Abstract
Preterm birth is a major public-health issue because of its increasing incidence combined with the frequent occurrence of subsequent behavioral, neurological, and psychiatric challenges faced by surviving infants. Approximately 10–15% of very preterm children (born < 30 weeks gestational age) develop cerebral palsy, and 30 – 60% of them experience cognitive impairments. These adverse outcomes are related to a confluence of abnormal brain development along with white (WM) and gray matter (GM) injury sustained during the neonatal period. It is becoming apparent that the extra-uterine environment during this critical period (24–40 weeks gestation) in brain development has a profound and long lasting impact on the premature infant. Magnetic resonance imaging in the neonatal period and infancy provides a non-invasive, “in vivo” assessment of brain development and extent of brain injury. This not only helps understand the extent and timing of injury but also identifies infants who may benefit from early intervention to minimize the impact of the injury.
Learning outcomes: (1) Readers will be able to appreciate the diverse impact of prematurity on neurodevelopmental outcome. (2) Readers will be able to recognize the biological vulnerability of the developing brain in premature infants born between 24–40 weeks of gestation. (3) Readers will be able to understand the role of magnetic resonance imaging (MRI) as a tool to detect abnormal development and brain injury in premature infants. (4) Readers will be able to see the potential role for novel MR imaging methods as biomarkers for brain development and injury in premature infants.
Keywords: Magnetic resonance imaging, neonatal brain injury, Premature infant
While the incidence of prematurity continues to rise, survival rates for very preterm infants (born < 30 weeks gestation) have improved dramatically in recent decades due to advances in perinatal and neonatal care. In contrast to this improvement in mortality, the long term neurodevelopmental outcomes have not improved and are highly problematic. Approximately 10–15% of very preterm children will develop cerebral palsy, whilst up to 40% will display mild motor deficits (1). The incidence of cognitive deficits is even higher, with 30 – 60% of very preterm children experiencing cognitive impairments and learning disabilities (1,2). These include visual-motor problems,(3) attentional difficulties,(4) impaired memory,(5) delayed language skills,(6) and executive dysfunction (7). Not surprisingly, learning disabilities are more prevalent in this population compared with controls, particularly in the area of mathematics (8). Awareness has also increased of the social and emotional difficulties faced by preterm children, such as poor social skills, higher rates of social withdrawal, anxiety and depression (9). In addition, an increased prevalence of developmental disorders (i.e., attention deficit/hyperactivity disorder (ADHD), autism and schizophrenia) has been described in the preterm population (10,11).
A greater understanding of the mechanisms responsible for these deficits in very preterm children is required in order to devise targeted interventions that can reduce the frequency and severity of these impairments. To date, numerous factors have shown correlations with these neurobehavioral impairments, including birth weight, gestational age, postnatal steroid use, prolonged ventilation, inotrope use and male gender (12–16). However, these perinatal risk factors correlate only modestly with neuro-behavioral outcomes, and the major neural mechanisms leading to these impairments are not yet understood. In order to better define the cerebral “lesions” associated with prematurity and neurodevelopmental impairments, it is important to identify both the nature/extent of direct cerebral injury and any secondary effect on subsequent cerebral development.
With regard to direct cerebral injury, the most common neuropathology reported in the preterm infant lies within the cerebral WM. WM lesions are characterized by loss of pre-myelinating O4+/O1+ oligodendrocytes (17,18) axonal damage, and gliosis (19,20). Autopsy studies in preterm infants with WM lesions have reported diffuse axonal damage in the frontal, parietal, and occipital lobes (21) which worsen over time as axonal degeneration progresses (19). A second region of vulnerability in the preterm infant for direct injury is the immature cortical subplate. The subplate is a transient developmental layer present in humans from 15 weeks gestation to 6 months post-natally (22,23). This region contains oligodendrocytes, the earliest population of neurons, GABAergic neurons migrating to the cortex, and traversing axons from multiple neuronal systems. Subplate neurons are required for normal development of thalamo-cortical projections to layer IV of the cortex (24), and lesions in the subplate early in development lead to a failure of these projections to reach cortical layer IV (25,26). Considering that the peak of subplate formation and function correlates with the peak period of vulnerability to injury in preterm infants and the crucial role of subplate neurons in cortical development, injury to subplate cells has been implicated in the causation of “cortical” deficits in preterm infants (27–29). In the cerebral cortex, direct cortical damage is not common, but pathological studies of postmortem preterm infants have demonstrated cortical abnormalities in association with WM lesions, including neuronal atrophy, neuronal hypertrophy, and a switch from predominantly trans-callosal and long circuits to local circuits (19,30). Delayed or arrested dendritic development has also been reported in preterm infants brains compared with term infants of equivalent post menstrual age (PMA) (31).
The mechanisms for a secondary disruption of cerebral development in cortical GM in association with WM injury in the preterm infant are not well understood but can be considered in relation to two published hypotheses. Based on analyses of pathological specimens, Marin-Padilla proposed that damage to underlying WM results in an acquired cortical dysplasia with de-afferentation of the overlying cortex and resulting cortical dysfunction (19, 30, 32). A second hypothesis is related to the tension-based theory of morphogenesis (33), which posits that cortical folding is driven by mechanical tension along long distance cortico-cortical connections. If lesions or delayed maturation of WM disrupt specific subsets of cortico-cortical, long distance connections, these altered connectivity patterns could in turn give rise to specific abnormalities in cortical folding patterns. Thus, WM injury resulting in a secondary disruption of cortical GM development may be a major mediator of cognitive deficits faced by preterm infants.
Finally, cortical development may be altered in the absence of WM or subplate injury in preterm infants. In rodent models, prenatal administration of toxins (34) or irradiation (35,36) during late gestation produce cortical abnormalities without WM lesions, resulting in a propensity to seizures. This suggests that damage to cortex without WM lesions during late gestation could be an important mechanism for “cortical” deficits associated with perinatal brain damage and preterm birth. Administration of postnatal dexamethasone to preterm infants has also been shown to be associated with a reduction in the volume of cortical GM (37) without WM injury.
Magnetic Resonance Imaging (MRI)
MR imaging offers a unique, non-invasive opportunity to study these complex neurobiological phenomena. For decades, MR imaging was only possible under sedation/anesthesia adding a layer of complexity in terms of safety and exposure to the vulnerable brain to yet another potentially injurious cocktail of medications. With the advent of unsedated MR scanning (62), these scans can be carried out with relative ease in most neonatal units. Recently, in addition to conventional MRI, volumetric analysis, diffusion tensor imaging (DTI) methods and novel surface based morphometry (SBM) methods are being developed that provide additional insights into the developing brain. This information provided by MR imaging has the potential to (i) improve current NICU clinical practice by identifying practices correlated with altered structure and poor outcome, (ii) allow development of early intervention strategies for therapy services by providing the means of identifying those infants who would most benefit from intervention, and (iii) promote the development of neuro-protective agents targeted in time and cerebral region.
Conventional MR Image Evaluation
MRI is a potent tool with high sensitivity for delineating alterations in the developing brain. Conventional MRI is more sensitive than cranial ultrasound for the detection of cerebral injury in the preterm infant, especially within the WM (40–42). The most common abnormality reported is in the cerebral WM, with 75% of preterm infants displaying qualitative abnormalities, including signal abnormalities in the WM, ventriculomegaly and thinning of the corpus callosum (43,44). Qualitative alterations in the cerebral WM are very common, with up to 70% of a large cohort of preterm children having abnormalities and 20% of the cohort displaying very significant abnormalities in WM (45). WM abnormality at term equivalent is the strongest independent predictor of neurodevelopment at 2 years of age when compared with other perinatal factors. Children with moderate to severe WM injury (Fig. 1) were 3 times more likely to exhibit severe cognitive impairment, 10 times more likely to have severe motor impairment, and 9 times more likely to have cerebral palsy (46). In addition, qualitative delay in gyral development was very common, occurring in 50% of infants, and was associated with a 4-fold increase in significant cognitive delay, motor delay and cerebral palsy.
Figure 1.
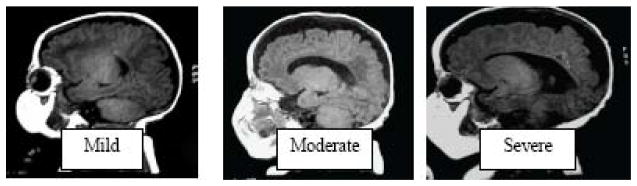
WMI score. T1 weighted sagittal images of three premature infants at term equivalent age showing Mild, Moderate and Severe WMI.(46)
These qualitative MRI scores were better predictors than any other clinical or imaging measure, there was clearly still a deficiency in their predictive power for adverse outcomes, with the most common abnormality of mild WM injury (50% of infants) being associated with very poor outcomes in 20% of infants.
Quantitative volumetric MRI techniques
Volume changes during normal maturation of cerebral tissue between 29 and 41 weeks gestation have been defined in vivo using MR tissue segmentation of the brain (47). Early data indicate that preterm infants have regional reductions in cortical GM volumes by term equivalent and in later childhood (38,39). In a study using volumetric MRI Inder et al showed that preterm infants (< 1500 g and < 32 weeks) with WM lesions had a 17% reduction in absolute cortical GM volume (p < 0.001) as well as a 25% reduction in absolute myelinated WM volume (p = 0.01) compared with preterm infants without WM lesions (48). Neurodevelopmental outcome assessed at 1 year showed that infants with moderate to severe disabilities had significantly smaller cortical GM volume at term equivalent, compared with former preterm infants without disabilities (48). Volumetric MRI studies conducted in school-aged preterm children have reported smaller corpus callosum (39,49), hippocampus (50), basal ganglia (39), and amygdala (39) as well as enlarged lateral ventricles (50). Altogether, these studies demonstrate that cerebral structural development is altered for many children born preterm. The functional significance of these volume reductions has been explored in school-aged preterm children, for whom regional reductions in brain volumes have been shown to be associated with neuropsychological impairment. For example, reductions in a) hippocampal volumes are associated with memory and learning deficits (51); b) corpus callosum volumes are related to impaired verbal fluency (49); and c) sensori-motor and temporal volumes correlate with lower IQ (39). These findings demonstrate that MRI volumetric abnormalities defined in childhood for preterm children are of clinical relevance, providing some insight into brain-behavior relationships.
Surface-based analyses of cerebral cortex
In adults, studies of cortical folding patterns and their variability across individuals and groups have been greatly aided by surface-based approaches. Surface-based analysis (cortical cartography) offers inherent advantages in terms of visualization. A recent study of Williams Syndrome (52) revealed statistically-significant cortical folding abnormalities compared to control subjects. Moreover, quantitative analyses of shape characteristics indicate that surface-based methods have diagnostic utility at the level of individual subjects. Folding abnormalities have also been demonstrated in autistic spectrum disorder (ASD) using this approach. This modality of MR imaging is currently being evaluated in neonates.
Diffusion MR
Diffusion tensor imaging (DTI) is based on measurement of the displacements of water molecules in tissue. One parameter describing these displacements is the “apparent” diffusion coefficient (ADC) which reflects the overall average water displacements in a tissue region. ADC values are commonly used clinically for the detection of acute cerebral injury. ADC also appears a sensitive marker for the presence of injury in preterm infants. High ADC values have been found for WM in preterm infants with diffuse signal abnormalities on T2-weighted images (53) and focal WM signal abnormalities on T1- and T2-weighted images (54). In the absence of injury, changes in ADC values reflect normal cerebral development. ADC values decline steadily during gestation, likely as a result of decreases in tissue water content (55,56).
Another parameter available from DTI is diffusion anisotropy, which provides information about the local environment. ADC values are higher when measured parallel to axons than perpendicular to them owing to the diffusion barrier imposed by myelin sheaths. This spatial variation in ADC is known as diffusion anisotropy. Anisotropy values for white matter show a steady increase during development, particularly when fiber tracts undergo myelination (55,56). Preliminary data reveal a reduction in white matter RA in preterm infants with WM lesions compared to those without WM lesions and term controls (47). Age-dependent increases in WM anisotropy values are smaller in preterm infants with WM lesions when compared to GA-matched infants with no evidence of WM lesions (57). Two important points are worth making: a) Preterm infants with no WM abnormality had cognitive outcomes that were identical to that of the term control cohort, emphasizing that preterm infants, even at extremes of immaturity, can have normal outcomes. b) Increasing severity of WM abnormality was associated with increasing impairment in a wide range of cognitive domains, emphasizing the association of WM pathology with cognitive impairments.
Anisotropy measured can also be applied to GM maturation. In adult human brain, water displacements in cortical GM are approximately isotropic (equal in all directions) suggesting that the distribution of glial, axonal, and dendritic processes restricts water displacement approximately equally in all directions. In developing cortex, cortical architecture is dominated by the radial orientation of the apical dendrites of pyramidal cells and radial glial cells (58). As a result, water displacements are greatest radially (59), parallel to these processes. The loss of anisotropy associated with maturation can be used to assess cortical development and reflects varying rates of development among different cortical areas (60). These gestationally regulated cortical DTI changes noted on MRI were correlated with histopathology in a primate model of neonatal brain injury confirming that the developing cortex has lower ADC values and anisotropy with increasing maturation, likely related to the increasing complexity in tissue organization in the mature cortex (61).
Conclusion
There is exponential growth in the human brain in the last trimester of pregnancy. It is often during this vulnerable time in gestation (24–40 weeks) that the premature neonate is in the NICU where components of the environment are relatively “hostile” to the developing brain. A combination of altered brain development and injury result in long term neurodevelopmental delay ranging from cerebral palsy to severe cognitive delay as well as ADHD and memory disorder in childhood.
MR imaging is emerging as the modality of choice in the anatomic diagnosis of this spectrum of abnormalities. While conventional MR imaging and DTI are widely available in clinical practice, volumetric analysis, tractography and SBM are available in research laboratories. These MR imaging “biomarkers” measured in the neonatal period may help identify premature infants who are at high risk for adverse neurodevelopmental outcomes. This information can then be used to target early intervention therapy services to these infants.
Appendix
Continuing education
-
The most common neurodevelopmental disability seen in premature infants on follow up is
Cerebral palsy
Mild motor deficits
Cognitive delay
Seizures
Language disorder
-
Which of the following components of the developing brain are most vulnerable to injury in premature infants?
Oligodendroglial cell lineage
Sub-plate neurons
Migrating neuronal cells
Axons
all of the above
-
The most common abnormality in premature infants detected on conventional MR imaging at term equivalent age is
Cerebellar injury
Basal ganglia injury
White matter injury
Hippocampal injury
Injury to the internal capsule
-
Diffusion tensor imaging yields apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values. Which of the following is true?
ADC values increase in white matter with advancing gestational age
FA values decrease in the white matter with maturity
ADC values decrease and FA values increase with advancing white matter maturation
ADC values increase and FA values decline with advancing white matter maturation
ADC and FA values in white matter remain relatively stable throughout gestation
-
DTI of the cerebral cortex shows a progressive decline in FA and ADC values with advancing gestational age
True
False
Answers:
c
e
c
c
a
References
- 1.Holsti L, Grunau R, MFMW Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23:9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Taylor H, Minich N, Klein N, Hack M. Longitudinal outcomes of very low birth weight: Neuropsychological findings. J Intern Neuropsychol Soc. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 3.Goyen T, Lui K, Woods R. Visual-motor, visual-perceptual, and fine motor outcomes in very-lowbirthweight children at 5 years. Dev Med Child Neurol. 1998;40:76–81. doi: 10.1111/j.1469-8749.1998.tb15365.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor H, Hack M, Klein N. Attention deficits in children with <750 gm birth weight. Child Neuropsychol. 1998;4:21–34. doi: 10.1076/0929-7049(200003)6:1;1-B;FT049. [DOI] [PubMed] [Google Scholar]
- 5.Rose SAFJ. Memory and processing speed in preterm children at eleven years: A comparison with fullterms. Child Dev. 1996;67:2005–2021. [PubMed] [Google Scholar]
- 6.Luoma L, Herrgard E, Martikainen A, Ahonen T. Speech and language development of children born at < or = 32 weeks’ gestation: a 5-year prospective follow-up study. Dev Med Child Neurol. 1998;40(6):380–387. [PubMed] [Google Scholar]
- 7.Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114(1):50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Anderson P, Doyle L. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 9.Zelkowitz P, Papageorgiou A, Zelazo P, Weiss MS. Behavioral adjustment in very low and normal birth weight children. J Clin Child Psychol. 1995;24(1):21–30. [Google Scholar]
- 10.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38(8):931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto H, Takei N, Saito F, Kachi K, Mori N. The association between obstetric complications and childhood-onset schizophrenia: a replication study. Psychol Med. 2001;31(5):907–914. doi: 10.1017/s0033291701003944. [DOI] [PubMed] [Google Scholar]
- 12.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 13.Ambalavanan N, Baibergenova A, Carlo WA, Saigal S, Schmidt B, Thorpe KE. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. J Pediatr. 2006;148(4):438–444. doi: 10.1016/j.jpeds.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117(4):1131–1135. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 15.Fily A, Pierrat V, Delporte V, Breart G, Truffert P. Factors associated with neurodevelopmental outcome at 2 years after very preterm birth: the population-based Nord-Pas-de-Calais EPIPAGE cohort. Pediatrics. 2006;117(2):357–366. doi: 10.1542/peds.2005-0236. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer B, Roth S, Riley K, Sellwood MW, Baudin J, Neville BG, Wyatt JS. Neurodevelopmental outcome of preterm infants with ventricular dilatation with and without associated haemorrhage. Dev Med Child Neurol. 2006;48(5):348–352. doi: 10.1017/S0012162206000764. [DOI] [PubMed] [Google Scholar]
- 17.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62(5):441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex. J Neuropathol Exp Neurol. 1997;56(3):219–235. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Iida K, Takashima S, Ueda K. Immunohistochemical study of myelination and oligodendrocyte in infants with periventricular leukomalacia. Pediatr Neurol. 1995;13(4):296–304. doi: 10.1016/0887-8994(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 21.Okoshi Y, Itoh M, Takashima S. Characteristic neuropathology and plasticity in periventricular leukomalacia. Pediatr Neurol. 2001;25(3):221–226. doi: 10.1016/s0887-8994(01)00309-5. [DOI] [PubMed] [Google Scholar]
- 22.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 23.Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271(3):355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- 24.Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347(6289):179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255(5050):1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- 27.Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267(1):1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- 28.Volpe JJ. Subplate neurons--missing link in brain injury of the premature infant? Pediatrics. 1996;97(1):112–113. [PubMed] [Google Scholar]
- 29.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30(4):227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. III: gray matter lesions of the neocortex. J Neuropathol Exp Neurol. 1999;58(5):407–429. doi: 10.1097/00005072-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Takashima S, Becker LE, Chan FW. Retardation of neuronal maturation in premature infants compared with term infants of the same postconceptional age. Pediatrics. 1982;69(1):33–39. [PubMed] [Google Scholar]
- 32.Marin-Padilla M. Perinatal brain damage, cortical reorganization (acquired cortical dysplasias), and epilepsy. Adv Neurol. 2000;84:153–172. [PubMed] [Google Scholar]
- 33.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 34.Colacitti C, Sancini G, DeBiasi S, Franceschetti S, Caputi A, Frassoni C, Cattabeni F, Avanzini G, Spreafico R, Di Luca M, Battaglia G. Prenatal methylazoxymethanol treatment in rats produces brain abnormalities with morphological similarities to human developmental brain dysgeneses. J Neuropathol Exp Neurol. 1999;58(1):92–106. doi: 10.1097/00005072-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Roper SN, Gilmore RL, Houser CR. Experimentally induced disorders of neuronal migration produce an increased propensity for electrographic seizures in rats. Epilepsy Res. 1995;21(3):205–219. doi: 10.1016/0920-1211(95)00027-8. [DOI] [PubMed] [Google Scholar]
- 36.Setkowicz Z, Klak K, Janeczko K. Long-term changes in postnatal susceptibility to pilocarpine-induced seizures in rats exposed to gamma radiation at different stages of prenatal development. Epilepsia. 2003;44(10):1267–1273. doi: 10.1046/j.1528-1157.2003.08203.x. [DOI] [PubMed] [Google Scholar]
- 37.Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107(2):217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 38.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Jama. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 40.Felderhoff-Mueser U, Rutherford MA, Squier WV, Cox P, Maalouf EF, Counsell SJ, Bydder GM, Edwards AD. Relationship between MR imaging and histopathologic findings of the brain in extremely sick preterm infants. AJNR Am J Neuroradiol. 1999;20(7):1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 41.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24(5):805–809. [PMC free article] [PubMed] [Google Scholar]
- 42.Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed. 1999;81(2):F116–121. doi: 10.1136/fn.81.2.f116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, Lewis SW, Kingsley DP, Moseley IF, Foster O, Murray RM. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet. 1999;353(9165):1653–1657. doi: 10.1016/s0140-6736(98)07130-x. [DOI] [PubMed] [Google Scholar]
- 44.Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54(5):672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- 45.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 46.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 47.Huppi PS. MR imaging and spectroscopy of brain development. Magn Reson Imaging Clin N Am. 2001;9(1):1–17. vii. [PubMed] [Google Scholar]
- 48.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 49.Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127(Pt 9):2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 50.Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125(Pt 7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 51.Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47(6):713–720. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26(20):5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112(1 Pt 1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, Barkovich AJ. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24(8):1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 55.Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aaronovitz JA, Miller JP, Lee BCP, Conturo TE. Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured using diffusion tensor imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 56.Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 57.Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16(6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 58.Marin-Padilla M. Cerebral Cortex. Vol. 7. New York: Plenum Press; 1988. Early ontogenesis fo the human cerebral cortex. Development and Maturation of Cerebral Cortex; pp. 1–34. [Google Scholar]
- 59.McKinstry RC, Mathur A, Miller JP, Ozcan AO, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ. Radial Organization of Developing Human Cerebral Cortex Revealed by Non-invasive Water Diffusion Anisotropy MRI. Cereb Cortex. 2002;12:1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 60.Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27(3):579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 61.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Modeling water diffusion anisotropy within fixed newborn primate brain using Bayesian probability theory. Magn Reson Med. 2006;55(1):187–197. doi: 10.1002/mrm.20728. [DOI] [PubMed] [Google Scholar]
- 62.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008 Mar;38(3):260–4. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]


