Abstract
Time estimation, within a range of seconds, involves cognitive functions which depend on multiple brain regions. Here we report on studies investigating the reproduction and production of three durations (5, 14, and 38 seconds) in four groups of patients. The amnesic patient underproduced the length of the long durations because of episodic memory deficit following bilateral medial temporal lesions. Epileptic patients (n = 9) with right medial temporal lobe resections underproduced the three durations because of a distorted representation of time in long-term memory. Traumatic brain injury patients (n = 15) made more variable duration productions and reproductions because of working memory deficits following frontal-lobe dysfunction. Patients with Parkinson's disease (n = 18) overproduced the short duration and underproduced the long duration because of a possible increase in internal clock speed following levodopa treatment, as well as working memory deficits associated with frontal-lobe damage. Further research, in neurological and psychiatric patients, is required to better understand the underlying mechanisms of time estimation.
Keywords: brain-damaged patient, time estimation, duration reproduction, duration production, memory
Abstract
La estimación del tiempo, para el rango de segundos, involucra funciones cognitivas que dependen de múltiples regiones cerebrales. En este artículo se presentan estudios que investigan la reproducción y producción de tres duraciones de tiempo (5, 14 y 38 segundos) en cuatro grupos de pacientes. El paciente amnésico subestimó la longitud de las duraciones más largas debido al déficit de la memo-ría episódica posterior a lesiones temporales mediales bilaterales. Los pacientes epilépticos (n = 9), con resecciones del lóbulo temporal medial derecho, subestimaron las tres duraciones debido a una representación distorsionada del tiempo en la memoria a largo plazo. Los pacientes con daño cerebral traumático (n = 15) tuvieron producciones y reproducciones de duración más variable debido a los déficit en la memoria de trabajo posteriores a disfunciones del lóbulo frontal. Los pacientes con Enfermedad de Parkinson (n = 18) sobreestimaron la producción más corta y subestimaron la producción más larga debido a un posible aumento en la velocidad del reloj interno después del tratamiento con levodopa, así como los déficit en la memoria de trabajo asociados con daños del lóbulo frontal. Se requiere de futuras investigaciones en pacientes neurológicos y psiquiátricos para una mejor comprensión de los mecanismos subyacentes a la estimación del tiempo.
Abstract
L'estimation temporelle implique des fonctions cognitives sous contrôle d'un ensemble de structures cérébrales. Nous rapportons les reproductions et productions de trois durées (5, 14 et 38 secondes) dans quatre groupes de patients. Le patient amnésique sous-estime les durées longues en reproduction car il présente un déficit de mémoire épisodique consécutif aux lésions médio-temporales bilatérales. Les patients épileptiques avec résection médio-temporales droites (n = 9) produisent des durées plus courtes du fait d'une représentation mnésique altérée des unités de temps. Les patients traumatisés crâniens (n = 15) fournissent des jugements temporels variables liés au déficit de mémoire de travail résultant d'un dysfonctionnement frontal. Les patients avec maladie de Parkinson (n = 18) surestiment la durée courte et sous-estiment la durée longue à cause d'une accélération probable de la vitesse d'horloge interne induite par le traitement dopaminergique ainsi que d'une altération de la mémoire de travail résultant d'un dysfonctionnement frontal. D'autres recherches menées chez des patients atteints de troubles neurologiques et psychiatriques devraient permettre de mieux comprendre les mécanismes sous jacents à l'estimation du temps.
Introduction
In daily life, people are required to estimate the duration of both external and internal events in order to anticipate significant changes and adapt their actions accordingly. Thus, time estimation plays an important role in adaptation to the environment. A number of factors can influence, time estimation, such as the size of the duration to be evaluated and the task used to elicit the duration judgment.1,2
Time estimation in the millisecond range is considered to be automatic and associated with the motor system, whereas time estimation in the second-to-minute range is considered to be controlled by cognition.3,4 As our interest is in the relationships between time estimation and cognition, we will focus this review on the longer duration range.
Studies of timing in the second-to-minute range have been influenced by the prominent Scalar Timing Theory.5,6 According to this theoretical model, temporal judgments are based on three information-processing stages: the clock stage, the memory stage, and the decision stage. The first stage is the clock stage, which refers to a pacemaker emitting pulses at a mean constant rate. These pulses are gated by a switch into an accumulator when the signal duration is being processed. Duration judgments depend on the pulse number, which increases as time elapses. The content of the accumulator, corresponding to the current time, is transferred and stored in a working memory system. The decision results in a comparison between the content of working memory and the content of reference memory. This long-term memory system is able to store representations of several number of pulses accumulated in past trials (Figure 1).
Figure.1. Scalar timing theory. Reproduced from ref 6: Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann N Y Acad Sci. 1984;423:52-77. Copyright © New York Academy of Sciences 1984.
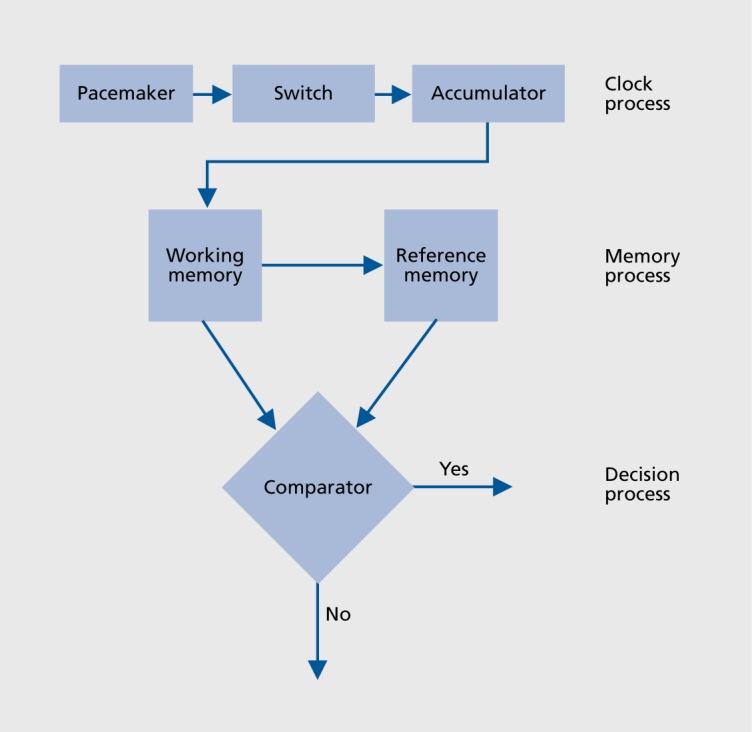
The temporal performance is also closely related to the task used to collect the duration judgments. For example, steady-state changes in the pacemaker rate will have no effects on the accuracy of duration judgments in a reproduction task. In this task, subjects have to evaluate and reproduce a target duration by comparing time passing during the evaluation phase with that elapsing during the reproduction phase. Both periods of time are typically coded by the same internal pacemaker pulsing at the same rate in the two phases of the task. In contrast, changes in the speed of the internal clock will be revealed in time estimation tasks requiring a translation between experienced duration and conventional time units, such as the production task (eg, press this button after what seems like 5 seconds to you) or the verbal estimation task (eg, how long have you been doing this task?).
The accuracy and variability of temporal judgments can also vary according to the conditions in which time estimation tasks are performed. Dual-task paradigms with time estimation and a concurrent task yield to shorter experienced durations.7-9 In these conditions, attention must be shared between processing temporal and non-temporal information, and fewer pulses are gated into the accumulator and transferred into the working memory store. Conversely, when participants can count for the stimulus duration, temporal judgments are generally accurate. The difference of temporal performance between these two conditions relies on the amount of attention allocated to time (ie, to the evaluation of duration).
Two indices of performances can be computed to assess accuracy and precision of temporal judgments. The first reflects the accuracy of temporal judgments and is computed by taking the ratio of the duration reproduced or produced to the target duration. The second is the coefficient of variation (CV), computed by taking the ratio of the standard deviation to the reproduction or production mean. This index represents the variability of temporal judgments of each participant, and allows evaluation of how consistent subjects are in their reproductions or productions of the same target duration.
Neuropsychological and neuroimaging studies have shown that many parts of the brain contribute to time estimation, the most commonly cited being the cerebellum, the right parietal cortex, the right prefrontal cortex, and the frontostriatal network.10,11 Different neural systems are probably implicated, depending on the temporal task and the duration range used. On one hand, time estimation in the millisecond range is related to motor areas of the brain including basal ganglia, supplementary motor area, and cerebellum. Patients with cerebellar lesions are generally impaired in motor timing tasks for durations in the range of milliseconds.12-15 Patients with Parkinson's disease, who have damage to the basal ganglia, also exhibit impaired temporal discrimination in the millisecond time range.16 On the other hand, time estimation in the second-to-minute range is related to the prefrontal and parietal cortices but also to a frontostriatal network, modulated by dopamine.10,11 Patients with prefrontal lesions,17,18 frontal lesions, or Parkinson's disease19-21 exhibit time estimation impairments for durations in the second-to-minute range. The activation of the vagus nerve, which stimulates the frontal lobe, also modulates time perception in patients with major depressive disorder.22 However, it is not clear whether these brain areas are directly related to time estimation, or if they take part in attention, working memory, and decision-making processes involved in time estimation of durations in the second range.10-21 Impairments of time estimation in schizophrenic patients could result from a combined effect of a disturbed central timing mechanism supported by dopamine in the basal ganglia and cognitive deficits mediated by a prefrontal-thalamic-striatal network23
Several studies suggest a predominant involvement of the right hemisphere in time estimation.22-24 Patients with right temporal lobe lesions are particularly affected in the estimation of durations in the second-to-minute range.25-28 Patients with lesions to the right prefrontal cortex also show time estimation deficits for the reproduction of long durations,18 and neuroimaging investigations confirm the activation of the right prefrontal cortex in time estimation.3,29 A specific dysfunction of the right putamen, right prefrontal cortex, and right thalamus underlie time estimation deficits in schizophrenia.23 The right hemispheric specificity for timing seems to depend on the temporal task used. Explicit timing tasks, such as the reproduction task, activates the right frontostriatal network, whereas implicit timing tasks, in which the subjects have to predict the offset of a duration, activate the left parietal and premotor cortex as well as the cerebellum.” Consequently, these studies underline that the brain structures and cognitive processes involved in time estimation may differ according to the paradigm used. In the last few years, a number of neuropsychological studies have been aimed at clarifying the relationships between time estimation and cognition by investigating time judgments in patients who present memory and attention dysfunctions following brain damage. In this article, we report on time estimation in the second range.
Time estimation: neuropsychological studies
We conducted a series of neuropsychological studies using the same paradigm in an amnesic patient,30 in epileptic patients with temporal lobe resections,31 in traumatic brain injury (TBI) patients,32 and in patients with Parkinson's disease.33 Two time estimation tasks were used (duration reproduction task and production task), in two conditions (control counting and concurrent reading conditions), with the same three target durations (5, 14, and 38 seconds). In the control counting condition, the participants were told to count aloud for the stimulus duration, as regularly as possible and at the rate they preferred, throughout the encoding and the reproduction phases of the reproduction task and the production task. In the concurrent reading condition, subjects were told to read aloud digits throughout the encoding phase of the reproduction task and until they felt that the target duration was over in the production task.
Results will be described and discussed in the frame of other neuropsychological studies using a similar paradigm.
Time estimation in an amnesic patient
The well-known patient “HM” was the first amnesic patient who was investigated regarding his duration judgments,34 and studies with other amnesic patients, or those with Korsakoff's syndrome, have led to the same findings.35-37 Our patient, “AC,” was profoundly amnesic with an impairment of episodic memory, while semantic memory was relatively spared.38
The performances of AC on the reproduction and production tasks revealed a clear dissociation. In the reproduction task, AC exhibited a consistent underestimation of the longer durations (14 s and 38 s), while he performed similarly to control subjects in the production for the same durations.
Considering the selectivity of AG's memory deficit due to his medial temporal lesions, the underestimations in the reproduction task could come from a failure in the retrieval of information from episodic memory.
Encoding temporal information (ie, time basis pulses) for the long durations would exceed the short-term memory capacity of AC. In contrast, duration productions were not impaired because representation of conventional units of time (eg, seconds) stored in a “reference memory” would be preserved in this patient. We propose that this “reference memory” could be assimilated to semantic memory, which stores general knowledge of the world, including time representation.
Time estimation in epileptic patients with right or left medial temporal resections
Several studies have suggested a predominant involvement of the right hemisphere in time estimation.23,25,29
In our study, eighteen patients had undergone a right or left medial temporal lobe resection for the relief of medically intractable epilepsy.
The results showed no difference between the time reproductions of patients with right or left medial temporal lesions and those of normal controls, whereas patients with right medial temporal resections produced shorter durations than both those produced by left temporal patients and by normal controls.
Our previous study on AC showed impaired reproductions of durations exceeding short-term memory capacity.
Contrarily to the reproduction task, the production task requires associating a given duration with a representation of durations or knowledge of conventional units. We proposed that the impaired duration productions in patients with right medial temporal lobe resection could come from a distorted representation of these time units in long-term memory (ie, reference memory in the scalar theory).
More recently, an impairment of duration productions has been extended to the minute range and to both medial temporal hemispheres.39 Patients with either left or right medial temporal lobe lesions overestimated durations of 1 to 8 minutes when they were asked to produce, them. According to the authors, the amount of attention allocated to time would have been poorer in patients with medial temporal lesions, leading to shorter subjective durations.
Time estimation following traumatic brain injury
Time estimation deficits have been reported in patients with frontal lobe lesions in the production task, for durations in the second range.19,37,40
We investigated temporal judgments of 15 patients with TBI.32 All participants also performed different neuropsychological tests in order to assess memory (short-term, working, and verbal episodic memories), as well as a simple, reaction task to assess information-processing speed.
The results showed that duration reproductions and productions of the three target durations were not less accurate in patients than in control subjects, in either the concurrent condition or in the control condition. Conversely, duration judgments were more variable in patients than in control subjects, on both tasks and in both conditions. Patients also exhibited slower reaction time tasks and poorer memory scores than control subjects.
More recent studies have investigated time estimation in TBI patients, using the verbal estimation task for durations in the seconds range.41,42 Although it is difficult to compare, results obtained in different time estimation tasks, it has been assumed that verbal estimation and production tasks may tap similar time perception mechanisms.43
In the first study, patients in the early stages of recovery following TBI performed verbal time estimates for 10-to 60-second intervals.41 The findings revealed no difference between time estimation in TBI and control subjects for durations less than 30 seconds, whereas TBI patients significantly underestimated the longer durations. The authors suggested that episodic memory dysfunction may account for the poorer accuracy of the TBI participants at durations that exceeded the time frame of working memory, a result that has also been found in other patients with long-term memory deficits.30,34,37
In the second study, the same verbal time estimation task was proposed in the early phase of recovery from injury and 1 year later.42 The results revealed that in the early phase of injury, patients underestimated the durations that exceed working memory, whereas at 12-month follow-up they exhibited normal time judgments despite a persistent episodic memory impairment. Moreover, in both phases of recovery, patients were not more variable than controls in their estimations. Measures of attention, speed processing, and executive functioning in TBI patients were still below these of normal controls at 1 year post-injury, but no significant correlations were found between the neuropsychological tests and time estimation accuracy. The authors suggested that patients could have relearned to accurately estimate time units during recovery, which is compatible with the hypothesis we proposed to explain the accurate duration productions in the amnesic patient AC.30
Time estimation in patients with Parkinson's disease
Parkinson's disease (PD) represents an excellent model to study the effects of dopaminergic dysfunctions on temporal judgments. Our patients performed like normal controls in the reproduction task, while they overproduced the short duration (5 s) and underproduced the long duration (38 s) in the production task: this temporal judgment bias, known as “the migration effect,”20 was correlated with short-term memory scores. Thus, the influence of durations on each other would occur between the different trials within a session rather than between the representations of durations in long-term memory. We also used a finger- tapping task, which is assumed to be a direct measure of internal clock speed, and we found that PD patients who produced the longer durations were those with the slowest 1-second tempo. Therefore, we proposed that levodopa administration in PD patients would have counteracted the slower rate of the internal clock typically reported in nonmedicated patients, without restoring all of the memory functions. Several other recent studies have shown a similar migration effect in PD patients.25,44,45
Time estimation in psychiatric patients
Patients with affective disorders have often been reported to exhibit impaired duration judgments.46-50 Depressive patients generally have the feeling of time passing slowly, and this feeling is correlated with the severity of depression. Conversely, manic patients have the feeling of time passing more quickly than it actually is.49,50 In the duration production tasks, depressive patients are particularly impaired for the longer durations. This result is explained by the requirement of supplementary cognitive processes, in particular attention and memory, in time estimation in the second-to-minute range.47 Time estimation impairments in depressive patients have also been associated with retardation. Indeed, depressive patients are known to show impaired executive functions as well as a slowing processing speed.51,52 The assessment of productions of long durations (35 and 90 s) reveal that both depressed and manic patients overestimate time, with the manic group overestimating even more prominently.48 The overestimation of time in manic patients (ie, shorter durations produced) appear compatible with the accelerated rate of mental events and agitation in these patients. Overestimation of time in depression is more difficult to explain. Although both groups of depressed and manic patients show greater retardation compared with controls, there were no significant correlations between retardation and time productions in none of both groups. Thus, impaired productions in patients with affective disorders could be due to memory deficits. In the reproduction of durations in the second range (1 s, 6 s, and 37 s), which is supposed to involve memory, manic patients underestimate the long duration and depressive patients overestimate the short duration.49 A recent study has shown a similar pattern of impaired reproductions in depressed and manic patients related to severity of illness.50 Additional measurement of short-term and longterm memory would be necessary to better understand the relationships between time estimation and memory in patients with affective disorders.
A time estimation deficit in patients with schizophrenia is also often reported.53-55,57 Also, schizophrenic patients exhibit attention and memory dysfunctions as well as metabolic alterations, including in the dopaminergic systems.56 The results show a tendency for patients with schizophrenia to overestimate time and to be less accurate in time estimation tasks than controls.53 Results have been interpreted as a deficit in a specific timing process. However, most of these studies used other time estimation paradigms than the production and reproduction tasks, as well as short time intervals.54,57 Some authors investigated time productions in patients with schizophrenia and manipulated the amount of attention allocated to time.55 The results showed that the negative effect of the dual task paradigm on the accuracy of time productions was higher for the schizophrenic patients than for the controls. Thus, the authors explained the altered temporal judgments in schizophrenia by working memory deficits. In a reproduction task, schizophrenic patients have been shown to be less accurate than controls and to overestimate time.53 According to the authors, these results suggest that attention and memory, which are impaired in schizophrenic patients and involved in the reproduction task, are confounding factors in schizophrenia. Moreover, dopamine dysregulation is involved in both schizophrenia and cognition.
Further studies are necessary to clarify how time estimation impairments in psychiatric patients are related to biochemical changes and to cognitive deficits.
Discussion
Our work is the first to compare time judgments obtained in several groups of patients with different neurological disorders using the same time estimation paradigm (ie, reproduction and production of three time durations in seconds). We can deduct from this the memory systems and the brain structures involved in each time estimation task (Table I).
TABLE I. The involvement of the memory systems and the associated brain structures in the reproduction and production of short (5 seconds) and long durations (14 and 38 seconds).
| Time estimation tasks | ||
| Duration | Reproduction | Production |
| 5 seconds | Short-term memory | Short-term memory |
| Working memory | Working memory | |
| Attention | Attention | |
| Frontal structures | Frontal structures | |
| delay | Comparison with a representation of time in semantic or procedural memory | |
| 14 and 38 seconds | Retrieval in episodic memory | |
| Bilateral medial temporal lobes | Right medial temporal and/or subcortical structures |
Short-term memory
Short-term and working memory would be involved in both reproductions and productions of durations in the second range. The accurate reproduction of short durations by the amnesic patients is due to the fact that short-term memory is preserved. Inversely, TBI and PD patients, who generally show working memory deficits, made respectively more variable duration reproductions and productions, and inaccurate productions. The frontal lobe would be necessary to maintain attention during the trial while accumulating temporal information in working memory.
Long-term memory
This would be differently involved in the productions and reproductions of durations in the second range.
In the reproduction task, episodic memory would be necessary to reproduce durations exceeding 10 to 15 seconds. The delay introduced between the encoding and the reproduction phases would require a retrieval of information from long-term memory when the target durations exceed the short-term memory span. In AC, the underestimation of long durations reflects a defective retrieval of information in episodic memory consecutive to bilateral medial temporal lesions.
A long-term memory system would also be required in the production task. When subjects have to produce a target duration given in conventional units of time, they must compare the content of time units accumulated and stored in working memory with a representation of time stored in a “reference memory.”5 Since the amnesic patient was able to produce durations as normal controls, we propose that this reference memory could be a semantic memory, which is preserved in AC. The representation of time would be stored with the general knowledge of the world in semantic memory. We would know what a second signifies, just as we know who is the president of the United States. Semantic memory involved in the production task would depend on the right medial temporal lobe, since epileptic patients are impaired in the production of durations. This explanation is consistent with recent studies which support the involvement of the medial temporal lobe in both episodic and semantic memory.58
Alternatively, since PD patients exhibit impaired duration productions, we can deduct from this that “reference memory” corresponds to procedural memory, which is generally altered in these patients.45 Thus, the representation of time would result in the past experience of time judgment in daily life. We would know what a second signifies, just as we know how to cook. The procedural memory necessary for the production task would thus depend on subcortical structures.
Attention and executive functions also seem to play an important role in duration reproduction and production tasks.
Further neuropsychological studies, combining neurological and psychiatric disorders, using the same time estimation tasks for the same duration range, will contribute to a better understanding of the complex interactions between cognition and time estimation.
REFERENCES
- 1.Zakay D. The evasive art of subjective time measurement: some methodological dilemmas. In: Block RA, ed. Cognitive Models of Psychological Time. Hillsdale, NJ: Erlbaum:59–84. [Google Scholar]
- 2.Block RA., Zakay D. Prospective and retrospective duration judgments: a meta-analytic review. Psychon Bull Rev. 1997;4:184–197. doi: 10.3758/BF03209393. [DOI] [PubMed] [Google Scholar]
- 3.Lewis PA., Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behav Proc. 2006;71:226–234. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ivry RB., Spencer RMC. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Church RM. Properties of the internal clock. In: Gibbon J, Allan LG, eds. Annals of the New York Academy of Sciences: Timing and Time Perception. New York, NY: New York Academy of Sciences. 1984:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibbon J., Church RM., Meek WH. Scalar timing in memory. Ann N Y AcadSci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 7.Zakay D., Block RA. Prospective and retrospective duration judgments: an executive-control perspective. Acta Neurobiol Exp (Wars). 2004;64:319–328. doi: 10.55782/ane-2004-1516. [DOI] [PubMed] [Google Scholar]
- 8.Casini L., Macar F. Effects of attention manipulation on judgments of duration and of intensity in the visual modality. Mem Cognit. 1997;25:812–818. doi: 10.3758/bf03211325. [DOI] [PubMed] [Google Scholar]
- 9.Fortin C. Attentional time-sharing in interval timing. In: Meek W, ed. Functional and Neural Mechanisms of Interval Timing CRC Press; 2003. Available at: http://www.crcnetbase.com/doi/abs/10.1201/9780203009574.ch9. Accessed August 2012. [Google Scholar]
- 10.Wittmann M. The inner experience of time. Philos Trans R Soc Lond, B Biol Sci. 2009;364:1955–1967. doi: 10.1098/rstb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coull JT., Cheng R-K., Meek WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology . 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casini L., Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13:10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Mangels JA., Ivry RB., Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 14.Ivry RB., Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 15.Harrington DL., Lee RR., Boyd LA., Rapcsak SZ., Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004;127(Pt 3):561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- 16.Harrington DL., Haaland KY., Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Kagerer FA., Wittmann M., Szelag E., Steinbuchel NV. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40:357–366. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 18.Koch G., Oliveri M., Carlesimo GA., Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59:1658–1658. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- 19.Nichelli P., Clark K., Hollnagel C., Grafman J. Duration processing after frontal lobe lesions. Ann N Y Acad Sci. 1995;769:183–190. doi: 10.1111/j.1749-6632.1995.tb38139.x. [DOI] [PubMed] [Google Scholar]
- 20.Malapani C., Deweer B., Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson's disease. J Cogn Neurosci. 2002;14:311–322. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- 21.Harrington DL., Castillo GN., Greenberg PA., et al. Neurobehavioral mechanisms of temporal processing deficits in Parkinson's disease. PLoS One. 2011;6:e17461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biermann T., Kreil S., Groemer TW., et al. Time perception in patients with major depressive disorder during vagus nerve stimulation. Pharmacopsychiatry. 201 1;44:179–182. doi: 10.1055/s-0031-1280815. [DOI] [PubMed] [Google Scholar]
- 23.Volz HP., Nenadic I., Gaser C., Rammsayer T., Hager F., Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- 24.Harrington DL., Haaland KY., Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18:1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch G., Costa A., Brusa L., et al. Impaired reproduction of second but not millisecond time intervals in Parkinson's disease. Neuropsychologia. 2008;46:1305–1313. doi: 10.1016/j.neuropsychologia.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Basso G., Nichelli P., Frassinetti F., di Pellegrino G. Time perception in a neglected space. NeuroReport. 1996;7:2111–2114. doi: 10.1097/00001756-199609020-00009. [DOI] [PubMed] [Google Scholar]
- 27.Vidalaki VN., Ho MY., Bradshaw CM., Szabadi E. Interval timing performance in temporal lobe epilepsy: differences between patients with left and right hemisphere foci. Neuropsychologia. 1999;37:1061–1070. doi: 10.1016/s0028-3932(98)00155-9. [DOI] [PubMed] [Google Scholar]
- 28.Rubia K., Schuri U., von Cramon DY., Poeppel E. Time estimation as a neuronal network property: a lesion study. NeuroReport. 1997;8:1273–1276. doi: 10.1097/00001756-199703240-00043. [DOI] [PubMed] [Google Scholar]
- 29.Harrington DL., Haaland KY. Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research. Rev Neurosci. 1999;10:91–116. doi: 10.1515/revneuro.1999.10.2.91. [DOI] [PubMed] [Google Scholar]
- 30.Perbal S., Pouthas V., Van der Linden M. Time estimation and amnesia: a case study. Neurocase. 2000;6:347–356. [Google Scholar]
- 31.Perbal S., Ehrle N., Samson S., Baulac M., Pouthas V. Time estimation in patients with right or left medial-temporal lobe resection. NeuroReport. 2001;12:939–942. doi: 10.1097/00001756-200104170-00015. [DOI] [PubMed] [Google Scholar]
- 32.Perbal S., Couillet J., Azouvi P., Pouthas V. Relationships between time estimation, memory, attention, and processing speed in patients with severe traumatic brain injury. Neuropsychologia. 2003;41:1599–1610. doi: 10.1016/s0028-3932(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 33.Perbal S., Deweer B., Pillon B., Vidailhet M., Dubois B., Pouthas V. Effects of internal clock and memory disorders on duration reproductions and duration productions in patients with Parkinson's disease. Brain Cogn. 2005;58:35–48. doi: 10.1016/j.bandc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Richards W. Time reproductions by H.M. Acta Psychologica. 1973;37:279–282. doi: 10.1016/0001-6918(73)90020-6. [DOI] [PubMed] [Google Scholar]
- 35.Williams JM., Medwedeff CH., Haban G. Memory disorder and subjective time estimation. J Clin Exp Neuropsychol. 1989;11:713–23. doi: 10.1080/01688638908400927. [DOI] [PubMed] [Google Scholar]
- 36.Shaw C., Aggleton JP. The ability of amnesic subjects to estimate time intervals. Neuropsychologia. 1994;32:857–873. doi: 10.1016/0028-3932(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 37.Mimura M., Kinsbourne M., O'Connor M. Time estimation by patients with frontal lesions and by Korsakoff amnesics. J Int Neuropsychol Soc. 2000;6:517–528. doi: 10.1017/s1355617700655017. [DOI] [PubMed] [Google Scholar]
- 38.Linden MV. Semantic memory and amnesia: a case study. Cogn Neuropsychol. 1996;13:391–414. [Google Scholar]
- 39.Noulhiane M., Pouthas V., Hasboun D., Baulac M., Samson S. Role of the medial temporal lobe in time estimation in the range of minutes. Neuroreport. 2007;18:1035–1038. doi: 10.1097/WNR.0b013e3281668be1. [DOI] [PubMed] [Google Scholar]
- 40.Wiener M., Coslett HB. Disruption of temporal processing in a subject with probable frontotemporal dementia. Neuropsychologia. 2008;46:1927–1939. doi: 10.1016/j.neuropsychologia.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitter-Edgecombe M., Rueda AD. Time estimation and episodic memory following traumatic brain injury. J Clin Exp Neuropsychol. 2008;30:212–223. doi: 10.1080/13803390701363803. [DOI] [PubMed] [Google Scholar]
- 42.Anderson JW., Schmitter-Edgecombe M. Recovery of time estimation following moderate to severe traumatic brain injury. Neuropsychology. 2011;25:36–44. doi: 10.1037/a0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craik Fl., Hay JF. Aging and judgments of duration: effects of task complexity and method of estimation. Percept Psychophys. 1999;61:549–560. doi: 10.3758/bf03211972. [DOI] [PubMed] [Google Scholar]
- 44.Wojtecki L., Elben S., Timmermann L., et al. Modulation of human time processing by subthalamic deep brain stimulation. PLoS One. 2011;6:e24589. doi: 10.1371/journal.pone.0024589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foerde K., Shohamy D. The role of the basal ganglia in learning and memory: insight from Parkinson's disease. Neurobiol Learn Mem. 2011;96:624–636. doi: 10.1016/j.nlm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitamura T., Kumar R. Time passes slowly for patients with depressive state. Acta Psychiatr Scand. 1982;65:415–420. doi: 10.1111/j.1600-0447.1982.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 47.Sevigny M-C., Everett J., Grondin S. Depression, attention, and time estimation. Brain Cogn. 2003;53:351–353. doi: 10.1016/s0278-2626(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 48.Bschor T., Ising M., Bauer M., et al. Time experience and time judgment in major depression, mania and healthy subjects. A controlled study of 93 subjects. Acta Psychiatr Scand. 2004;109:222–229. doi: 10.1046/j.0001-690x.2003.00244.x. [DOI] [PubMed] [Google Scholar]
- 49.Mahlberg R., Kienast T., Bschor T., Adli M. Evaluation of time memory in acutely depressed patients, manic patients, and healthy controls using a time reproduction task. Eur Psychiatry. 2008;23:430–433. doi: 10.1016/j.eurpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Q., Ji Y., Wang K., Zhang L., Liu P., Jiang Y. [Time perception in depressed and manic patients], Zhonghua Yi XueZaZhi. 2010;90:332–336. [PubMed] [Google Scholar]
- 51.Fossati P., Ergis AM., Allilaire JF. [Executive functioning in unipolar depression: a review], Encephaie. 2002;28:97–107. [PubMed] [Google Scholar]
- 52.Baudic S., Benisty S., Dalla Barba G., Traykov L. [Impairment of executive function in elderly patients with major unipolar depression: influence of psychomotor retardation], Psychol Neuropsychiatr Vieil. 2007;5:65–71. [PubMed] [Google Scholar]
- 53.Bonnot O., de Montalembert M., Kermarrec S., Botbol M., Walter M., Coulon N. Are impairments of time perception in schizophrenia a neglected phenomenon? J Physiol Paris. 2011;105:164–169. doi: 10.1016/j.jphysparis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Elvevag B., McCormack T., Gilbert A., Brown GDA., Weinberger DR. Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- 55.Tracy JI., Monaco C., McMichael H., et al. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- 56.Barch DM., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davalos DB., Kisley MA., Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 58.Hoscheidt SM., Nadel L., Payne J., Ryan L. Hippocampal activation during retrieval of spatial context from episodic and semantic memory. Behav Brain Res. 2010;212:121–132. doi: 10.1016/j.bbr.2010.04.010. [DOI] [PubMed] [Google Scholar]


