Abstract
Environmental light synchronizes the primary mammalian biological clock in the suprachiasmatic nuclei, as well as many peripheral clocks in tissues and cells, to the solar 24-hour day. Light is the strongest synchronizing agent (zeitgeber) for the circadian system, and therefore keeps most biological and psychological rhythms internally synchronized, which is important for optimum function. Circadian sleep-wake disruptions and chronic circadian misalignment, as often observed in psychiatric and neurodegenerative illness, can be treated with light therapy. The beneficial effect on circadian synchronization, sleep quality, mood, and cognitive performance depends on timing, intensity, and spectral composition of light exposure. Tailoring and optimizing indoor lighting conditions may be an approach to improve wellbeing, alertness, and cognitive performance and, in the long term, producing health benefits.
Keywords: circadian rhythm, daylight, bright light, indoor lighting, zeitgeber, entrainment, intrinsically photosensitive retinal ganglion cell (ipRGC), light therapy, circadian rhythm sleep disorder, affective disorder
Abstract
La luz ambiental sincroniza el reloj biológico primario de los mamíferos en el núcleo supraquiasmático, así como muchos relojes periféricos en tejídos y células, para el día solar de 24 horas. La luz es el agente sincronizador más potente (zeitgeber=dador de tiempo) para el sistema circadíano, y por consiguiente mantiene la mayoría de los ritmos biológicos y psicológicos que se sincronizan internamente, lo que es importante para una óptima función. Las disrupciones circadianas sueño-vigilia y los desajustes circadianos crónicos que se observan con frecuencía en enfermedades psiquiátricas y neurodegenerativas pueden ser tratados con fototerapía. Los efectos favorables sobre la sincronización circadíana, la calidad del sueño, el ánimo y el rendimiento cognitivo dependen de la duración, intensidad y composición espectral de la exposición a la luz. La adaptación y optímízacíón de las condíciones de luz interior pueden constituir una manera de mejorar el bienestar, el alerta y el rendimiento cognitivo, y a largo plazo producir beneficios para la salud.
Abstract
La lumière environnementale synchronise l'horloge biologique primaire des mammifères dans le noyau suprachiasmatique, ainsi que de nombreuses horloges périphériques dans les tissus et les cellules, au jour solaire de 24 h. La lumière est l'agent synchronisant le plus fort (zeitgeber) du système circadien, conservant donc la plupart des rythmes biologiques et psychologiques synchronisés en interne, ce qui est important pour un fonctionnement optimal. Les troubles circadiens veille-sommeil et le décalage circadien chronique, souvent observés dans les maladies psychiatriques et neurodégénératives, peuvent être traités par luminothérapie. L'effet bénéfique sur la synchronisation circadienne, la qualité du sommeil, l'humeur et la performance cognitive dépend de la chronologie, de l'intensité et de la composition spectrale de l'exposition à la lumière. Personnaliser et optimiser les conditions de lumière en intérieur pourraient permettre d'améliorer le bien-être, la vigilance et la performance cognitive et, à long terme, être bénéfique pour la santé.
Introduction
The solar 24-hour cycle has existed for more than 4 billion years, and it has led to the evolution of circadian rhythms in most organisms. In mammals, a circadian master clock in the brain has evolved in the suprachiasmatic nuclei (SCN) of the mammalian hypothalamus.1 This master clock has a genetically determined endogenous period length which slightly differs from 24 hours2 and has to be adjusted to the exact 24-hour rhythm day by day. Environmental light is the strongest synchronizer for the circadian system, and phase-resetting capacities to light mainly depend on time of day, light intensity, and spectral composition.3 Table I illustrates illuminance ranges (lx) under different natural and electrical lighting conditions.
Table I. Approximate illuminance ranges of different lighting environments (measured on a horizontal plane). Illuminance indicates the flux density of a light source and is measured in lux (symbol: Ix). Lux is defined as lumen per unit area (lumen per square meter: 1 lx=lm/m2). Please note that in most cases, where the light source is located above a human observer, much less illuminance is measured on a vertical plane at the approximate eye level (viewing direction).
| Environmental lighting situation | Typical illuminance range [lx] |
| Daylight, clear sky | 50 000-100 000 |
| Daylight, overcast sky | 10 000-20 000 |
| Light therapy lamp | 5000-10 000 |
| Precise indoor workbench | 1000-2000 |
| Typical indoor office setting | 300-500 |
| Living room lighting | 50-200 |
| Street and walkway lighting | 5-20 |
| Full moonlight | <1 |
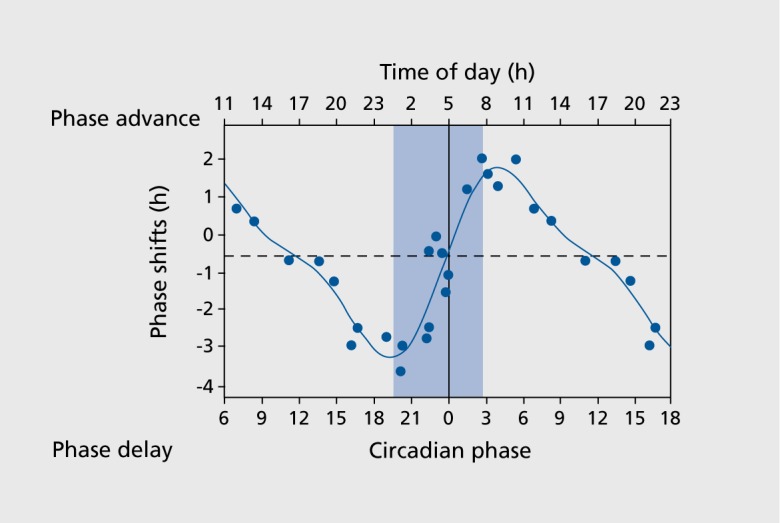
Under controlled laboratory conditions, the impact of timing light exposure has been shown to shift the human biological clock, as illustrated in the phase-response curve to light (Figure 1.):4 The strongest circadian phase delays were induced when light exposure occurred in the evening or night hours before the core body temperature nadir (usually around 5 am). Maximum advances occurred in the early morning hours after the core body temperature minimum, whereas around noon, bright light exposure exerted only small effects. The impact of light intensity on circadian phase was studied using light pulses from 0.1 to 10 000 lx to describe and quantify dose-response curves.5,6 When different light intensities and durations were combined, longer exposures with moderate light intensity resulted in larger phase shifts than shorter exposures to brighter light.7 Most recently, a duration-response curve to a single bright light pulse of 10 000 lx demonstrated a nonlinear relationship for circadian phase shifts in humans after different durations (0.2 h to 4 h).8 Shorter light exposures were more effective.
Figure 1. The human phase response curve, where phase advances are indicated with positive values, and delays with negative values. The data are plotted against the timing of the center of the light exposure, relative to the melatonin midpoint on the pre-stimulus circadian phase assessments (defined to be 22 h). Data points from circadian phases 6 to 18 are double plotted. The filled circles represent data from melatonin samples. The solid cuive is a dual harmonic function fitted through all of the data points. The horizontal dashed line represents the anticipated 0.54-h average delay drift of the pacemaker between the pre- and post-stimulus phase assessments. The vertical line represents the core body temperature minimum (= circadian phase 0), and the blue bar represents biological night. Adapted from ref 4: Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945-952, Copyright © Cambridge University Press 2003.
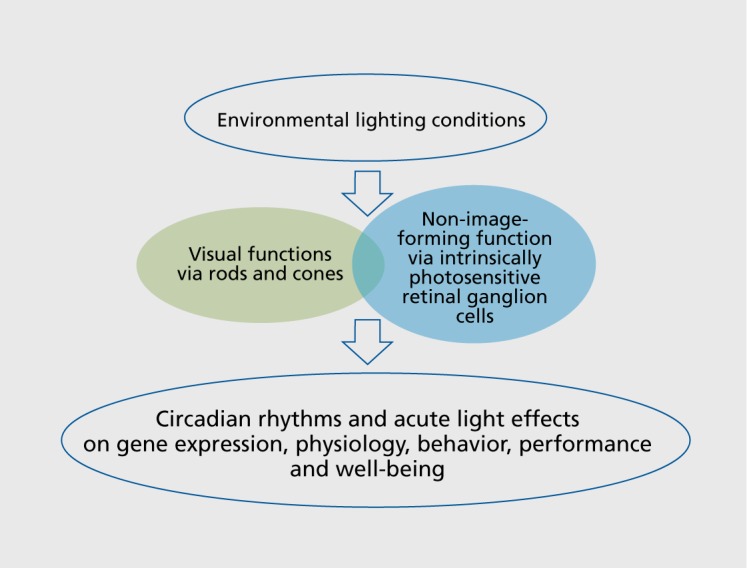
Light through the eyes is perceived by rods, cones, and also intrinsically photosensitive retinal ganglion cells (ipRGC)9,10 (Figure 1.) containing the photo-pigment melanopsin. Only 1% to 2% of ipRGCs are directly light-sensitive, and to date five subtypes of ipRGCs (M1-M5) are anatomically and functionally distinguishable. 11 The ipRGCs integrate incoming light information in two different ways: directly by intrinsic photosensitivity and via afferent synaptic input from rods and cones. Melanopsin-dependent neuronal projections connect the retina with deeper brain areas such as the SCN, the pretectal olivary nucleus (pupillary light reflex), pineal gland, habenula, thalamus, and many more.12,13 The ipRGC's response is shifted towards the blue light spectra, showing a sensitivity peak around 480 nm, whereas the photopic sensitivity maximum of the three types of cones is in the green part of visible light (at 555 nm).14
Figure 2. Schematic summary of the general impact of light on both visual and non-image-forming biological functions.
Acute light effects
Light also exerts acute effects on subjective alertness and cognitive performance, and it inhibits the secretion of melatonin by the pineal gland.3,5,15,16 Salivary or plasma melatonin concentrations are commonly used to assess circadian phase or to quantify the magnitude of light-induced melatonin suppression. Acute light effects are dependent on the photopigment melanopsin, and are stronger when light contains a greater proportion of blue light.3,17 For example, light exposure with monochromatic blue light had a greater alerting effect, increased heart rate, core body temperature, cognitive performance, pupil light reflex, and clock gene expression compared with green light (for reviews see refs 3,17). Several functional magnetic resonance imaging (fMRI) studies have revealed higher brain activity16 and stronger effects on mood-related brain areas to monochromatic blue than to green light.18 Even a low-lit computer screen, which contains more blue light, had stronger effects on subjective alertness and cognitive performance than a conventional screen.19 Most of these studies were performed during nighttime, with prior dim or dark adaptation. Some also showed acute light effects during daytime and evening with polychromatic white light20-22 or blue-enriched light sources.23,24 Acute light effects are at least partly conveyed by the ascending reticular arousal system, projecting to higher cortical areas via the brain stem, hypothalamus, thalamic nuclei, and other brain regions,16,22 known to convey visual and nonvisual information (such as the lateral geniculate nuclei of the thalamus25). There is growing evidence that genetic factors, for example the clock gene PER3 polymorphism, play a role in responsiveness to acute light effects in humans.26 Prior light history modulates subsequent light effects, as has been shown on circadian phase shifts, melatonin suppression, and cognitive performance.27-29 Light exposure during the day impacts on sleep during the night,30 with different effects on sleep latency, non-rapid eye movement sleep, slow-wave activity, and wakefulness during scheduled sleep, as well as on rapid eye movement sleep latency. These changes depend on the light source, exposure duration, and timing.30-32
Light and age
There is conflicting information as to whether healthy older adults undergo a general attenuation in non-image-forming light perception. At the level of the eye, a substantial proportion of visible blue light is filtered out due to physiological yellowing of the aging lens and smaller baseline pupil size.33 Some evidence for functionally intact (blue) light sensitivity with age comes from a recent study, in which steady-state pupil constrictions to monochromatic blue light were similar in young and older subjects.34 Another study showed that sustained pupil contraction to a bright blue light stimulus increased, and redilation to baseline took longer, in older than younger subjects.35 This may indicate a compensatory response to the lower blue light transmission through the aging lens. The fact that older patients with cataracts exhibited faster reaction times under blue-enriched light after cataract surgery (with clear UV-only blocking intraocular lens replacement) when compared with presurgery performance36 indicates that attenuation of blue light sensitivity might be an epiphenomenon caused by reduced light transmission of the lens, and not by a change in sensitivity of melanopsin-dependent function itself.
Likewise, older subjects suppressed melatonin less in response to monochromatic blue than green light37,38 and exhibited reduced responsiveness with respect to mood and alertness when compared with a young group.37 Healthy older subjects showed a reduction in the phase-delaying response to moderate light39 and, evening exposure of healthy elderly subjects to either polychromatic blue-enriched or polychromatic white light did not differ in its effects on phase delay, evening alertness, and sleep architecture.40 One interpretation might be that non-image-forming light perception undergoes different age-related modifications of functionally separated ipRGC dependent pathways.
Light treatment of misaligned circadian rhythms
Scheduled bright light exposure is an effective countermeasure for sleepiness and fatigue, ie, in shift workers during night work and for re-entrainment after return to daytime shifts, or jet lag. Other circadian rhythm sleep disorders, such as delayed and advanced sleep phase syndrome, can be treated with light therapy as well (for reviews see refs 41,42). Light therapy is used alone or as adjuvant therapy in a growing number of psychiatric and neurodegenerative diseases, where alterations of sleep-wake cycles are often observed:43-45 Light has antidepressant properties and is the treatment of choice for seasonal affective disorder (SAD),46 and is increasingly used in nonseasonal depression,45-47 particularly efficient when combined with serotonin reuptake inhibitors.48-50
Moreover, beneficial effects of light on mood, agitation, sleep quality, and/or cognitive performance have been found in patients with ante-partum depression,51 borderline personality disorder,52,53 bulimia nervosa,54 adult attention-deficit/hyperactivity disorder,55 and Parkinson's disease56 as well as Alzheimer's disease and other dementias,57,58 by applying open trial designs and double-blind protocols. The control condition in those studies was either no light treatment, or lower light intensities (ie, 70 lx,51 500 lx red light54), or lower lighting (±300 lx) compared with whole-day bright lighting installations (±1000 lx) in elderly care units.58 Regular outdoor morning light exposure has led to similar antidepressant effects in SAD as with artificial light therapy.59
Optimizing indoor lighting conditions
Despite the multitude of studies investigating light effects on humans, it is still unclear how much light is needed during daytime to stay fully entrained to the environmental light-dark cycle. This becomes an important topic in our round-the-clock society, since the time we spend outside during the day progressively decreases, whereas the time we spend with light-emitting devices during the night increases. Another factor is the substantial interindividual difference in light requirements by the circadian system to stay synchronized with the external 24-hour rhythm, for example between extreme morning or evening types (“larks” and “owls”). The definition of extreme chronotypes is based on subjective preferences for very early (morning type) or very late (evening type) habitual sleep and wake (times), which usually differ by 2 to 4 hours.60 Both chronotypes are usually obliged to follow similar scheduled work hours in spite of being at different endogenous circadian phases,60 and thus, respond differently to the daytime light exposure, which has to be considered when designing indoor lighting conditions.
Conclusion
Chronobiological knowledge of how light affects human behaviour has begun to be implemented at work places,24 in schools,61 and in clinical environments (such as residential care homes for the elderly, and intensive care and neonatal units24,51). There is still much work to do: to test, predict and apply optimal lighting conditions for different populations and patients, in terms of spectral composition, light intensity, and dynamics. Also, geographical latitude, building exposure, and building properties play an important role. Only synergistic interdisciplinary work between (neuro-) scientists, physicians, architects, and engineers will allow us to better assess and optimize lighting conditions, and to foster the translation into practical applications.
Acknowledgments
Dr M. Münch is supported by the Velux Foundation (Switzerland), and Dr V. Bromundt by the AXA Research Fund.
Contributor Information
Mirjam Münch, Laboratory for Solar Energy and Building Physics, Ecole Polytechnique Federale de Lausanne, Switzerland.
Vivien Bromundt, Centre for Chronobiology, Psychiatric Hospital of the University of Basel, Switzerland.
REFERENCES
- 1.Moore RY., Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Czeisler CA., Duffy JF., Shanahan TL., et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 3.Lockley SW. Circadian rhythms: influence of light in humans. In: Larry RS, ed. Encyclopedia of Neuroscience. Oxford, UK: Academic Press; 2009:971–988. [Google Scholar]
- 4.Khalsa SB., Jewett ME., Cajochen C., Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeitzer JM., Dijk DJ., Kronauer RE., Brown EN., Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Hilaire MA., Gooley JJ., Khalsa SB., Kronauer RE., Czeisler CA., Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590(Pt 13):3035–3045. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewan K., Benloucif S., Reid K., Wolfe LF., Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang AM., Santhi N., St Hilaire M., et al. Human responses to bright light of different durations. J Physiol. 2012;S90(Pt 13):3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berson DM., Dunn FA., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 10.Ruby NF., Brennan TJ., Xie X., et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt TM., Chen SK., Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooley JJ., Lu J., Chou TC., Scammell TE., Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S., Kumar M., Park A., et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatori M., Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16:435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cajochen C., Zeitzer JM., Czeisler CA., Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human-alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 16.Vandewalle G., Maquet P., Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Vandewalle G., Schwartz S., Grandjean D., et al. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci U S A. 2010;107:19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cajochen C., Frey S., Anders D., et al. Evening exposure to a light emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 20.Phipps-Nelson J., Redman JR., Dijk DJ., Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 21.Rüger M., Gordijn MC., Beersma DG., de Vries B., Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 22.Vandewalle G., Balteau E., Phillips C., et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Chellappa SL., Steiner R., Blattner P., Oelhafen P., Götz T., Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PloS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola AU., James LM., Schlangen LJ., Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 25.Dacey DM., Liao HW., Peterson B., et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 26.Chellappa SL., Viola AU., Schmidt C., et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–E437. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- 27.Hébert M., Martin SK., Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang AM., Scheer FA., Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Münch M., Linhart F., Borisuit A., Jaeggi SM., Scartezzini JL. Effects of prior light exposure on cognitive performance, subjective sleepiness, and hormonal secretion in the evening. Behav Neurosci. 2011;126:196–203. doi: 10.1037/a0026702. [DOI] [PubMed] [Google Scholar]
- 30.Dijk DJ., Archer SN. Light, sleep, and circadian rhythms: together again. PloS Biol. 2009;7:e1000145. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münch M., Kobialka S., Steiner R., Oelhafen P., Wirz-Justice A., Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1421–R1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 32.Santhi N., Thorne HC., van der Veen DR., et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 33.Turner PL., Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol. 2008;92:1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daneault V., Vandewalle G., Hébert M., et al. Does pupil constriction under blue and green monochromatic light exposure change with age? J Biol Rhythms. 2012;27:257–264. doi: 10.1177/0748730412441172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbst K., Sander B., Lund-Andersen H., et al. Intrinsically photosensitive retinal ganglion cell function in relation to age: a pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 2012;12:4. doi: 10.1186/1471-2415-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmoll C., Tendo C., Aspinall P., Dhillon B. Reaction time as a measure of enhanced blue-light mediated cognitive function following cataract surgery. Br J Ophthalmol. 2011;95:1656–1659. doi: 10.1136/bjophthalmol-2011-300677. [DOI] [PubMed] [Google Scholar]
- 37.Sletten TL., Revell VL., Middleton B., Lederle KA., Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 38.Herljevic M., Middleton B., Thapan K., Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Duffy JF., Zeitzer JM., Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Münch M., Scheuermaier KD., Zhang R., et al. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav Brain Res. 2011;224:272–278. doi: 10.1016/j.bbr.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arendt J. Shift work: coping with the biological clock. Occup Med (Lond). 2010;60:10–20. doi: 10.1093/occmed/kqp162. [DOI] [PubMed] [Google Scholar]
- 42.Gooley JJ. Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singapore. 2008;37:669–676. [PubMed] [Google Scholar]
- 43.Wulff K., Gatti S., Wettstein JG., Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 44.Wirz-Justice A., Bromundt V., Cajochen C. Circadian disruption and psychiatric disorders: the importance of entrainment. Sleep Med Clin. 2009;4:273–284. [Google Scholar]
- 45.Wirz-Justice A., Terman M. Chronotherapeutics (light and wake therapy) as a class of interventions for affective disorders. Handb Clin Neurol. 2012;106:697–713. doi: 10.1016/B978-0-444-52002-9.00042-5. [DOI] [PubMed] [Google Scholar]
- 46.Terman M., Terman J. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–663. doi: 10.1017/s1092852900019611. [DOI] [PubMed] [Google Scholar]
- 47.Tuunainen A., Kripke DF., Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004;CD004050 doi: 10.1002/14651858.CD004050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benedetti F., Colombo C., Pontiggia A., Bernasconi A., Florita M., Smeraldi E. Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J Clin Psych. 2003;64:648–653. doi: 10.4088/jcp.v64n0605. [DOI] [PubMed] [Google Scholar]
- 49.Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psych Scan. 2004;425:7–28. doi: 10.1111/j.1600-0447.2004.00460_2.x. [DOI] [PubMed] [Google Scholar]
- 50.Kripke DF. Light treatment for nonseasonal depression: speed, efficacy, and combined treatment. J Affect Disord. 1998;49:109–117. doi: 10.1016/s0165-0327(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 51.Wirz-Justice A., Bader A., Frisch U., et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psych. 2011;72:986–993. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- 52.Bromundt V., Wirz-Justice A., Kyburz S., Opwis K., Dammann G., Cajochen C. Circadian sleep-wake cycles, well-being, and light therapy in borderline personality disorder. J Pers Disord. 2012; doi: 10.1521/pedi_2012_26_057. doi: 10.1521/pedi_2012_26_057. [DOI] [PubMed] [Google Scholar]
- 53.Prasko J., Bruovsky M., Latalova K., et al. Augmentation of antidepressants with bright light therapy in patients with comorbid depression and borderline personality disorder. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:355–361. doi: 10.5507/bp.2010.053. [DOI] [PubMed] [Google Scholar]
- 54.Lam RW., Goldner EM., Solyom L., Remick RA. A controlled study of light therapy for bulimia nervosa. Am J Psychiatry. 1994;151:744–750. doi: 10.1176/ajp.151.5.744. [DOI] [PubMed] [Google Scholar]
- 55.Rybak YE., McNeely HE., Mackenzie BE., Jain UR., Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psych. 2006;67:1527–1535. doi: 10.4088/jcp.v67n1006. [DOI] [PubMed] [Google Scholar]
- 56.Willis GL., Moore C., Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson's disease. Rev Neurosci. 2012;23:199–226. doi: 10.1515/revneuro-2011-0072. [DOI] [PubMed] [Google Scholar]
- 57.Van Someren EJ., Kessler A., Mirmiran M., Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 58.Riemersma-van der Lek RF., Swaab DF., Twisk J., Hol EM., Hoogendijk WJ., Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 59.Wirz-Justice A., Graw P., Kräuchi K., et al. “Natural” light treatment of seasonal affective disorder. J Affect Disord. 1996;37:109–120. doi: 10.1016/0165-0327(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 60.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 61.Barkmann C., Wessolowski N., Schulte-Markwort M. Applicability and efficacy of variable light in schools. Physiol Behav. 2012;105:621–627. doi: 10.1016/j.physbeh.2011.09.020. [DOI] [PubMed] [Google Scholar]


