Transmission electron microscopy
Abstract
Glutathione is an important antioxidant and redox buffer in plants. It fulfills many important roles during plant development, defense and is essential for plant metabolism. Even though the compartment specific roles of glutathione during abiotic and biotic stress situations have been studied in detail there is still great lack of knowledge about subcellular glutathione concentrations within the different leaf areas at different stages of development.
In this study a method is described that allows the calculation of compartment specific glutathione concentrations in all cell compartments simultaneously in one experiment by using quantitative immunogold electron microscopy combined with biochemical methods in different leaf areas of Arabidopsis thaliana Col-0 (center of the leaf, leaf apex, leaf base and leaf edge). The volume of subcellular compartments in the mesophyll of Arabidopsis was found to be similar to other plants. Vacuoles covered the largest volume within a mesophyll cell and increased with leaf age (up to 80% in the leaf apex of older leaves). Behind vacuoles, chloroplasts covered the second largest volume (up to 20% in the leaf edge of the younger leaves) followed by nuclei (up to 2.3% in the leaf edge of the younger leaves), mitochondria (up to 1.6% in the leaf apex of the younger leaves), and peroxisomes (up to 0.3% in the leaf apex of the younger leaves). These values together with volumes of the mesophyll determined by stereological methods from light and electron micrographs and global glutathione contents measured with biochemical methods enabled the determination of subcellular glutathione contents in mM.
Even though biochemical investigations did not reveal differences in global glutathione contents, compartment specific differences could be observed in some cell compartments within the different leaf areas. Highest concentrations of glutathione were always found in mitochondria, where values in a range between 8.7 mM (in the apex of younger leaves) and 15.1 mM (in the apex of older leaves) were found. The second highest amount of glutathione was found in nuclei (between 5.5 mM and 9.7 mM in the base and the center of younger leaves, respectively) followed by peroxisomes (between 2.6 mM in the edge of younger leaves and 4.8 mM in the base of older leaves, respectively) and the cytosol (2.8 mM in the edge of younger and 4.5 mM in the center of older leaves, respectively). Chloroplasts contained rather low amounts of glutathione (between 1 mM and 1.4 mM). Vacuoles had the lowest concentrations of glutathione (0.01 mM and 0.14 mM) but showed large differences between the different leaf areas. Clear differences in glutathione contents between the different leaf areas could only be found in vacuoles and mitochondria revealing that glutathione in the later cell organelle accumulated with leaf age to concentrations of up to 15 mM and that concentrations of glutathione in vacuoles are quite low in comparison to the other cell compartments.
1. Introduction
The tripeptide glutathione (γ-glutamyl-cysteinyl-glycine) is the major non-protein thiol in plant cells and is present in many cellular compartments at millimolar concentrations (Diaz Vivancos et al., 2010a,b). As an antioxidant it is involved in detoxifying reactive oxygen species (ROS) through the ascorbate-glutathione cycle (Foyer and Noctor, 2009, 2011; Noctor et al., 2012). Glutathione is also involved in the detoxification of xenobiotics, herbicides (Cummins et al., 2011; DeRidder and Goldsbrough, 2006; Edwards et al., 2005; Mohsenzadeh et al., 2011) and heavy metals (Ammar et al., 2008; DalCorso et al., 2008; Jozefczak et al., 2012; Nocito et al., 2008; Seth et al., 2012; Tan et al., 2010; Zawoznik et al., 2007) and protects proteins from oxidation through glutathionylation (Zaffagnini et al., 2012a,b). Additionally, glutathione is a key regulator of redox signaling which controls gene expression and contributes to cell survival (Foyer et al., 2001; Maughan and Foyer, 2006). Glutathione is also essential for proper plant development. The glutathione deficient Arabidopsis mutant rml1 which contains up to 97% less glutathione than the wildtype lacks a root meristem and develops severe growth defects (Cairns et al., 2006; Vernoux et al., 2000; Zechmann and Müller, 2010). Additionally, it has been shown recently that pollen grains with inhibited glutathione synthesis resulting in low glutathione contents during germination show a strong decrease in germination rates (Zechmann et al., 2011a,b). High and stable levels of glutathione in mitochondria have been found to be of significant importance in Arabidopsis as the glutathione deficient pad2-1 mutant which shows a phenotype similar to the wildtype (Zechmann et al., 2008) contains control levels of glutathione in mitochondria despite a drop of glutathione contents in all other cell compartments of up to 90%.
Generally, the compartmentation of metabolites in different compartments, tissues and organs is of great importance for many physiological processes and metabolic regulation (Bowsher and Tobin, 2001; Hartmann et al., 2003). To get a greater understanding of the function of metabolites, such as glutathione, it is important to analyze the subcellular localization and measure compartment specific concentrations. There are different approaches which have given more detailed insight about the subcellular distribution of glutathione. It has been measured with biochemical methods (high performance liquid chromatography; HPLC) in different compartments (like mitochondria, chloroplasts, peroxisomes, apoplast, and vacuoles) after isolation and fractionation (Jiménez et al., 1997, 1998; Krueger et al., 2009; Kuźniak and Sklodowska, 2001, 2004, 2005; Ohkama-Ohtsu et al., 2007; Vanacker et al., 1998a,b,c). By using these methods glutathione has been detected at a concentration between 0.5 and 5 mM in chloroplasts (Foyer and Halliwell, 1976; Noctor et al., 2002; Krueger et al., 2009), between 1 and 3.52 mM in the cytosol (Noctor et al., 2002; Krueger et al., 2009) and 0.73 mM in the vacuole (Krueger et al., 2009). Nevertheless, biochemical methods face the problems that large amounts of plant material are needed for the isolation of organelles, and that it is not possible to isolate all cell compartments simultaneously in one experiment. Additionally, as postulated by Krueger et al. (2009) the isolation procedure can also lead to a loss of glutathione from organelles and to contaminations of non-organelle specific substances. Thus, it remains unclear how well the obtained results reflect situations in vivo (Chew et al., 2003; Krueger et al., 2009; Noctor et al., 2002). Other methods for the detection of glutathione include light microscopy, and staining of glutathione with monochloro- or monobromobimane (Fricker et al., 2000; Meyer and Fricker, 2000; Müller et al., 2005). By using these methods glutathione could be detected in nuclei, cytosol and vacuoles (Meyer et al., 2007; Müller et al., 2005). The concentration of glutathione in the cytosol was estimated to be between 2.7 and 3.2 mM (Meyer et al., 2001). However, these studies were limited by a resolution of about 200 nm and the inability of the stains to infiltrate certain organelles. Thus, it was not possible to obtain more information about the localization of glutathione in plastids, peroxisomes, mitochondria, dictyosomes and endoplasmic reticulum (ER) and to quantify compartment specific differences with these methods. Ratiometric redox-sensitive green fluorescence protein (GFP) expressed in Arabidopsis thaliana (Gutscher et al., 2008; Meyer et al., 2007) has been used recently to detect glutathione in the cytosol and also in smaller organelles such as the ER as well as changes in glutathione contents in the cytosol during environmental stress situations (Jubany-Marí et al., 2010). Additionally, this method has been used to analyze the redox state of the cytosol and other cell compartments (Gutscher et al., 2008; Maughan et al., 2010; Maughan and Foyer, 2006; Meyer et al., 2007). Nevertheless, these methods are limited by the fact that it is difficult to investigate the situation in deeper cell layers (mesophyll) or tissues (e.g. vascular cells) due to background fluorescence and therefore it remains unclear how changes observed in epidermal cells, isolated cells or cell cultures reflect the situation in other cell layers. Additionally, it has not been possible so far and will remain technically challenging to measure changes in glutathione contents in all cell compartments simultaneously in one experiment and to translate the obtained signal into compartment specific glutathione concentrations. One powerful method that has been developed recently is the study of the subcellular distribution of glutathione based on immunogold-cytochemistry and computer-supported transmission electron microscopy which allows the simultaneous detection of glutathione in all cell compartments at a high level of resolution (Queval et al., 2011; Zechmann et al., 2006, 2008; Zechmann and Müller, 2010). This method has been applied on different plant species and revealed that the highest amount of glutathione was always detected in mitochondria, followed by nuclei, peroxisomes, the cytosol and plastids. None or very little glutathione was detected in vacuoles and the apoplast (Zechmann and Müller, 2010).
Many studies showed differences in glutathione contents either between organs or cell compartments, but the subcellular distribution in different leaf areas as well as compartment specific glutathione concentrations throughout the leaf remain unclear. Such information could be essential in order to study differences in the stress response between different leaf areas, during leaf development, and in the accumulation of glutathione in certain leaf areas. Thus, the aim of the present study was to develop a method that allows the determination of compartment specific glutathione concentrations in all cell compartments simultaneously in one experiment. This method was further used to determine differences in the subcellular distribution of glutathione in A. thaliana Col-0 leaves between different stages of development (older and youngest fully developed leaves) and different leaf areas (center of the leaf, leaf apex, leaf base, and leaf edge). To analyze the subcellular distribution of glutathione quantitative immunogold-cytochemistry was combined with biochemical measurements of global glutathione contents (LC/MS/MS) in order to measure compartment specific concentrations of glutathione. Together with the mesophyll volume, which was analyzed by light microscopy, fresh and dry weight of the particular leaf area, and the relative compounds of the compartments the concentration was calculated in mM. Some information about the mesophyll volume has already been determined in other studies with species such as barley (Winter et al., 1993), spinach (Winter et al., 1994) and wheat (Bowsher and Tobin, 2001; Hopkins, 1997) but data is still missing for Arabidopsis leaves. Thus, the described method gives compartment specific glutathione concentrations in mM on a high level of resolution by a combination of stereological, quantitative cytohistochemical and biochemical methods.
2. Materials and methods
2.1. Plant material and growth conditions
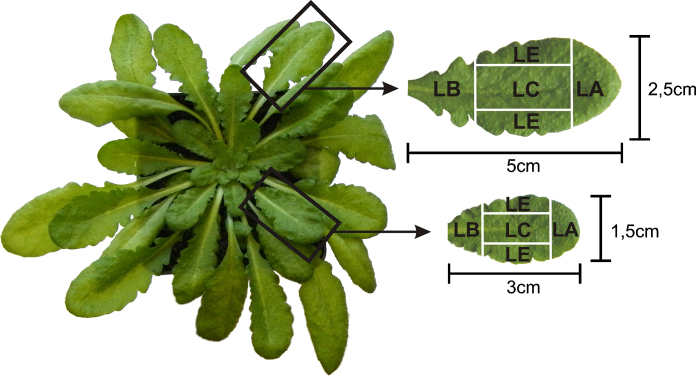
After stratification for 4 days at 4 °C seeds of A. thaliana accession Col-0 from the European Arabidopsis stock centre (NASC, Loughborough, UK) were grown on soil in growth chambers with a 9/15 h day/night photoperiod. The temperatures during the days were 22 °C and 18 °C during the night. While the relative humidity was 60% the plants were kept at 100% relative soil water content. Light intensity was 150 μmol m−2 s−1. Six weeks after stratification samples of different leaf areas from older leaves (5 cm × 2.5 cm from the second rosette after cotyledons) and youngest fully developed leaves (3 cm × 1.5 cm from the youngest fully developed rosette) were harvested 2 h after the onset of the light period (see Fig. 1 for details about leaf area and size). Harvesting of the samples was performed in the growth chambers. The experiments were performed twice and the results represent a mixture of two independent experimental setups.
Fig. 1.
Different leaf areas from Arabidopsis thaliana Col-0 used in this study. LC = leaf center, LA = leaf apex, LB = leaf base and LE = leaf edge.
2.2. Sample preparation for transmission electron microscopy
Samples of about 1 mm2 from the different leaf areas (leaf center, leaf apex, leaf base and leaf edge) were cut on a modeling wax plate in a drop of fixative solution. Samples for ultrastructural investigations were cut in a drop of 2.5% glutardialdehyde in 0.06 M Sørensen phosphate buffer (pH 7.2), whereas samples for cytohistochemical analysis in 2.5% paraformaldehyde/0.5% glutardialdehyde in 0.06 M Sørensen phosphate buffer (pH 7.2). Samples were then transferred into glass vials with the same solutions mentioned above and fixed for 90 min at room temperature (RT). Samples for ultrastructural analysis were rinsed 4 times for 10 min in phosphate buffer and transferred into a 1% osmium tetroxide solution in 0.06 M Sørensen phosphate buffer for 90 min. Then the samples were washed for 4 times each 10 min and where dehydrated in increasing concentrations of acetone (50%, 70%, 90% and 100%) for 20 min per step at RT. Pure acetone was then exchanged with propylene oxide and the samples were infiltrated for a minimum of 3 h per step with increasing concentrations of Agar 100 epoxy resin (30%, 60% and 100%) mixed with propylene oxide. In the last step the samples were transferred into fresh Agar 100 epoxy resin (Agar Scientific Ltd., Stansted, UK) and polymerized for 48 h at 60 °C. Ultrathin sections (80 nm) for ultrastructural investigations were cut with a Reichert Ultracut S ultramicrotome (Leica, Microsystems) and post-stained for 5 min with lead citrate and 15 min with uranyl acetate.
The specimens for immunogold analysis were dehydrated with increasing concentrations of acetone after fixation and were then transferred from 90% acetone to increasing concentrations of LR-White resin (30%, 60%, 100%; London Resin Company Ltd., Berkshire, UK) mixed with acetone (90%) for at least 3 h at each step at RT. Finally, the samples were embedded in pure, fresh LR-White resin and polymerized at 50 °C for 48 h in small plastic cups under anaerobic conditions.
2.3. Determination of cell volumes and cellular volumes
To determine cell volumes the ultrastructure was investigated in a Philips CM 10 transmission electron microscope (FEI, Eindhoven, Netherlands) and images of randomly chosen whole cells at 1650-fold magnification were taken. The relative content for mitochondria, chloroplasts, nuclei, peroxisomes, cytosol, vacuoles and cell walls was evaluated using the image analysis program Cell D (Olympus, Life and Material Science Europe GmbH, Hamburg, Germany; Table 1). These data were used to determine the relative subcellular volumes, rcomp, according to the principle of Delesse: “The areal density of profiles on sections is an unbiased estimate of the volume density of structures” (Weibel and Bolender, 1973; Winter et al., 1993). The total volume of mesophyll cells was determined by light microscopy of embedded samples by measuring the aquatic volume (defined as all cellular tissues without intercellular spaces) and subtracting the amount of epidermis cells, vascular bundle cells and the cell wall (Table 1). For this purpose images were taken with an OLYMPUS Provis AX-70 light microscope (Olympus Optical Co. Ltd., Tokyo) from randomly chosen sections of embedded plant material at 40-fold magnification (Olympus Uplan Apo 40-fold objective lens). The relative content of mesophyll cells as well as the relative water content (difference between fresh and dry weight, Table 1) were calculated to determine the mesophyll volume in μl/g FW for Arabidopsis leaves.
Table 1.
Structural parameters of older (O) and youngest fully developed leaves (Y; LC = center of the leaf, LA = leaf apex, LB = leaf base, LE = leaf edge) of Arabidopsis thaliana Col-0. Mesophyll volume was determined from light micrographs of embedded plant material. Data for ultrastructural investigations was determined from transmission electron micrographs. P < 0.05 was regarded as significant as analyzed by Kruskal–Wallis test followed by post hoc comparison according to Conover. Statistical differences were calculated for each cell compartment in the different leaf areas from either older or younger leaves. Different lower case letters indicate significant differences. n > 20 for peroxisomes and nucleus and n > 60 for other structures.
| OLC | OLA | OLB | OLE | YLC | YLA | YLB | YLE | |
|---|---|---|---|---|---|---|---|---|
| Structural leaf parameters | ||||||||
| Fresh weight (mg) | 240.7 | 156.0 | 151.2 | 147.8 | 63.7 | 40.9 | 40.2 | 38.7 |
| Dry weight (mg) | 11.4 | 10.8 | 9.7 | 11.2 | 4.8 | 4.0 | 5.4 | 4.7 |
| Mesophyll volume (μl/g fresh weight) | 697.68 | 691.08 | 644.43 | 577.54 | 621.15 | 606.70 | 582.27 | 589.93 |
| Relative compartment volume rcomp (%) | ||||||||
| Mitochondria | 0.47a | 0.49a | 0.48a | 0.49a | 0.48c | 1.57a | 1.20b | 1.10b |
| Chloroplasts | 15.63a | 14.03a | 14.23a | 14.51a | 17.13b | 16.26b | 19.58a | 19.95a |
| Nuclei | 0.47c | 0.71b | 0.16d | 1.32a | 0.67c | 1.56ab | 1.93ab | 2.28a |
| Peroxisomes | 0.14a | 0.12a | 0.14a | 0.06a | 0.14c | 0.28a | 0.19bc | 0.25b |
| Cytosol | 4.10c | 4.70bc | 5.49ab | 5.88a | 3.77b | 5.69a | 6.80a | 7.03a |
| Vacuole | 79.19a | 79.95a | 79.50a | 77.74a | 77.81a | 74.64ab | 70.30b | 69.39b |
2.4. Immunogold labeling of glutathione
Ultrathin sections of about 80 nm were blocked with 2% bovine serum albumin (BSA; Sigma–Aldrich) in phosphate buffered saline (pH 7.2). Subsequently the samples were treated with the primary antibodies (anti-glutathione rabbit polyclonal IgG, Millipore Corp., Billerica, Ma, USA) diluted 1:50 in phosphate buffer with 1% goat serum for 2 h at RT. Then the specimens were rinsed with 1% BSA and treated with a 10 nm gold-conjugated secondary antibody (goat anti-rabbit IgG, British BioCell International, Cardiff, UK) diluted 1:50 in phosphate buffer for 90 min at RT. Next the samples were washed two times in distilled water and post-stained with uranyl acetate for 15 s.
Afterwards the samples were analyzed with a Philips CM10 transmission electron microscope where micrographs of randomly photographed immunogold-labeled sections at 8900-fold magnification were digitized and gold particles were counted automatically using the software package Cell D with the particle analysis tool in manually identified cell structures (mitochondria, plastids, nuclei, peroxisomes, cytosol, vacuole and cell wall).
2.5. Direct and simultaneous determination of reduced and oxidized glutathione by liquid chromatography-electrospray/mass spectrometry
Additionally to the investigation of the subcellular distribution of glutathione the glutathione content was determined biochemically according to Rellán-Álvarez et al. (2006). Therefore, the different leaf areas were cut out from the whole leaves and the fresh weight of these samples was determined before they were frozen in liquid nitrogen. The middle vein was cut out from the leaf parts taken from the center of the leaves before determination of the fresh weight and freezing. The frozen leaf parts were thoroughly fine-ground with a micro-mortar in liquid N2, and the plant material was immediately homogenized with 1 mL of cold (4 °C) extraction solution (5% (w/v) metaphosphoric acid (MPA), 1 mM EDTA in 0.1% formic acid) supplemented with 1% (m/v) polyvinyl–polypyrrolidone just before use. The homogenates were centrifuged at 15,000 × g for 20 min at 4 °C. The supernatants were transferred to single-use syringes while the pellets were resuspended and extracted again with 0.5 mL of extraction solution under the same conditions. Supernatants were combined and filtered through 0.2 μm polyvinylidene fluoride filters and immediately analyzed or stored at −80 °C until analysis.
2.5.1. Standard solutions
Stock solutions containing 10 mM reduced glutathione and 10 mM oxidized glutathione (both purchased from AppliChem, Darmstadt, Germany) were prepared in a solution containing 2.5% (w/v) MPA, 1 mM EDTA, and 0.1% formic acid. Aliquots were stored at −80 °C and only thawed once to prepare the standards for the calibration curve. Eight standards were mixed containing 10–20–30–50–70–80–90–100 μmol L−1 reduced glutathione and 0.1–0.2–0.5–1.0–1.5–2.0–3.0–5.0 μmol L−1 oxidized glutathione. Standards were again prepared in 2.5% MPA-solution containing 1 mM EDTA and 0.1% formic acid.
2.5.2. Equipment
Chromatography was performed using an Agilent 1200 high pressure liquid chromatography (HPLC) system comprised of a G1312A binary pump, a G1379 B online vacuum degasser, a G1367B autosampler, a G1330B autosampler thermostat and a G1316A heated column compartment (Wilmington, DE, USA). The detector for the system was an Applied Biosystems MDS Sciex 4000 Q-Trap LC/MS/MS system with an electro-spray ionization (ESI) source (Toronto, ON, Canada). Data acquisition, peak integration and calculation were assigned to a computer work station running Analyst™ Software 1.4.2.
Chromatographic separation was performed on a Kinetex-C18 column (150 mm × 3 mm, 2.6 μm) (Phenomenex, Torrance, CA, USA) protected by a similar phase guard column (2.1 mm × 4.6 mm). 10 μL of the sample was injected at a flow rate of 0.4 mL min−1, under isocratic conditions (1 mM ammonium acetate, 3% acetonitrile, 0.5% formic acid). The column oven and auto-injector sample tray were maintained at 25 and 6 °C, respectively.
Mass spectrometric data were acquired in positive ion mode with the following ESI-MS parameters: ion spray voltage: 5500 V; temperature: 600 °C; curtain gas (N2): 35; ion source gas 1 (N2): 75; ion source gas 2 (N2): 65. The entrance potential was set to 10 V. The optimized instrument settings are shown in Supplementary Table 1. Quadrupole one and three were set to unit resolution. Data were recorded in multiple reactions monitoring (MRM) mode. The following transitions were monitored: m/z 308.1 to 76.1, 162.1 and 179 for reduced glutathione and m/z 613.2 to 355.1, 484.1 and 231.0 for oxidized glutathione. The underlined transition was used for quantification the other two transitions served as qualifier.
The LC/MS/MS runtime was 13 min.
2.6. Data calculations
The compartment volumes Vcomp in μl/g FW were calculated by multiplying the relative compartment volume rcomp (principle of Delesse) with the mesophyll volume. Subsequently, the relative glutathione content per compartment, gcomp, was obtained using the corresponding labeling density and the relative compartment volume:
where the sum runs over all compartments. Multiplying the result with the total glutathione content from the biochemical analysis yielded the glutathione content per compartment (in nmol/g FW). The concentration was finally calculated by dividing this result by the compartment volume Vcomp (see Supplementary Table 2 for an example on how the calculations were performed).
2.7. Statistics
For each treatment and experimental setup at least two different samples were examined. For ultrastructural investigations, a minimum of 10 different cells were analyzed, for cytohistochemical investigations a minimum between 20 (peroxisomes and vacuoles) and 60 (other cell structures) of at least 10 different cells were analyzed for gold particle density. The obtained data were then statistically evaluated using Statistica (Stat-Soft Europe, Hamburg, Germany) in which the data were presented as the number of gold particles per μm2. For statistical analyses the non-parametric Kruskal–Wallis test followed by a post hoc comparison according to Conover was used. P < 0.05 was considered as significant (Bortz et al., 2008).
3. Results
3.1. Cell volumes and subcellular volumes
In older leaves the mesophyll content was found to be higher than in youngest fully developed leaves (Table 1). The largest compartment in all cells was the vacuole which covered between 69 and 80% in older and younger leaves (Table 1; Supplementary Fig. 1). The second largest compartment was the chloroplast (between 14 and 20%) followed by the cytosol which covered between 4 and 7%. Nuclei covered between 0.16 and 2.28%. Rather small compartments are mitochondria which covered up to 0.47% in older and up to 1.57% in younger mesophyll cells and peroxisomes which covered around 0.06 and 0.28% (Table 1; Supplementary Fig. 1). In older leaves significant differences among the different leaf areas were only observed between the nuclei and the cytosol. Nuclei at the leaf edge (1.32%) covered a significantly larger volume than nuclei at the other leaf areas (0.16–0.71%; Table 1). A similar situation was found for the cytosol which covered a significantly larger volume at the leaf edge (5.88%), than at the other leaf areas (Table 1).
In younger leaves larger differences in subcellular volumes between the cell volumes in mesophyll cells were found (Table 1). The chloroplasts covered between 19.95% of a mesophyll cell within the leaf edge and 16.26% in leaf apex. The cytosol covered between 5.69 and 7.03% in the leaf apex, leaf base and leaf edge, whereas the center of the leaf contained significantly lower amounts (3.77%) of cytosol (Table 1). Significant differences were also found in mitochondria, which covered between 0.48% and 1.57% of the cells in the leaf center and apex, respectively. Nuclei covered between 2.28% in cells of the leaf edge and 0.67% in the center of the leaf. Peroxisomes at the leaf apex (0.28%) covered a significantly larger volume than peroxisomes at the other leaf areas (Table 1).
3.2. Subcellular distribution of glutathione
The subcellular distribution of glutathione was similar as previously described (Zechmann et al., 2008; Zechmann and Müller, 2010; Queval et al., 2011). Gold particles were detected in all cell compartments but were absent in the apoplast (Figs. 2 and 3). In older and the youngest fully developed leaves the highest glutathione gold particle density was always found in mitochondria and the lowest one in the vacuoles (Figs. 2 and 3; Table 2). In older leaves the distribution of glutathione throughout the different leaf areas showed significant differences in many cell compartments. In mitochondria the highest glutathione gold particle density was found in the center of the leaf (601.12 gold particles per μm2) followed by the leaf edge, the leaf apex, and the leaf base which showed the lowest labeling density (376.90 gold particles per μm2). In nuclei highest labeling density was found in the center of the leaf, whereas the leaf apex showed the lowest one (Table 2). A similar distribution of gold particles bound to glutathione could be analyzed in the peroxisomes, where the leaf center, the leaf base and the leaf edge showed a gold particle density between 156.36 and 178.77 and the leaf apex 133.42 gold particles per μm2. In chloroplasts and the cytosol the lowest glutathione gold particle density was always found in the leaf apex. The highest gold particle density in the chloroplasts was detected at the leaf edge (61.49 gold particles per μm2). The leaf center and the base showed intermediate labeling density. In the cytosol the center of the leaf showed highest glutathione gold particle density (181.49 gold particles per μm2) whereas the distribution in the leaf apex was much lower (107.60%; Table 2).
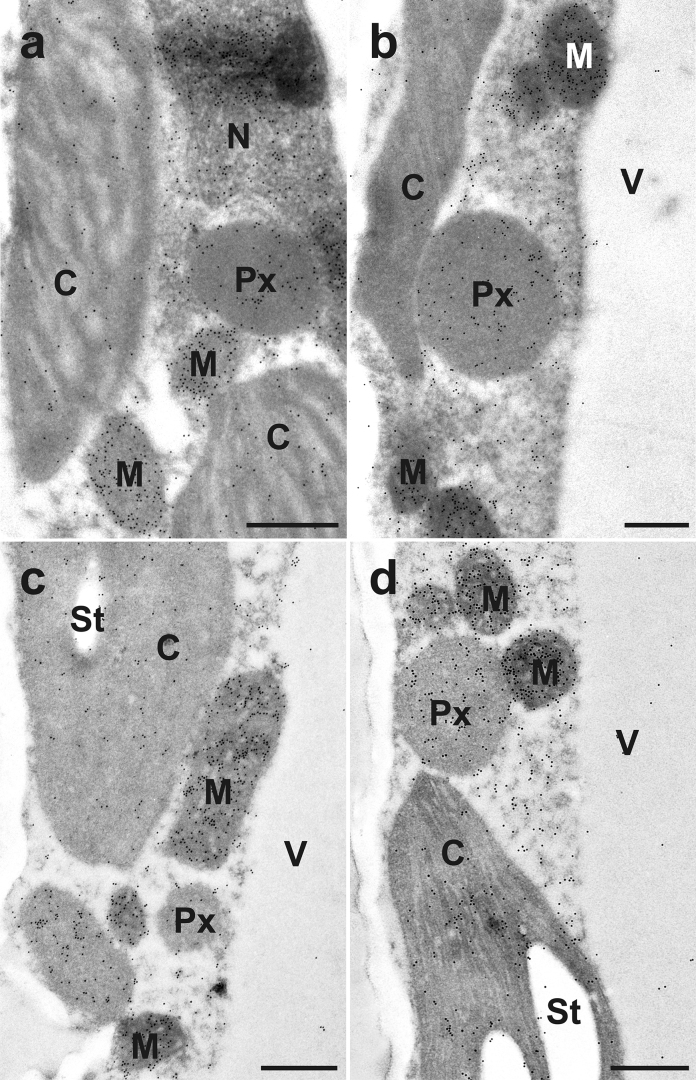
Fig. 2.
Transmission electron micrographs showing the subcellular distribution of glutathione in different areas of older leaves from Arabidopsis thaliana Col-0. Images show parts of mesophyll cells from (a) center of the leaf, (b) leaf apex, (c) leaf base, and (d) leaf edge. C = chloroplasts with or without starch (St), M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 0.5 μm.
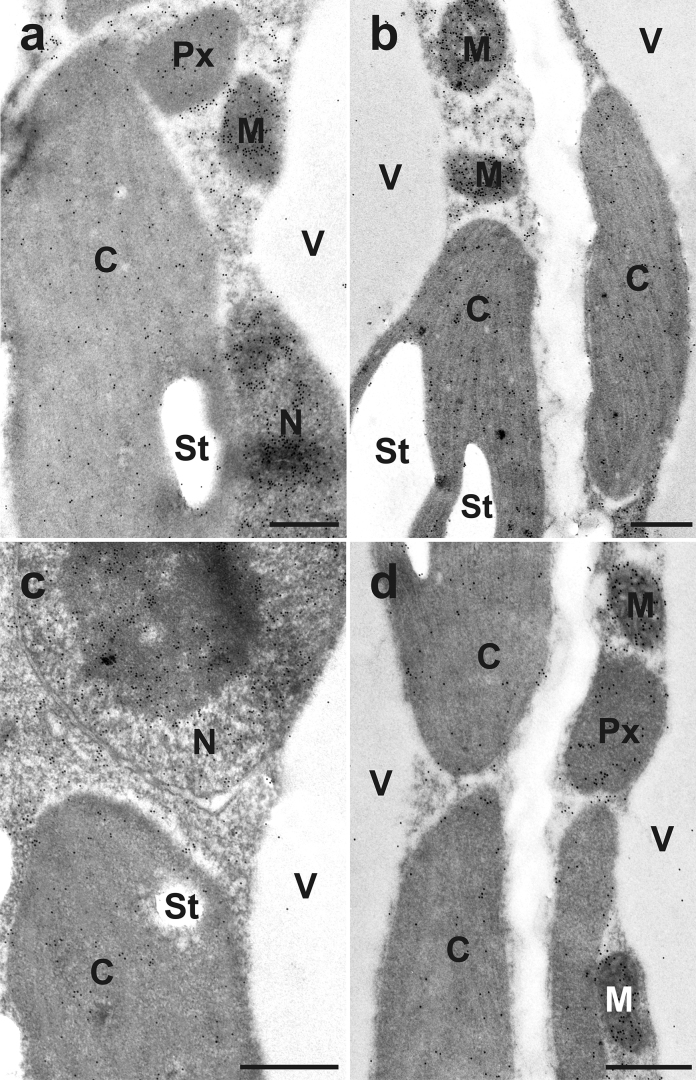
Fig. 3.
Transmission electron micrographs showing the subcellular distribution of glutathione in different areas of younger leaves from Arabidopsis thaliana Col-0. Images show parts of mesophyll cells from (a) center of the leaf, (b) leaf apex, (c) leaf base, and (d) leaf edge. C = chloroplasts with or without starch (St), M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 0.5 μm.
Table 2.
Values document glutathione gold particle density per μm2 in different cell compartments of older (O) and younger leaves (Y; LC = center of the leaf, LA = leaf apex, LB = leaf base, LE = leaf edge) of Arabidopsis thaliana Col-0. Significant differences between the samples are indicated by different lowercase letters, samples which are significantly different from each other have no letter in common. P < 0.05 was regarded as significant as analyzed by the Kruskal–Wallis test followed by post hoc comparison according to Conover. Statistical differences were calculated for each cell compartment in the different leaf areas. Different lower case letters indicate significant differences from either older or younger leaves. n > 20 for peroxisomes and nuclei, n > 60 for other cell structures.
| Labeling density (gold particles per μm2) |
||||||||
|---|---|---|---|---|---|---|---|---|
| OLC | OLA | OLB | OLE | YLC | YLA | YLB | YLE | |
| Mitochondria | 601.12a | 533.67a | 376.90b | 594.88a | 442.12a | 362.39b | 330.61c | 383.85ab |
| Chloroplasts | 46.90b | 38.99c | 47.28b | 61.49a | 43.90a | 40.75a | 34.76a | 43.88a |
| Nuclei | 259.84a | 238.25b | 254.77a | 253.56ab | 367.28a | 272.96b | 194.82c | 248.49b |
| Peroxisomes | 178.77a | 133.42b | 162.49a | 156.36a | 148.20b | 179.54a | 161.05ab | 113.84c |
| Cytosol | 181.49a | 107.60c | 120.94b | 166.62a | 155.00a | 158.85a | 149.06a | 121.45b |
| Vacuole | 3.18c | 3.82b | 4.40a | 1.82d | 4.99a | 2.06b | 0.46c | 1.98b |
In youngest fully developed leaves the leaf center showed highest glutathione gold particle density in mitochondria (442.12 gold particles per μm2), the nuclei (367.28 gold particles per μm2), and in the vacuole (4.99 gold particles per μm2). Lowest density was always detected in the leaf base (Table 2). The other leaf areas showed intermediate labeling density. Major differences in gold particle density could also be found in the peroxisomes of younger leaves (Table 2). There the leaf apex contained highest glutathione labeling density (179.54 gold particles per μm2) and the leaf edge the lowest (113.84 gold particles per μm2). Chloroplasts showed no significant differences in the distribution of glutathione throughout the different leaf areas. In the cytosol the number of gold particles was significantly lower in the leaf edge (121.45 gold particles per μm2), whereas no significant differences could be observed between the center of the leaf, the leaf apex, and the leaf base (labeling density between 149.06 and 158.85 gold particles per μm2; Table 2).
3.3. Biochemistry
The data of biochemical analysis performed by LC/MS/MS-measurements revealed no significant differences between the different leaf areas at different developmental stages and varied between 358.49 nmol/g FW in the leaf edge of older leaves (Table 3) and 412.23 nmol/g FW in the leaf base of younger leaves (Table 3). Oxidized glutathione contents (GSSG) varied between 17.71 and 32.16 nmol/g FW. It covered between 4.8% and 8.5% of the total glutathione content in the apex and base of older leaves, respectively (Table 3).
Table 3.
Estimates of subcellular glutathione concentrations from immunogold labeling densities for older (O) and youngest fully developed leaves of Arabidopsis thaliana Col-0 (Y; LC = center of the leaf, LA = leaf apex, LB = leaf base, LE = leaf edge). The concentrations were based on total leaf GSH + GSSG content for each leaf area in nmol/g fresh weight.
| Measured glutathione and calculated subcellular glutathione concentrations |
||||||||
|---|---|---|---|---|---|---|---|---|
| OLC | OLA | OLB | OLE | YLC | YLA | YLB | YLE | |
| Glutathione [nmol/g fresh weight] | ||||||||
| Total leaf content | 371.57 | 368.46 | 365.35 | 358.49 | 362.71 | 401.82 | 412.23 | 391.51 |
| GSHred | 344.40 | 350.75 | 334.18 | 334.33 | 336.01 | 378.50 | 390.74 | 359.35 |
| GSSGox | 27.17 | 17.71 | 31.17 | 24.16 | 26.70 | 23.32 | 21.49 | 32.16 |
| Mitochondria | 48.2 | 51.0 | 34.4 | 39.6 | 35.2 | 82.8 | 64.5 | 57.1 |
| Chloroplasts | 126.4 | 106.7 | 127.1 | 120.7 | 123.6 | 96.2 | 110.9 | 118.9 |
| Nuclei | 20.8 | 33.1 | 7.9 | 45.1 | 40.4 | 61.9 | 61.2 | 77.1 |
| Peroxisomes | 4.5 | 3.2 | 4.4 | 1.3 | 3.5 | 7.3 | 5.1 | 3.8 |
| Cytosol | 128.3 | 98.4 | 125.4 | 132.6 | 96.1 | 131.3 | 165.1 | 115.9 |
| Vacuole | 43.4 | 75.6 | 66.1 | 19.2 | 63.9 | 22.3 | 5.3 | 18.7 |
| Glutathione-concentration (mM) | ||||||||
| Mitochondria | 14.86 | 15.10 | 11.05 | 13.93 | 11.71 | 8.67 | 9.25 | 8.84 |
| Chloroplasts | 1.16 | 1.10 | 1.39 | 1.44 | 1.16 | 0.98 | 0.97 | 1.01 |
| Nuclei | 6.42 | 6.74 | 7.47 | 5.94 | 9.72 | 6.53 | 5.45 | 5.72 |
| Peroxisomes | 4.42 | 3.76 | 4.76 | 3.66 | 3.92 | 4.30 | 4.51 | 2.62 |
| Cytosol | 4.49 | 3.03 | 3.54 | 3.90 | 4.10 | 3.80 | 4.17 | 2.80 |
| Vacuole | 0.08 | 0.14 | 0.13 | 0.04 | 0.13 | 0.05 | 0.01 | 0.05 |
Subcellular glutathione concentration calculated by using the data obtained by LC/MS/MS-measurements and electron microscopical studies revealed highest glutathione concentrations in mitochondria, where values in a range between 11.05 and 15.10 mM were found in older leaves (Table 3) and contents between 8.67 and 11.71 mM were found in younger ones (Table 3). The second highest amount of glutathione could be found in nuclei where the concentration was between 5.45 and 9.72 mM in older leaves as well as in younger ones. In peroxisomes the values differed between 3.66 and 4.76 mM in older leaves (Table 2) and between 2.62 and 4.51 mM in younger leaves. In the cytosol glutathione concentrations between 3.03 and 4.49 mM in older leaves and between 2.80 and 4.17 mM in younger leaves could be analyzed. The concentration in chloroplasts was between 1.10 and 1.44 mM in older leaves (Table 3) and around 1 mM in younger ones (Table 3). In the vacuole the highest concentration was found in the apex and base of older leaves (0.13–0.14 mM) and in the center of younger ones (0.13 mM), whereas the lowest concentration were found in the leaf edge of older leaves (0.04 mM) and the leaf base of younger leaves (0.01 mM).
4. Discussion
4.1. Cell and organelle volumes
Relative volumes of subcellular compartments within the mesophyll of Arabidopsis were similar to obtained data from other plant species such as barley (Winter et al., 1993), spinach (Winter et al., 1994), peach, celery, common and sea plantain (Nadwodnik and Lohaus, 2008). In this study vacuole contents were evaluated between 69 and 80% in younger and older leaves, respectively. Similar values for vacuoles (between 68 and 85%) have been found for mesophyll cells of leaves from peach, barley, celery, and spinach. Chloroplast contents were evaluated in this study between 14 and 20% which are similar to data obtained for barley, celery, spinach and peach (between 16 and 21%). Slightly lower values for chloroplast contents were found in different species of plantain (10–12%). The cytosol made up between 4 and 7% of the leaf volume in the mesophyll within the different leaf areas. Similar values (between 3.4 and 6.7%) have been found in plantain, spinach, and barley. Higher values for the cytosol have been calculated for peach and celery (11 and 12%, respectively). Mitochondria contents in this study (around 0.5% in older leaves, between 0.5 and 1.6 in the different leaf areas of younger leaves) were similar to data obtained for spinach and barley (Winter et al., 1993, 1994; Nadwodnik and Lohaus, 2008). Nuclei contents in Arabidopsis varied between 0.16% (leaf base of older leaves) to 2.3% (leaf edge of younger leaves). In both spinach and barley nuclei contents were calculated of about 0.3% of the total cell volume (Winter et al., 1993, 1994). In this study vacuoles reached their largest size in older leaves (up to 80%) with comparable values in the leaf center of the youngest fully developed leaves (78%). The other areas of the youngest fully developed leaves showed a gradient of vacuole content from the leaf edge (69%) to the leaf apex (75%) with intermediate volume in the leaf base (70%). Considering that vacuole size increases with the age of a cell (Marty et al., 1980; Wink, 1993) it seems that the leaf center of the youngest fully developed leaves reached a similar developmental stage as the different leaf areas in the older leaves.
4.2. Subcellular glutathione concentrations
In order to calculate subcellular glutathione contents in the different leaf areas of older and younger Arabidopsis leaves subcellular volume data of the different cell compartments were combined with quantitative immunogold labeling of glutathione and global glutathione concentrations which were measured in whole leaves by biochemical methods. The accuracy and specificity of the immunogold labeling approach has been described previously and demonstrated that glutathione is not redistributed during fixation (Zechmann and Müller, 2010) and that differences in the compartment specific distribution of glutathione can be successfully visualized on a high level of resolution (Zechmann et al., 2008; Zechmann and Müller, 2010). Cellular glutathione contents based on glutathione labeling density and concentrations in mM in the different leaf areas demonstrated that mitochondria always contained the highest levels of glutathione, followed by nuclei, peroxisomes or the cytosol, plastids and vacuoles. These results are in accordance to previous glutathione labeling results observed in yeast cells (Zechmann et al., 2011a,b) and in different plant species including Arabidopsis (Fernandez-García et al., 2009; Queval et al., 2011; Zechmann et al., 2008; Zechmann and Müller, 2010) and the alga Micrasterias (Volland et al., 2012) but expand the knowledge about relative subcellular glutathione labeling to concentrations in millimolar range for different leaf areas which have not been studied in detail before. In this context mitochondria contained glutathione contents between 8.7 mM and 15.1 mM. These results are somewhat in accordance to recently calculated glutathione concentrations between 6 and 10 mM in mitochondria of A. thaliana Col-0 (Queval et al., 2011; Han et al., in press). Similar values have also been obtained for animal tissue where glutathione concentrations between 8.5 and 12 mM were measured in isolated mitochondria with biochemical methods (Wahllander et al., 1979; Garcia-Ruiz et al., 1994). Highest contents of glutathione, relative to the other cell compartments, have also been observed with similar methods used in this study in mitochondria of yeast cells (Zechmann et al., 2011a,b), the green alga Micrasterias (Volland et al., 2012) and different animal tissues (Hjelle et al., 1994; Huster et al., 1998) which indicates an important role of glutathione in mitochondria for the detoxification of ROS produced during the mitochondrial respiratory chain. High and stable levels of glutathione in mitochondria have an important function for cell survival as glutathione is of critical importance for the maintenance of the mitochondrial genome and suppresses the accumulation of ROS (Mari et al., 2010; Ayer et al., 2010). The depletion of glutathione in mitochondria favors the accumulation of ROS and induces the release of cytochrome c which triggers caspase induced cell death (Mari et al., 2010; Ayer et al., 2010). Thus it is not surprising that in yeast, cell death induced by severe oxidative stress could be correlated with a strong drop of glutathione in mitochondria and severe swelling of this cell compartment (Zechmann et al., 2011a,b). Similar results have been obtained with plants where distorted plant development was correlated with low glutathione contents in mitochondria as the glutathione deficient rml1 mutant which develops a severe phenotype (Cheng et al., 1995; Vernoux et al., 2000; Cairns et al., 2006) shows a decrease in glutathione contents in all cell compartments including mitochondria of up to 97% (Zechmann and Müller, 2010). In opposite the pad2-1 mutant, which develops a phenotype similar to the wild type, is characterized by glutathione levels similar to Col-0 in mitochondria, despite a strong drop of glutathione in all other cell compartments of up to 90% (Parisy et al., 2007; Zechmann et al., 2008). Thus we can conclude that high and stable levels of glutathione in mitochondria are essential for cell and plant survival. In this respect it is interesting that glutathione concentrations inside mitochondria of older leaves were much higher than in younger ones indicating that mitochondria in older tissues are of special need for glutathione for protection against ROS. Even though the physiological relevance of high glutathione contents in mitochondria during ageing is not clearly established for plants yet results from animal tissue indicates higher ROS production in ageing cells due to mitochondrial dysfunction (Cui et al., 2012). In Arabidopsis mutants lacking a mitochondrial protease distorted leaf development at the later stages of leaf development could be correlated with mitochondrial dysfunction leading to elevated levels of ROS, increased amounts of oxidatively damaged mitochondrial proteins and impairment of organelle development (Gibala et al., 2009). Thus it is very well likely that the accumulation of glutathione in mitochondria of older leaf tissue in comparison to younger ones is an important strategy of plants to protect mitochondria from oxidative damage induced by higher ROS production during ageing.
In the cytosol glutathione concentrations were calculated between 2.8 and 4.5 mM which fit well with concentrations recently obtained from biochemical measurements in A. thaliana Col-0 (3.52 mM for the cytosol) after the isolation of different cell compartments by non-aqueous fractionation from whole leaves (Krueger et al., 2009). Similar concentrations between 2.7 and 3.2 mM have also been obtained for the cytoplasm with light microscopical methods after labeling of glutathione in cells of A. thaliana Col-0 with monochlorobimane in situ (Meyer et al., 2001). Lower concentrations (between 1 and 2 mM) have been calculated for wheat (Noctor et al., 2002) indicating different glutathione concentrations in different plant species within the cytosol. The concentrations found in plants are lower to what has been found in animal tissue where cytosolic concentrations between 6 and 8.2 mM were measured biochemically after fractionation (Wahllander et al., 1979; Garcia-Ruiz et al., 1994). In plants the cytosol and plastids are the only compartments able to synthesize glutathione as the necessary enzymes are exclusively targeted to these cell compartments (Wachter et al., 2005). Thus, the other cell compartments need to import glutathione from the cytosol through glutathione transporters which still have to be clearly identified in plants for mitochondria, peroxisomes, and nuclei.
In chloroplasts we calculated glutathione concentrations up to 1.4 mM which are well below the concentration measured in isolated chloroplasts from A. thaliana Col-0 (3.2 mM) and spinach (3.5 mM) after non-aqueous and aqueous fractionation, respectively (Foyer and Halliwell, 1976; Krueger et al., 2009). Nevertheless, the range for glutathione concentrations measured biochemically in isolated chloroplasts from different plant species in the literature varies between 0.5 and 5 mM as discussed in Noctor et al. (2002). Considering that glutathione is water soluble and that glutathione transporters in the chloroplast membranes allow the import and export of glutathione (Noctor et al., 2002; Maughan et al., 2010) it is well likely that glutathione gets redistributed during the isolation process as discussed in previous studies using these methods (Noctor et al., 2002; Krueger et al., 2009). Physiologically it is interesting that chloroplasts which are considered to be a major source for ROS in plants (Halliwell, 2006; Karuppanapandian et al., 2011; Kangasjärvi et al., 2012) contained relatively little glutathione in comparison to other cell compartments and that concentrations did not differ much between the different leaf areas. It has been shown in previous studies that glutathione accumulates during oxidative stress in chloroplasts to much higher concentrations than in other cell compartments (Queval et al., 2011). As chloroplasts are able to synthesize and import glutathione through transporters (Noctor et al., 2002; Maughan et al., 2010) it seems that, despite low concentrations of glutathione in non-stressed conditions, a rapid import and higher glutathione synthesis leading to glutathione accumulation in chloroplasts is possible and most probably an important defense strategy of plants in situation of oxidative stress.
Glutathione concentrations in nuclei were similar in the different leaf areas but in general higher than what was found in the cytosol demonstrating that glutathione accumulates in this cell compartment. It has been suggested that glutathione is imported into the nucleus through the activation or incorporation of nuclear pore proteins (Diaz Vivancos et al., 2010a,b). Within nuclei glutathione could serve to protect DNA and redox-sensitive nuclear proteins from oxidation, as well as driving glutaredoxin-related processes. This will influence the binding of transcription factors which will result in adaptations of gene expression patterns. Additionally, glutathione can bind to nuclear proteins and protect them from oxidation (Mou et al., 2003; Gomez et al., 2004; Foyer and Noctor, 2009; Green et al., 2006; Diaz Vivancos et al., 2010a,b). An accumulation of glutathione in nuclei, followed by a depletion from the cytosol has also been related to increased synthesis and rapid accumulation of cellular glutathione contents (Diaz Vivancos et al., 2010a,b). Thus, it is well likely that high levels of glutathione in nuclei as observed in this study play important roles in the glutathione homeostasis, redox regulation, signaling and gene expression.
Vacuoles showed much lower concentrations of glutathione in the different leaf areas in this study (between 0.01 and 0.14 mM) in comparison to higher concentrations measured after non-aqueous fractionation (0.7 mM; Krueger et al., 2009). As described above the discrepancy in compartment specific glutathione concentrations found in this study and biochemical measurement after isolation of organelles may be caused by redistribution of glutathione during the isolation process (Noctor et al., 2002; Krueger et al., 2009). Such effects can be excluded with the method used in this study as we have shown in a previous study that glutathione contents and distribution remain similar between microwave fixed samples where the ultrastructure is fixed within 30 s and conventional fixation at RT which can take up to 90 min (Zechmann and Müller, 2010). Vacuoles are well known to import glutathione and glutathione conjugates (Noctor et al., 2011). Additionally, an accumulation of GSSG has been observed in vacuoles during situations of oxidative stress indicating an important mechanism to maintain a reduced environment in the cytosol (Tommasini et al., 1993; Queval et al., 2011). Thus, high concentrations of glutathione in vacuoles can be used as an indicator for oxidative stress. In this respect large differences (0.01 and 0.14 mM) in glutathione contents have been observed in vacuoles between the different areas in this study. Nevertheless, the physiological relevance of these observations remains unclear as no clear conclusions could be drawn from the results. Additionally, it seems that even though glutathione contents in vacuoles varied between the different leaf areas between 0.01 and 0.14 mM in this study it seems unlikely that certain leaf areas suffered oxidatives stress as much higher concentrations in vacuoles (0.32 mM) were found when plants suffered oxidative stress (Queval et al., 2011).
5. Conclusions
Considering the distribution of glutathione throughout different leaf areas we can conclude that clear differences in subcellular glutathione concentrations can mainly be found in mitochondria and vacuoles. In mitochondria of older leaves highest concentrations (around 15 mM) were found in the leaf center (together with the leaf apex). A similar situation was found in younger leaves where glutathione also accumulated in mitochondria at the leaf center. Such effects could also be found for other cell compartments in the leaf center of the youngest fully developed leaves as glutathione contents were highest in chloroplasts, nuclei, the cytosol and vacuoles reaching or succeeding levels found in the older leaves. This observation might be correlated with leaf age as the ultrastructure of the leaf center from the youngest fully developed leaves showed a similar ratio of the cellular compartments as the older leaves. The other leaf areas of the youngest fully developed leaves (leaf apex, edge and base) showed much higher volumes of the cytosol (up to 7%) and lower volumes of vacuoles (as low as 69%) which indicates that the center of younger leaves was in a similar developmental stage as the leaf center in the older leaf. As cells from older tissues have in general larger vacuoles and less cytosol (Marty et al., 1980; Wink, 1993) it seems that glutathione accumulates in older tissues and leaf areas such as the leaf center. The physiological relevance behind this observation is unclear. As we have shown recently high and stable levels of glutathione are important for cell survival and plant development (Zechmann et al., 2008; Zechmann and Müller, 2010), high levels of glutathione in mitochondria of older leaves and tissues might fulfill similar roles.
Summing up, we can conclude that the method described in this study allowed the measurement of subcellular glutathione concentrations in different areas of A. leaves by combining stereological methods with global glutathione contents determined by quantitative immunogold labeling and biochemical methods, respectively.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
This study was financially supported by the Austrian Science Fund (FWF, P20619 and P22988).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.micron.2012.11.006.
Contributor Information
Barbara E. Koffler, Email: barbaraeva.koffler@edu.uni-graz.at.
Elke Bloem, Email: elke.bloem@jki.bund.de.
Günther Zellnig, Email: guenther.zellnig@uni-graz.at.
Bernd Zechmann, Email: bernd.zechmann@uni-graz.at.
Appendix A. Supplementary data
The following are the supplementary data to this article:
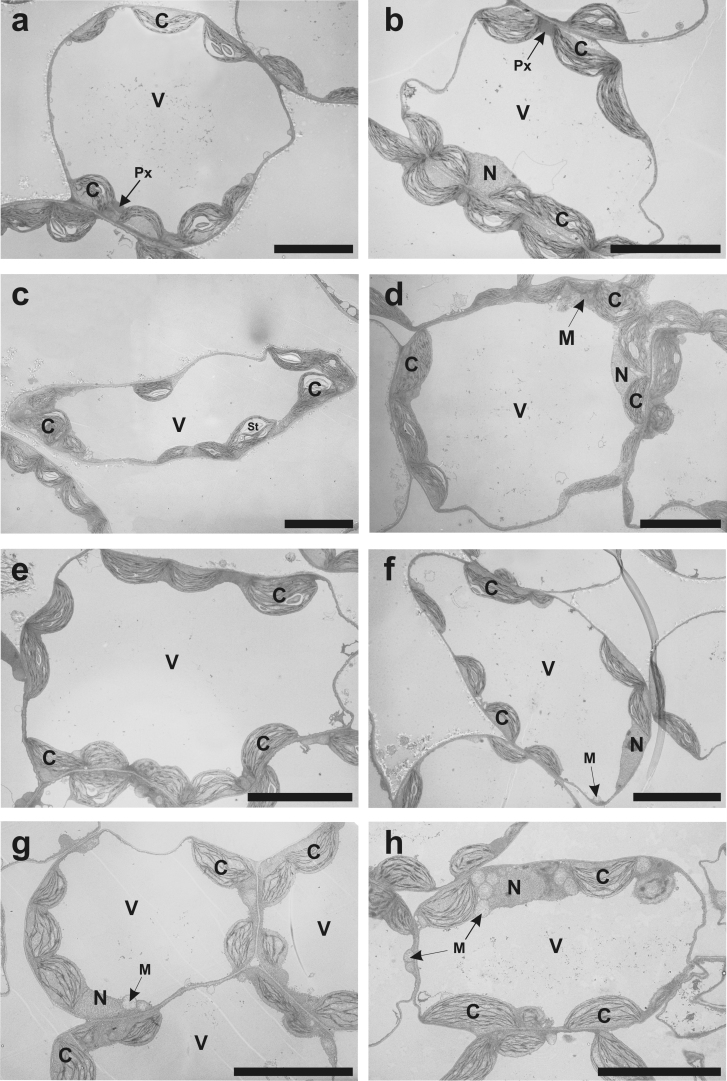
Supplementary Fig. 1.
Transmission electron micrographs showing mesophyll cells of older (a–d) and younger leaves (e–h) from Arabidopsis thaliana Col-0. Transmission electron microscopical images show sections of whole mesophyll cells from center of the leaf (a and e), leaf apex (b and f), leaf base (c and g), and leaf edge (d and h). Bar = 10 μm.
References
- Ammar W.B., Mediouni C., Tray B., Ghorbel M.H., Jemal F. Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biologia Plantarum. 2008;52:314–320. [Google Scholar]
- Ayer A., Tan S.X., Grant C.M., Meyer A.J., Dawes I.W., Perrone G.G. The critical role of glutathione in maintenance of the mitochondrial genome. Free Radical Biology and Medicine. 2010;49:1956–1968. doi: 10.1016/j.freeradbiomed.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Bortz J., Lienert G.A., Bohenke K. Springer; Heidelberg: 2008. Verteilungsfreie Methoden in der Biostatistik. [Google Scholar]
- Bowsher C.G., Tobin A.K. Compartmentation of metabolism within mitochondria and plastids. Journal of Experimental Botany. 2001;52:513–527. [PubMed] [Google Scholar]
- Cairns N.G., Pasternak M., Wachter A., Cobbett C.S., Meyer A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiology. 2006;141:446–455. doi: 10.1104/pp.106.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.C., Seeley K.A., Sung Z.R. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiology. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew O., Whelan J., Millar A.H. Molecular definition of ascorbate–glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. Journal of Biological Chemistry. 2003;278:46869–46877. doi: 10.1074/jbc.M307525200. [DOI] [PubMed] [Google Scholar]
- Cui H., Kong Y., Zhang H. Oxidative stress, mitochondria dysfunction, and aging. Journal of Signal Transduction. 2012 doi: 10.1155/2012/646354. Article ID 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins I., Dixon D.P., Freitag-Pohl S., Skipsey M., Edwards R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metabolism Reviews. 2011;43:226–280. doi: 10.3109/03602532.2011.552910. [DOI] [PubMed] [Google Scholar]
- DalCorso G., Farinati S., Maistri S., Furini A. How plants cope with cadmium: staking all on metabolism and gene expression. Journal of Integrative Plant Biology. 2008;50:1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- DeRidder B.P., Goldsbrough P.B. Organ-specific expression of glutathione S-transferases in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Physiology. 2006;140:167–175. doi: 10.1104/pp.105.067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Vivancos P., Dong Y., Ziegler K., Markovic J., Pallardo F.V., Pellny T.K., Verrier P.J., Foyer C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant Journal. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- Diaz Vivancos P., Wolff T., Markovic J., Pallardo F.V., Foyer C.H. A nuclear glutathione cycle within the cell cycle. Biochemical Journal. 2010;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- Edwards R., Brazier-Hicks M., Dixon D.P., Cummins I. Chemical manipulation of antioxidant defences in plants. Advances in Botanical Research. 2005;42:1–32. [Google Scholar]
- Fernandez-García N., Martí M.C., Jimenez A., Sevilla F., Olmos E. Sub-cellular distribution of glutathione in Arabidopsis mutant (vtc1) deficient in ascorbate. Journal of Plant Physiology. 2009;166:2004–2012. doi: 10.1016/j.jplph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiology. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. Redox regulation and photosynthetic organisms: signaling, acclimation and practical implications. Antioxidants and Redox Signaling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Theodoulou F.L., Delrot S. The functions of inter- and intracellular glutathione transport systems in plants. Trends in Plant Science. 2001;6:486–492. doi: 10.1016/s1360-1385(01)02086-6. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fricker M.D., May M., Meyer A.J., Sheard N., White N.S. Measurement of glutathione levels in intact roots of Arabidopsis. Journal of Microscopy. 2000;198:162–173. doi: 10.1046/j.1365-2818.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C., Morales A., Ballesta A., Rodes J., Kaplowitz N., Fernandez-Checa J.C. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. Journal of Clinical Investigation. 1994;94:193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala M., Kica M., Sakamoto W., Gola E.M., Kubrakiewicz J., Smakowska E., Janska H. The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage or rosette development under short-day photoperiod. Plant Journal. 2009;59:685–699. doi: 10.1111/j.1365-313X.2009.03907.x. [DOI] [PubMed] [Google Scholar]
- Gomez L.D., Noctor G., Knight M.R., Foyer C.H. Regulation of calcium signalling and expression by glutathione. Journal of Experimental Botany. 2004;55:1851–1859. doi: 10.1093/jxb/erh202. [DOI] [PubMed] [Google Scholar]
- Green R.M., Graham M., O’Donovan M.R., Chipman J.K., Hodges N.J. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- Gutscher M., Pauleau A.L., Marty L., Brach T., Wabnitz G.H., Samstag Y., Meyer A.J., Dick T.P. Real-time imaging of the intracellular glutathione redox potential. Nature Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants, Redox biology is a fundamental theme of aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y., Mhamdi, A., Chaouch, S., Queval, G., Zechmann, B., Noctor, G. Glutathione acts independently of its antioxidant function to orchestrate defence phytohormone signalling triggered by intracellular H2O2. Antioxidants and Redox Signaling, in press, http://dx.doi.org/10.1089/ars.2012.5052.
- Hartmann T.N., Fricker M.D., Rennenberg H., Meyer A.J. Cell-specific measurements of cytosolic glutathione in poplar leaves. Plant, Cell and Environment. 2003;26:965–975. doi: 10.1046/j.1365-3040.2003.01031.x. [DOI] [PubMed] [Google Scholar]
- Hopkins, L., 1997. The effects of elevated ultraviolet-B radiation on the growth and development of the primary leaf of wheat (Triticum aestivum L. cv. Maris Huntsman). Dissertation or Thesis, University of St. Andrews.
- Hjelle O.P., Chaudhry F.A., Ottersen O.P. Antisera to glutathione: characterization and immunocytochemical application to the rat cerebellum. European Journal of Neuroscience. 1994;6:791–804. doi: 10.1111/j.1460-9568.1994.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Huster D., Hjelle O.P., Haug F.M., Nagelhus E.A., Reichelt W., Ottersen O.P. Subcellular compartmentation of glutathione and glutathione precursors. A high resolution immunogold analysis of the outer retina of guinea pig. Anatomy and Embryology. 1998;198:277–287. doi: 10.1007/s004290050184. [DOI] [PubMed] [Google Scholar]
- Jiménez A., Hernández J.A., Pastori G., del Rio L.A., Sevilla F. Role of the ascorbate–glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiology. 1998;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A., Hernández J.A., del Rio L.A., Sevilla F. Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiology. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczak M., Remans T., Vangronsfeld J., Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. International Journal of Molecular Sciences. 2012;13:3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubany-Marí T., Prinsen E., Munné-Bosch S., Alegre L. The timing of methyl jasmonate, hydrogen peroxide and ascorbate accumulation during water deficit and subsequent recovery in the Mediterranean shrub Cistus albidus L. Environmental and Experimental Botany. 2010;69:47–55. [Google Scholar]
- Karuppanapandian T., Moon J.C., Kim C., Manoharan K., Kim W. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Australian Journal of Crop Sciences. 2011;5:709–725. [Google Scholar]
- Kangasjärvi S., Neukermans J., Shengchun L., Aro E.M., Noctor G. Photosynthesis, photorespiration, and light signalling in defence responses. Journal of Experimental Botany. 2012;63:1619–1636. doi: 10.1093/jxb/err402. [DOI] [PubMed] [Google Scholar]
- Krueger S., Niehl A., Lopez Martin M.C., Steinhauser D., Donath A., Hildebrandt T., Romero L.C., Hoefgen R., Gotor C., Hesse H. Analysis of cytosolic and plastidic serine acetyltransferase mutants and subcellular metabolite distributions suggests interplay of the cellular compartments of cysteine biosynthesis in Arabidopsis. Plant, Cell and Environment. 2009;32:349–367. doi: 10.1111/j.1365-3040.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Kuźniak E., Sklodowska A. Compartment-specific role of the ascorbate–glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. Journal of Experimental Botany. 2005;56:921–933. doi: 10.1093/jxb/eri086. [DOI] [PubMed] [Google Scholar]
- Kuźniak E., Sklodowska A. Comparison of two methods for preparing mitochondria from tomato leaves to study the ascorbate–glutathione cycle activity. Biologia Plantarum. 2004;48:537–542. [Google Scholar]
- Kuźniak E., Sklodowska A. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinera. Plant Science. 2001;160:723–731. doi: 10.1016/s0168-9452(00)00457-x. [DOI] [PubMed] [Google Scholar]
- Mari M., Morales A., Colell A., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxidant and Redox Signaling. 2010;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F., Branton D., Leigh R.A. vol. 1. Macmillan Press; 1980. (The Biochemistry of Plants: A Comprehensive Treatise). pp. 625–658. [Google Scholar]
- Maughan S., Foyer C.H. Engineering and genetic approaches to modulating the glutathione network in plants. Physiologia Plantarum. 2006;126:382–397. [Google Scholar]
- Maughan S.C., Pasternak M., Cairns N., Kiddle G., Brach T., Jarvis R., Haas F., Nieuwland J., Lim B., Müller C., Salcedo-Sora E., Kruse C., Orsel M., Hell R., Miller A.J., Bray P., Foyer C.H., Murray J.A.H., Meyer A.J., Cobbett C.S. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proceedings of the National Academy of Sciences of United States of America. 2010;107:2331–2336. doi: 10.1073/pnas.0913689107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A.J., Brach T., Marty L., Kreye S., Rouhier N., Jacquot J.H., Hell R. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant Journal. 2007;52:973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- Meyer A.J., May M.J., Fricker M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant Journal. 2001;27:67–78. doi: 10.1046/j.1365-313x.2001.01071.x. [DOI] [PubMed] [Google Scholar]
- Meyer A.J., Fricker M.D. Direct measurement of glutathione in epidermal cells of intact Arabidopsis roots by 2-photon laser scanning microscopy. Journal of Microscopy. 2000;198:174–181. doi: 10.1046/j.1365-2818.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Mohsenzadeh S., Esmaeili M., Moosavi F., Shahrtash M., Saffari B., Mohabatkar H. Plant glutathione S-transferase classification, structure and evolution. African Journal of Biotechnology. 2011;43:8160–8165. [Google Scholar]
- Mou Z., Fan W., Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;27:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Müller M., Zellnig G., Urbanek A., Zechmann B. Recent developments in methods intracellulary localizing glutathione within plant tissues and cells (a minireview) Phyton (Horn, Austria) 2005;45:45–55. [Google Scholar]
- Nadwodnik J., Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta. 2008;227:1079–1089. doi: 10.1007/s00425-007-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito F.F., Espen L., Crema B., Cocucci M., Sacchi G. Cadmium induces acidosis in maize root cells. New Phytology. 2008;179:700–711. doi: 10.1111/j.1469-8137.2008.02509.x. [DOI] [PubMed] [Google Scholar]
- Noctor G., Mhamdi A., Chaouch S., Han Y., Neukermans J., Marquez-Garcia B., Queval G., Foyer C.H. Glutathione in plants: an integrated overview. Plant, Cell and Environment. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Noctor G., Gomez L., Vanacker H., Foyer C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. Journal of Experimental Botany. 2002;53(372):1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- Noctor G., Queval G., Mhamdi A., Chaouch S., Foyer C.H. The Arabidopsis Book; 2011. Glutathione. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N., Radwan S., Peterson A., Zhao P., Badr A.F., Xiang C., Oliver D.J. Characterization of the extracellular γ-glutamyl transpeptidases, GGT1 and GGT2, in Arabidopsis. Plant Journal. 2007;49:865–877. doi: 10.1111/j.1365-313X.2006.03004.x. [DOI] [PubMed] [Google Scholar]
- Parisy V., Poinssot B., Owsianowski L., Buchala A., Glazebrook J., Mauch F. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant Journal. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- Queval G., Jaillard D., Zechmann B., Noctor G. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant, Cell and Environment. 2011;34:21–32. doi: 10.1111/j.1365-3040.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- Rellán-Álvarez R., Hernández L.E., Abadía J., Álvarez-Fernández A. Direct and simultaneous determination of reduced and oxidized glutathione and homoglutathione by liquid chromatography-electrospray/mass spectrometry in plant tissue extracts. Analytical Biochemistry. 2006;356:254–264. doi: 10.1016/j.ab.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Seth C.S., Remans T., Keunen E., Jozefczak M., Gielen H., Opdenakker K., Weyens N., Vangronsfeld J., Cuypers A. Phytoextraction of toxic metals: a central role for glutathione. Plant, Cell and Environment. 2012;35:334–346. doi: 10.1111/j.1365-3040.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- Tan J.F., O’Toole N., Taylor N.L., Millar A.H. Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiology. 2010;152:747–761. doi: 10.1104/pp.109.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R., Martinoia E., Grill E., Dietz K.-J., Amrhein N. Transport of oxidized glutathione into barley vacuoles: evidence for the involvement of the glutathione-S-conjugate ATPase. Zeitschrift für Naturforschung. 1993;48c:867–871. [Google Scholar]
- Vanacker H., Carver T.L.W., Foyer C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiology. 1998;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H., Foyer C.H., Carver T.L.W. Changes in apoplastic antioxidants induced by powdery mildew attack in oat genotypes with race non-specific resistance. Planta. 1998;208:444–452. [Google Scholar]
- Vanacker H., Harbinson J., Ruisch J., Carver T.L.W., Foyer C.H. Antioxidant defences of the apoplast. Protoplasma. 1998;205:129–140. [Google Scholar]
- Vernoux T., Wilson R.C., Seeley K.A., Reichheld J.P., Muroy S., Brown S., Maughan S.C., Cobbett C.S., Van Montagu M., Inzé D., May M.J., Sung Z.R. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volland S., Lütz C., Michalke B., Lütz-Meindl U. Intracellular chromium localization and cell physiological response in the unicellular alga Micrasterias. Aquatic Toxicology. 2012;109:59–69. doi: 10.1016/j.aquatox.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A., Wolf S., Steininger H., Bogs J., Rausch T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant Journal. 2005;41:15–30. doi: 10.1111/j.1365-313X.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- Wahllander A., Soboll S., Sies H. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Letters. 1979;97:138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- Weibel E.R., Bolender R.P. Biological applications. In: Hayat M.A., editor. Principles and Techniques of Electron Microscopy. Van Nostrand Reinhold Company; New York: 1973. pp. 237–296. [Google Scholar]
- Wink M. The plant vacuole: a multifunctional compartment. Journal of Experimental Botany. 1993;44:231–246. [Google Scholar]
- Winter H., Robinson D.G., Heldt H.W. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193:530–535. [Google Scholar]
- Winter H., Robinson D.G., Heldt H.W. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Zaffagnini M., Bedhomme M., Lemaire S.D., Trost P. The emerging roles of protein glutathionylation in chloroplasts. Plant Science. 2012;185–186:86–96. doi: 10.1016/j.plantsci.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C.H., Morisse S., Trost P., Lemaire S.D. Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxidants and Redox Signaling. 2012;16:567–586. doi: 10.1089/ars.2011.4255. [DOI] [PubMed] [Google Scholar]
- Zawoznik M.S., Groppa M.D., Tomaro M.L., Benavides M.P. Endogenous salicylic acid potentiates cadmium-induces oxidative stress in Arabidopsis thaliana. Plant Science. 2007;173:190–197. [Google Scholar]
- Zechmann B., Liou L.C., Koffler B.E., Horvat L., Tomašić A., Fulgosi H., Zhang Z. Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Research. 2011;11:631–642. doi: 10.1111/j.1567-1364.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Koffler B.E., Russell S. Glutathione synthesis is essential for pollen germination in vitro. BMC Plant Biology. 2011;11:54. doi: 10.1186/1471-2229-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Müller M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma. 2010;246:15–24. doi: 10.1007/s00709-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Mauch F., Sticher L., Müller M. Subcellular immunocytochemical analysis detects the highest concentration of glutathione in mitochondria and not in plastids. Journal of Experimental Botany. 2008;59:4017–4027. doi: 10.1093/jxb/ern243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Müller M., Zellnig G. Intracellular adaptations of glutathione content in Curcubita pepo L. induced by treatment with reduced glutathione and buthionine sulfoximine. Protoplasma. 2006;227:197–209. doi: 10.1007/s00709-005-0129-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.