Abstract
Infiltration of immune cells, specifically macrophages, into the tumor microenvironment has been linked to increased mammary tumor formation and progression. Activation of growth factor receptor signaling pathways within mammary epithelial cells, such as the fibroblast growth factor receptor 1 (FGFR1) pathway, induces recruitment of macrophages to the mammary epithelium. These macrophages promote increased epithelial cell proliferation and angiogenesis. However, the specific mechanisms by which these macrophages are regulated by the preneoplastic epithelial cells and the mechanisms of action of the macrophages within the developing FGFR1-driven tumor microenvironment remain unknown. In this study, we demonstrate that activation of inducible FGFR1 in mammary glands leads to decreased activity of the transforming growth factor beta (TGFβ)/Smad3 pathway in macrophages associated with early stage lesions. Further studies demonstrate that macrophages have increased expression of inflammatory chemokines that bind Cxcr2 following exposure to conditioned media from mammary epithelial and tumor cells in which the FGF pathway had been activated. The increase in these ligands is inhibited following activation of the TGFβ pathway, suggesting that decreased TGFβ signaling contributes to the upregulation of these chemokines. Using co-culture studies, we further demonstrate that macrophages are capable of promoting epithelial and tumor cell migration and invasion through activation of Cxcr2. These results indicate that macrophage-derived Cxcr2 ligands may be important for promoting mammary tumor formation regulated by FGFR signaling. Furthermore, these results suggest that targeting Cxcr2 may represent a novel therapeutic strategy for breast cancers that are associated with high levels of infiltrating macrophages.
Keywords: Mammary tumor, breast cancer, FGFR1, CXCR2
Introduction
Breast tumor formation and progression involve complex interactions between tumor cells and their surrounding environment. Infiltration of immune cells into the tumor microenvironment has been linked to tumor formation and progression (1). Specifically, increased numbers of tumor associated macrophages are linked to poor prognosis in breast cancer patients (2). While macrophages were initially expected to inhibit tumor growth via their cytotoxic functions, it is now clear that exposure to the tumor microenvironment polarizes macrophages towards a tumor-promoting phenotype (3). Therefore, obtaining a better understanding of the mechanisms through which macrophages regulate tumor growth and progression may result in the development of strategies that either inhibit the activities of tumor-promoting macrophages or re-program the macrophages to increase their tumor cytotoxic activities. Numerous functions have been ascribed to macrophages during tumor progression, including promotion of tumor cell invasion, angiogenesis and immune suppression (4). While published studies have identified specific mechanisms through which macrophages contribute to mammary tumor metastasis in mouse models (5, 6), less is known regarding the mechanisms of macrophage function during mammary tumor initiation.
Macrophages associated with late stage tumors have been shown to express high levels of transforming growth factor-beta (TGFβ) (7). TGFβ, which is a key regulator of development, immune function and wound healing, acts primarily through a core signaling pathway involving the Smad family of transcription factors (8). During mammary gland development, TGFβ is a potent inhibitor of epithelial cell proliferation and branching morphogenesis (9). In premalignant lesions, TGFβ acts as a tumor suppressor by inhibiting proliferation and promoting apoptosis. Paradoxically, malignant tumors are associated with high levels of TGFβ, which promote later stages of tumor progression and metastasis (8, 10). Pleiotropic effects of TGFβ have also been observed in macrophages, depending on their stage of differentiation. While TGFβ acts as a chemoattractant for monocytes, it inhibits phagocytosis and production of proinflammatory mediators in differentiated macrophages (11). TGFβ expression levels are high in macrophages associated with late stage tumors (7), which is thought to influence many aspects of malignant progression including invasion, angiogenesis and immune suppression (10). However, the functional consequences of regulating the TGFβ/Smad signaling pathway within macrophages during different stages of tumorigenesis have not been investigated.
Inappropriate activation of growth factor signaling pathways has been strongly linked to breast cancer formation and progression. Recent studies have implicated the fibroblast growth factor (FGF) pathway in tumor growth, progression and resistance to standard therapies (12, 13). Amplification of the chromosomal region of 8p12 that includes the FGF receptor 1 (FGFR1) gene is associated with poor prognosis, and approximately 10% of breast cancers exhibit amplified FGFR1 (13, 14). Transgenic mice expressing an inducible FGFR1 (iFGFR1) transgene in mammary epithelial cells develop early epithelial lesions that progress to alveolar hyperplasia, ultimately resulting in mammary tumor formation (15). Activation of iFGFR1 leads to alterations in the microenvironment, including increased angiogenesis and a rapid inflammatory response characterized by infiltrating macrophages (15, 16). Macrophage depletion in this model leads to reduced epithelial cell proliferation and angiogenesis associated with early stage lesions demonstrating that in an FGFR1-dependent model of mammary tumor formation, macrophages are capable of promoting the development of early stage epithelial lesions (16).
In these studies, we have further utilized the iFGFR1 model to identify mechanisms that regulate the pro-tumor functions of macrophages during early stage tumor formation. We demonstrate here that macrophages associated with iFGFR1-driven early stage epithelial lesions exhibit decreased activation of the TGFβ/Smad3 pathway. The decrease in TGFβ-associated genes within macrophages correlates with increased expression of macrophage-derived chemokines that bind to the chemokine receptor Cxcr2. Restoration of TGFβ signaling leads to inhibition of expression of these chemokines in macrophages. These studies suggest that repressed TGFβ/Smad3 signaling may be functionally important for regulating the pro-tumorigenic function of macrophages in early stages of tumor formation. Furthermore, these studies demonstrate that macrophage-derived chemokines, specifically Cxcr2 binding chemokines, promote migration and invasion of preneoplastic mammary epithelial cells, suggesting a potential therapeutic target for early stage breast tumors.
Materials and Methods
Animals
Generation of mouse mammary tumor virus (MMTV)-iFGFR1 transgenic mice has been described previously (15) and the mice were obtained from Dr. Jeffrey Rosen (Baylor College of Medicine, Houston, TX, USA). Animal care and procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and were in accordance with the procedures detailed in the Guide for Care and Use of Laboratory Animals.
Cell sorting and RT-PCR analysis
Six-week-old female MMTV-iFGFR1 transgenic mice and non-transgenic littermates were injected intraperitoneally (i.p.) with 1 mg/kg B/B dimerizer (Clontech, Mountain View, CA, USA). Mice were sacrificed 48 hours later and mammary glands were collected for analysis. The tissue was dissociated using 2 mg/ml collagenase A (Roche Applied Science, Indianapolis, IN, USA) for 45 minutes at 37°C with rocking at 200 rpm. The solutions were vigorously shaken every 15 minutes and the dissociated cells were collected by centrifuging for 5 minutes at 1500 rpm. The cells were washed 3 times with DMEM/F12 containing 5% fetal bovine serum (FBS) at 1500 rpm and 2 times at 800 rpm for 5 minutes each. The cells were stained with either Cd11b-APC (Life Technologies, Grand Island, NY, USA) at a dilution of 1:200 or isotype control antibody at the same concentration for 1 hour at RT. The cells were then washed, filtered through a 40 micron filter and sorted using a triple laser MoFlo (Cytomation, Fort Collins, CO, USA). RNA was isolated from Cd11b-positive cells sorted from 6 mice per timepoint as described above and pooled into duplicate samples. RNA was extracted using the Arcturus PicoPure RNA Isolation Kit (Life Technologies) and RT-PCR analysis was performed using primers specific for ArgI and iNOS as described below. qRT-PCR analysis was performed for TGFβ1 as described below. Primer sequences are listed in Supplemental Table 1. Cyclophilin was used to normalize gene expression levels.
Immunohistochemistry and immunofluorescence
Mammary glands from MMTV-iFGFR1 transgenic mice, or non-transgenic littermate controls, that were treated with B/B for 48 hours as described above were fixed for 2 hours in 4% paraformaldehyde and embedded in paraffin. Five micron sections were used for immunohistochemistry and immunofluorescent staining using the following antibodies and dilutions: rat monoclonal F4/80 (Invitrogen, Carlsbad, CA, USA), 1:50; phospho-Smad3 (Cell Signaling, Danvers, MA, USA), 1:200. Immunostaining was performed as described previously (17) in the absence of antigen retrieval. F4/80 and phospho-Smad3 positive cells were counted and double positive cells were calculated relative to the total number of F4/80 positive cells. A minimum of 600 cells from a total of three mice per treatment group was counted for each dataset. All statistical analyses were performed using the unpaired student's t-test to compare two means.
Cell culture
HC-11 cells were maintained in RPMI containing 10% FBS (Invitrogen), 1% penicillin-streptomycin, 5 μg/ml insulin (Sigma-Aldrich, St. Louis, MO) and 10 ng/ml epidermal growth factor (EGF) (Invitrogen). HC-11 cells stably expressing the iFGFR construct (HC-11/R1) were generated as previously described (18) and were acquired from Dr. Jeffrey Rosen. HC-11/R1 cells were maintained in HC-11 medium with the addition of 0.7 μg/ml puromycin (Sigma-Aldrich). We received SUM225 and MCF10DCIS.com from Dr. Fariba Behbod (University of Kansas City Medical Center, Kansas City, KS, USA) and maintained as described (19). All other cells lines were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and maintained in the suggested media. All cell lines were used for fewer than 6 months after resuscitation. HC-11 cells and HC-11/R1 cells, which are not commercially available, are not maintained for longer than 20 passages and are tested for mycoplasma, β-casein expression and iFGFR1 expression regularly.
Two-dimensional co-culture assays
HC-11/R1 cells were grown to confluence, washed with PBS and incubated overnight in serum-free RPMI. The cells were treated with 30 nM B/B or an equal amount of ethanol as solvent control for 24 hours. Conditioned media was collected (R1-CM) and added to RAW 264.7 cells that had been incubated overnight in serum-free DMEM. To determine gene expression, cells were collected in Trizol after 2 hours of treatment with R1-CM supplemented with or without 10 ng/ml recombinant TGFβ (R&D Systems, Minneapolis, MN, USA) and analyzed as described below. For signaling studies, R1-CM was added to RAW264.7 cells for the indicated time and lysates were collected for immunoblotting. Then, RAW 264.7 cells were pretreated for 30 minutes with 2.5 or 10 μM of the MEK1 inhibitor U0126 (Cell Signaling) or solvent control (DMSO) prior to incubation with R1-CM and the inhibitor or control for an additional 2 hours. For migration assays, RAW 264.7 cells were incubated for 24 hours with R1-CM and CM was collected (R1/RAW-CM). Migration assays were performed using cell culture inserts with 8μm pore size (BD Biosciences, San Jose, CA, USA). HC-11 cells were washed with PBS and incubated overnight in serum-free RPMI. Cells were plated on the top of the insert and either R1-CM or R1/RAW-CM was placed on the bottom of the insert as a chemoattractant. The CXCR2 inhibitor, SB225002 (20 or 200 μM- Cayman Chemical, Ann Arbor, MI, USA), or the solvent control, ethanol, was added with the HC-11 cells to the top of the well. After 18 hours, cells on the bottom of the insert were fixed with 4% paraformaldehyde and stained with hematoxylin. The number of cells migrated in four fields of the insert were counted. Similar experiments were done with human cell lines in which MCF-7 cells were treated with 50ng/ml basic fibroblast growth factor (bFGF, Invitrogen). After 24 hours, the conditioned media (MCF-CM) was then incubated with the human monocyte cell line THP-1 that had been treated with 5 ng/ml PMA 24 hours and then starved overnight. Gene expression of CXCR2 ligands in macrophages was determined by collecting THP-1 cells treated with MCF-CM for 2 or 4 hours and analyzed as described below. For migration assays, MCF-CM was added to THP-1 cells for 24 hours and the conditioned media (MCF/THP-CM) was used as a chemoattractant for MCF-7 cells in the cell culture inserts as described above. The CXCR2 inhibitor, SB225002 (100, 200, or 400 μM) or solvent control, ethanol, was added with MCF-7 cells in the top of the well.
Quantitative reverse transcription-PCR
RNA was extracted from primary macrophages or macrophage cell lines (RAW 264.7 and THP-1, ATCC) using Trizol (Invitrogen) as described in the manufacturer protocol. cDNA was prepared using the Quantitect reverse transcription kit (Qiagen, Valencia, CA, USA). Quantitative RT-PCR (qRT-PCR) was performed using SYBR green (Bio-Rad, Hercules, CA, USA) and the Bio-Rad iQ5 system. The 2-ΔΔCt method (20) was used to determine the relative quantification of gene expression normalized to cyclophilin. The primers used for these studies are listed in Supplemental Table 1.
qRT-PCR Array
RNA was isolated from RAW 264.7 cells treated with R1-CM for 2 hours in the presence and absence of 10 ng/ml TGFβ, and cDNA was made, as described above. The RT2Profiler PCR Array: Mouse chemokines and receptors from SA Biosciences (Valencia, CA, USA) was performed as described for other qRT-PCR experiments.
ELISA analysis and activity assays
R1-CM was added to starved RAW 264.7 cells in the presence or absence of 10 ng/ml TGFβ. After 2 hours the media was removed and serum free RPMI was added. The R1/RAW-CM was collected after an additional 24 hours and ELISAs were performed to quantify the level of Cxcl1, Cxcl5, IL-10 and IL-12 following the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA). In addition, the RAW 264.7 were collected in lysis buffer (10 mM Tris-HCl (pH 7.4) containing 1μM pepstatin A, 1 μM leupeptin, and 0.4% Triton X-100) and the supernatant was used for an arginase activity assay (BioAssay Systems, Hayward, CA, USA). For the Smad3 luciferase assay, RAW 264.7 cells were transduced with a lentivirus expressing a Smad3 reporter construct (Qiagen) and were then treated with conditioned media from B/B-treated HC-11/R1 cells for 6 hours. Luciferase activity was measured using standard procedures (Promega, Wadison, WI, USA). For analysis of human chemokine expression, THP-1 cells were incubated with MCF-CM for 2 hours and then serum free DMEM for 24 hours. The levels of secreted CXCL1 and CXCL8 in the MCF/THP-CM were measured by ELISA following the manufacturer's protocol (R&D Systems).
Immunoblot analysis
Cells were lysed in RIPA buffer and equal amounts of protein were analyzed by SDS-PAGE. Immunoblotting was done using the following antibodies: CXCR2 (AHR1532Z; Biosource, Camarillo, CA, USA), β-tubulin (2146; Cell Signaling), pERK1/2 (9101; Cell Signaling) and ERK1/2 (sc-94; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Three-dimensional co-culture assay
Mammary glands were isolated from 8-12 week old MMTV-iFGFR1 transgenic mice as described above. Bone marrow was collected from femurs of 6-10 week old wild-type mice. Cells were differentiated into macrophages with the addition of 20% conditioned media from L929 cells that have a high concentration of granulocyte colony-stimulating factor (G-CSF). 10,000 mammary epithelial cells (MECs) were plated on growth-factor reduced matrigel (BD Biosciences) in 8 well chamber slides as described previously (21) After 2 days, 3000 bone marrow derived macrophages (BMDM) were added to the 3D culture. Also, the following treatments began at this time: -/+ 30 nM B/B and -/+ 200 nM SB225002, CXCR2 inhibitor (with (-) being ethanol, solvent control). After 10 days, cells were fixed with 2% paraformaldehyde and co-stained for macrophages (F4/80, Invitrogen) and epithelial cells (cytokeratin 8 (ab59400- Abcam, Cambridge, MA, USA) and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Images were taken at confocal microscopy facility at the Masonic Cancer Center (University of Minnesota, Minneapolis, MN).
Results
Regulation of pro- and anti-inflammatory genes in macrophages following exposure to conditioned media from iFGFR1-activated HC-11/R1 cells
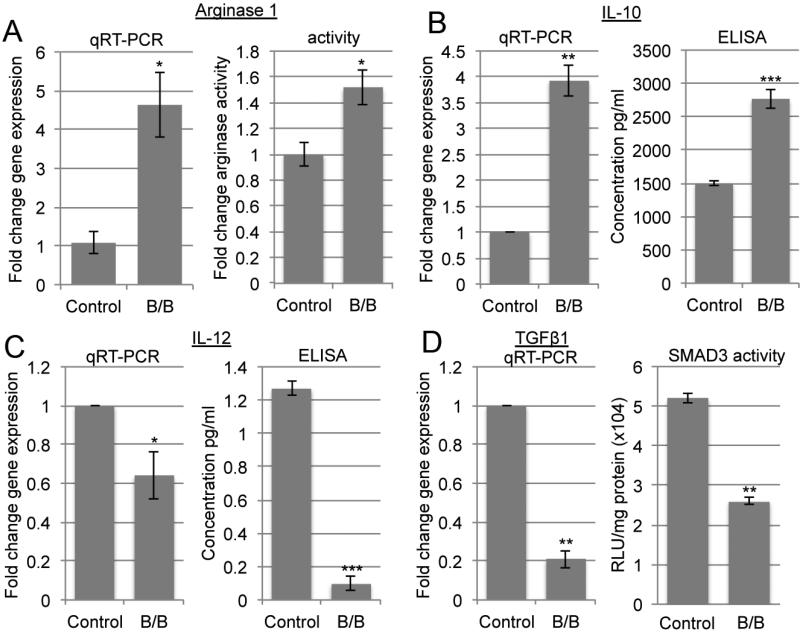
As described previously, activation of iFGFR1 in mammary epithelial cells promotes rapid recruitment of macrophages to epithelial structures in vivo and in vitro (16). Furthermore, depletion of macrophages leads to delayed formation of early hyperplastic lesions in vivo (16). Therefore, we further utilized this model to delineate the mechanisms that mediate the interactions between epithelial cells and macrophages. We have previously shown that exposure of macrophages to conditioned media from HC-11/R1 cells following activation of iFGFR1 leads to increased production of the proinflammatory cytokine IL-1β by macrophages in vitro and that IL-1β contributes to the formation of early stage lesions in vivo (17). To extend our analysis of macrophage response to iFGFR1 activation in epithelial cells, we further analyzed the expression of various genes known to be preferentially regulated in tumor associated macrophages, including IL-10, IL-12, Arginase I (ArgI) and TGFβ. For these studies, RAW 264.7 cells were exposed to conditioned media from HC-11/R1 cells treated with either the B/B homodimerizer, which activates the iFGFR1, or ethanol as a solvent control. After exposure to conditioned media, RAW 254.7 cells were analyzed for expression and activity of Arg1, IL-10, IL-12 and TGFβ. As shown in Figure 1A and 1B, expression levels of IL-10 and gene expression and activity of ArgI, which are typically increased in tumor associated macrophages, were induced whereas expression levels of IL-12, which are normally reduced in tumor associated macrophages, were decreased. Interestingly, expression of the TGFβ1 gene, which is usually induced in tumor associated macrophages, was reduced in these studies. In addition, the transcriptional activity of Smad3 also decreased (Figure 1D) suggesting an overall decrease in the TGFβ/Smad3 pathway. These results, together with our previously published studies (17), demonstrate that exposure of macrophages to conditioned media from epithelial cells with activated iFGFR1 leads to regulation of a number of genes associated with macrophage polarization.
Figure 1. Exposure to conditioned media from mammary epithelial cells with activated iFGFR1 leads to regulation of inflammation-associated genes in vitro.
RAW 264.7 cells were exposed to conditioned media from B/B-treated HC-11/R1 cells for 2 hours and either cells were collected for gene expression or serum free media was added for 24 hours to examine secreted protein expression or activity. A) qRT-PCR analysis of ArgI gene expression levels (left panel) and arginase activity (right panel). B) qRT-PCR analysis of IL-10 gene expression levels (left panel) and secreted protein level by ELISA (right panel). C) qRT-PCR analysis of IL-12 gene expression levels (left panel) and secreted protein level by ELISA (right panel). D) qRTPCR analysis of TGFβ1 gene expression levels (left panel) and Smad3 transcriptional activity by luciferase assay (right panel). Expression levels were normalized to cyclophilin for qRT-PCR. Error bars represent standard error of the mean (SEM). *P<0.05, **P<0.005, ***P<0.001.
Decreased TGFβ gene expression and Smad3 activity in macrophages from MMTV-iFGFR1 transgenic mice
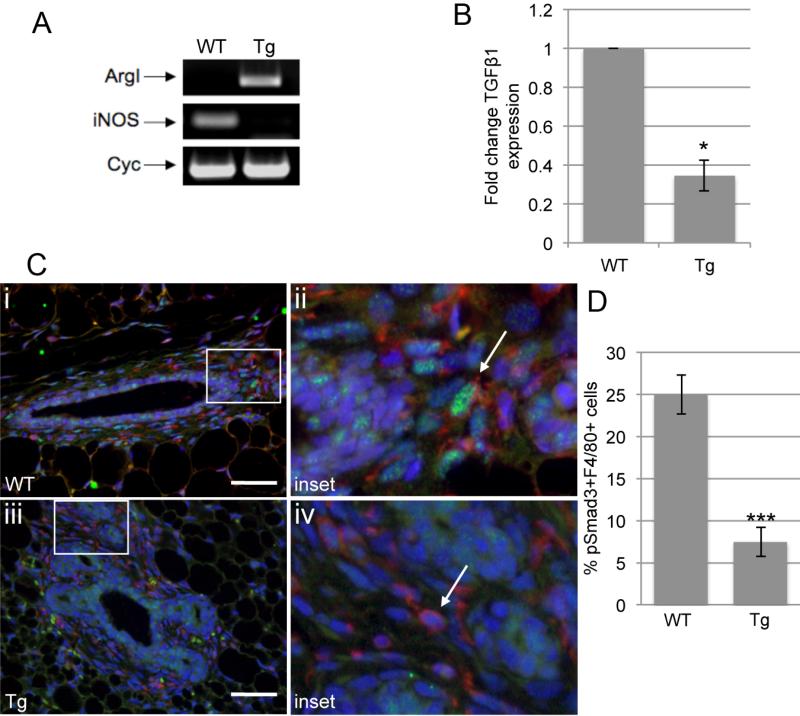
To examine gene expression of macrophages in vivo, macrophages were isolated from the mammary glands of wild-type and MMTV-iFGFR1 transgenic mice following 48 hours of B/B treatment, at which time macrophage recruitment to epithelial structures is consistently observed (16). Following a brief enzymatic digestion, macrophages were isolated by sorting with anti-Cd11b, which stains both monocytes and macrophages. Approximately 8.5% of the cells isolated from the mammary glands were positive for the Cd11b antigen compared with the isotype control antibody (Supplemental Figure 1A). These cells were collected for both immunostaining and RNA extraction. To determine the percentage of the population of sorted cells that represents mature macrophages, staining was performed using the F4/80 antibody (Supplemental Figure 1B). Approximately 85% of the sorted cells were positive for F4/80, suggesting that the preparation was significantly enriched for macrophages. To examine gene expression in the macrophages, RT-PCR analysis was performed and expression levels of ArgI, a marker of pro-tumor macrophages, and iNOS, a marker of tumor-inhibitory macrophages, were examined. As shown in Figure 2A, ArgI expression was increased and iNOS expression was decreased in macrophages isolated from MMTV-iFGFR1 transgenic mice treated with B/B, consistent with a polarization of the macrophages towards the tumor-promoting phenotype. Further studies using qRT-PCR revealed a decrease in expression of TGFβ1 in these macrophages (Figure 2B), consistent with the results observed in vitro (Figure 1).
Figure 2. Alterations in the TGFβ gene expression and Smad3 activity in macrophages isolated from mammary glands following FGFR1 activation.
A) Cd11b-positive sorted cells were analyzed for expression of the indicated genes using RT-PCR. The lanes represent macrophages sorted from three MMTV-iFGFR1 transgenic (Tg) or three non-transgenic wild-type (WT) littermates and pooled. B) Expression of TGFβ1 in macrophages isolated from MMTV-iFGFR1 transgenic mice or non-transgenic wild-type littermates treated with B/B for 48 hours. Expression levels were normalized to cyclophilin. C) Non-transgenic (i,ii) and transgenic (iii,iv) mice were treated with B/B for 48 hours and mammary glands were collected. Sections were co-stained with the F4/80 and phospho-Smad3 antibodies. F4/80 positive cells (red) are found in the stroma surrounding the epithelial structures (blue=DAPI staining of nuclei). Phospho-Smad3 (green) is detected in nuclei of F4/80 positive cells in sections from the non-transgenic (arrow, ii) but not transgenic (arrow, iv), mice. Scale bars=50 μm D) Quantification of the percentage of F4/80+/phospho-Smad3+ cells. Error bars represent SEM. *P<0.05, ***P<0.0001.
To determine whether decreased TGFβ production correlated with altered Smad3 activity in the macrophages, mammary gland sections from wild-type and MMTV-iFGFR1 transgenic mice following 48 hours of B/B treatment were immunostained with an antibody to phosphorylated Smad3 (pSmad3) (Figure 2C). The sections were co-stained with an F4/80 antibody to visualize the macrophages and the percentage of F4/80 cells positive for pSmad3 expression was determined (Figure 2D). In wild-type mice, approximately 25% of the F4/80+ cells located within the stroma surrounding the ductal structures were positive for pSmad3. Interestingly, there was a significant decrease in the number of cells positive for pSmad3 present in the stroma following 48 hours of B/B treatment of iFGFR1 mice. This observation suggests that along with decreased expression of TGFβ in the macrophages, there is also decreased TGFβ signaling within macrophages following iFGFR1 activation in mammary epithelial cells, consistent with the in vitro studies.
Effects of TGFβ on chemokine expression in macrophages
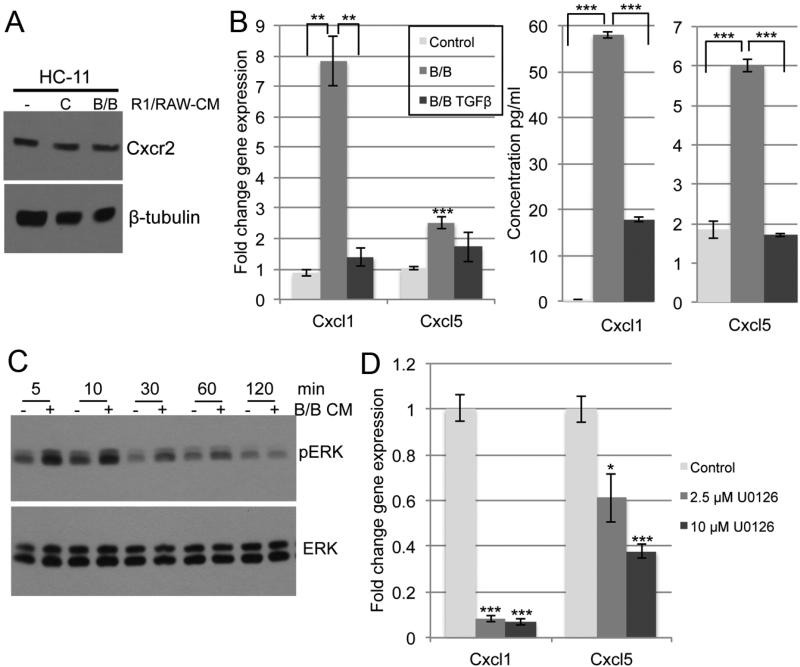
Based on our results, we hypothesized that decreased TGFβ signaling in macrophages may be linked to pro-tumorigenic macrophage function in early stages of tumorigenesis. Activation of the TGFβ/Smad3 pathway has been shown to inhibit the expression of inflammatory cytokines and chemokines (22, 23), suggesting that repression of this pathway leads to induction of these factors. Furthermore, recent studies demonstrated that loss of signaling through Tgfbr2 led to increased expression of chemokines in a model of pancreatic ductal adenocarcinoma (24). Therefore, we used a qRT-PCR-based chemokine array to analyze chemokine gene expression in macrophages exposed to conditioned media from B/B treated HC-11/R1 cells in the absence or presence of exogenous TGFβ. Several chemokines were found to be either up- or down-regulated in the macrophages following exposure to these conditions (Table 1). The chemokines were divided into 5 clusters based on the change in their expression patterns. While a number of genes were either induced or repressed with B/B alone and with B/B and TGFβ combined, we focused on genes in cluster 1, which were up-regulated with B/B treatment and down-regulated with the addition of TGFβ. Interestingly, 4 of these chemokines (Cxcl1, Cxcl2, Cxcl5, and Cxcl7) are ligands for the Cxcr2 receptor. Before validating expression levels of these chemokines, we confirmed that Cxcr2 is expressed in the HC-11 mammary epithelial cell line by immunoblot analysis (Figure 3A). We validated the results from the array using distinct primer sets to determine gene expression and ELISAs to measure secreted proteins. We observed similar gene and protein expression patterns for both Cxcl1 and Cxcl5 (Figure 3B). Induction of Cxcl2 and Cxcl7 was not detected by ELISA (data not shown). These data suggest that macrophages respond to FGFR1-induced soluble factors by increasing the production of chemokines that bind Cxcr2, primarily Cxcl1 and Cxcl5, and that this induction can be inhibited by restoring TGFβ signaling.
Table 1.
Macrophage chemokines regulated by conditioned media from FGFRI-induced mammary epithelial cells in the presence or absence of TGFβ.
| Gene ID | Fold change B/B CM | Fold change B/B CM+TGFβ | |
|---|---|---|---|
| Cluster 1 | |||
| CCL8 | 3.9 | 0.93 | |
| CCR1 | 1.86 | 0.863 | |
| CCR1/1 | 2.04 | 0.833 | |
| CXCL1 | 1.44 | 0.81 | |
| CXCL2 | 1.79 | 0.716 | |
| CXCL5 | 3.25 | 1.85 | |
| CXCL7 | 1.47 | 0.94 | |
| Cluster 2 | |||
| Bmp6 | 0.65 | 1.07 | |
| CCL2 | 0.172 | 0.776 | |
| CCL20 | 0.429 | 1.18 | |
| Rgs3 | 0.425 | 2.02 | |
| Cluster 3 | |||
| CX3CL1 | 1.76 | 3.15 | |
| Trem1 | 2.32 | 8.05 | |
| Cluster 4 | |||
| Ccbp2 | 0.39 | 0.3 | |
| CCR8 | 0.54 | 0.53 | |
| Ecgf1 | 0.42 | 0.55 | |
| Cluster 5 | |||
| CCR6 | 3.14 | 3.75 | |
| TNfsf14 | 1.97 | 1.73 | |
| Xcl1 | 1.82 | 2.02 |
Figure 3. Activation of iFGFR1 in mammary epithelial cells leads to increased expression of Cxcr2-binding chemokines in macrophages.
A) HC-11 cells were treated with R1/RAW-CM with or without B/B. Whole cell lysates were collected and the expression of Cxcr2 and β-tubulin (loading control) was analyzed by immunoblotting. B) RAW 264.7 cells were treated with R1-CM in the presence or absence of 10 ng/ml TGFβ for 2 hours. Expression of the given genes was determined by qRT-PCR and normalized to cyclophilin (left panel). For protein expression, RAW 264.7 cells were incubated with R1-CM for 2 hours followed by serum free media for 24 hours. The conditioned media samples were analyzed using ELISAs (middle and right panel). C) RAW 264.7 cells were treated with R1-CM for the given time. Whole cell lysates were collected and the expression of pERK1/2 and ERK1/2 (loading control) was analyzed by immunoblotting. D) RAW 264.7 cells were pre-treated with DMSO (solvent control) or U0126 for 30 min. and then treated for an additional 2 hours with the same conditions in the presence of B/B R1-CM. The expression of the given genes was analyzed by qRT-PCR and normalized to cyclophilin. Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001
Next, we wanted to determine the signaling pathway by which the Cxcr2 ligands are regulated in the macrophages. Expression of Cxcr2 ligands can be regulated by various signaling pathways depending on cell type and stimulus, including the NFκB and ERK pathways (24, 25). Interestingly, we were unable to detect an increase in NFκB activity in macrophages exposed to conditioned media from B/B-treated HC-11/R1 cells (data not shown). Further analysis focused on examining the ERK signaling pathway. For these studies, RAW 264.7 cells were exposed to conditioned media from HC-11/R1 cells treated with or without B/B. Protein lysates were collected at different time points and the expression of phosphorylated and total ERK1/2 was examined. There was a rapid induction of ERK1/2 activation in macrophages treated with B/B conditioned media in comparison to solvent controls (Figure 3C). Further studies were performed to determine the contribution of the ERK1/2 pathway to expression of the Cxcr2 ligands. As shown in Figure 3D, blocking ERK activation with the MEK1 inhibitor U0126 led to a decrease in gene expression of the Cxcr2 ligands. Inhibition of Cxcl1 was observed at the lowest concentration of U0126, whereas there was a dose-dependent decrease in Cxcl5 expression (Figure 3D). These results suggest that although both ligands are regulated by the ERK pathway, they may be regulated via slightly different mechanisms. Overall, these results indicate that the ERK pathway contributes to the regulation of Cxcr2 ligand expression.
Macrophages induce migration of epithelial cells, which is blocked by Cxcr2 inhibition
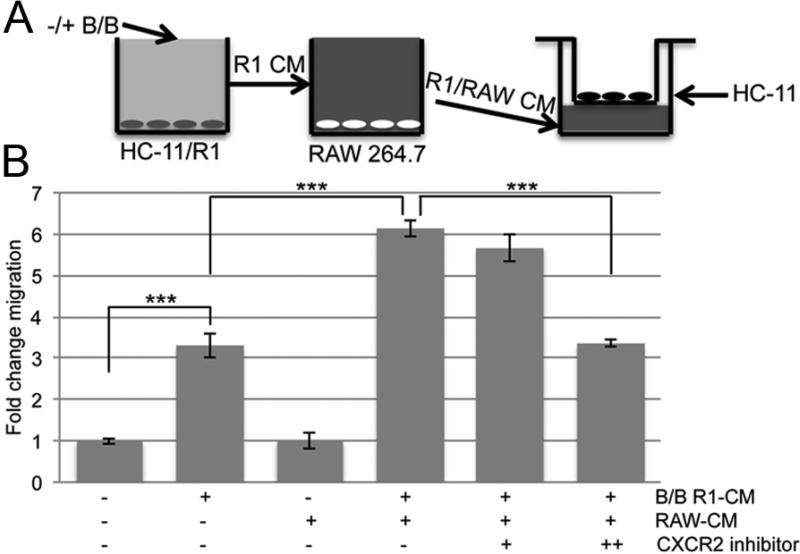
To determine the effects of Cxcr2 ligand secretion from macrophages, we developed a co-culture migration assay (Figure 4A). In this assay, conditioned medium from the HC-11/R1 cells containing FGFR1-induced soluble factors was added to RAW 264.7 cells and allowed to incubate overnight. The ability of the soluble factors collected from the treated RAW 264.7 cells to promote migration of parental HC-11 cells, which do not respond to B/B treatment, was determined using a transwell assay. The ability of macrophages to promote epithelial cell migration following exposure to conditioned media from cells with an activated FGFR1 reflects the ability of the stimulated macrophages to act in a tumor-promoting manner. Conditioned media from B/B treated HC-11/R1 cells alone was able to induce migration of HC-11 cells, suggesting that activation of FGFR1 in these cells leads to the production of migratory factors (Figure 4B). However, the conditioned media isolated from the treated macrophages led to a significant increase in migration of HC-11 cells compared with conditioned media from the HC-11/R1 cells alone (Figure 4B). Therefore, treatment of macrophages with conditioned media following FGFR1 activation leads to an increase in the ability of macrophages to produce migratory factors. To determine whether the increase in migration required Cxcr2, we further assessed the effects of blocking Cxcr2 activity on HC-11 cell migration using the Cxcr2-specific inhibitor SB225002. As shown in Figure 4B, the increase in migration was blocked when SB225002 was incubated with the HC-11 cells in the presence of the macrophage conditioned media. These results demonstrate an important role for macrophage-derived Cxcr2 ligands in the promotion of mammary epithelial cell migration.
Figure 4. Cxcr2 inhibition leads to decreased macrophage-induced migration of HC-11 cells.
A) Model of the migration co-culture assay. B) R1-CM, -/+ B/B, was exposed to RAW 264.7 cells for 24 hours and the conditioned media, R1/RAW-CM was used as the chemoattractant for a transwell migration assay. HC-11 cells were plated on top of the insert with ethanol (solvent control) or CXCR2 inhibitor (+ 20 nM and ++ 200nM). After 18 hours, the migrated cells on the bottom of the insert were stained with hematoxylin and counted. Error bars represent SEM. ***P<0.001.
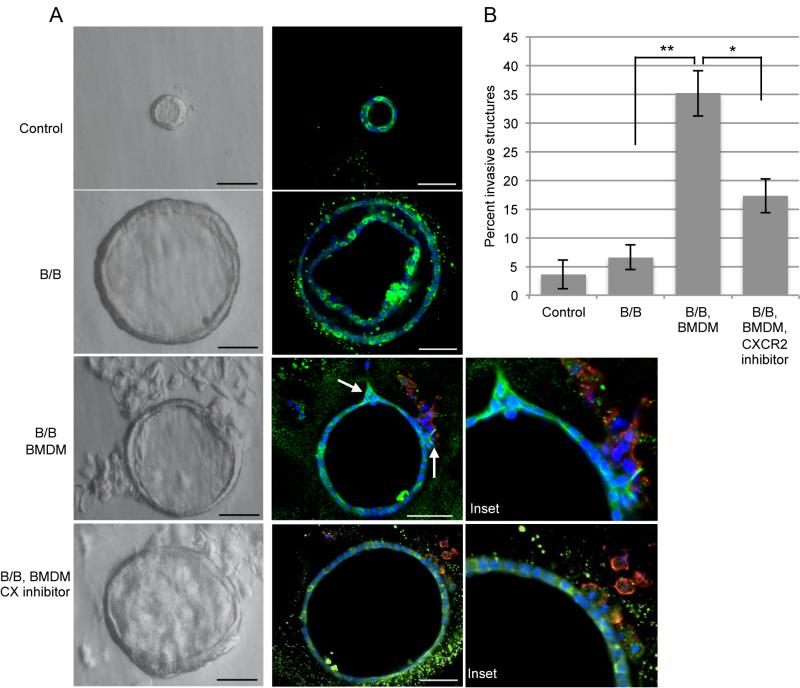
Macrophages promote invasion of primary mammary epithelial cells in a 3D co-culture assay
Primary mammary epithelial cells (MECs) form acinar-like structures when grown in 3D culture and more closely represent characteristics of mammary epithelium in vivo (21). We took advantage of the ability of primary MECs isolated from MMTV-iFGFR1 transgenic mice to grow in 3D culture (21) and developed a co-culture model to study the interactions between MECs and macrophages. For these studies, we isolated MECs from iFGFR1 transgenic mice and plated them in growth factor reduced Matrigel™. After 2 days, bone marrow derived macrophages (BMDM) that were isolated from wild type mice, were added to MECs. The structures were grown in the presence or absence of B/B and the CXCR2 inhibitor SB225002. After 10 days of treatment, cells were fixed and stained for epithelial (cytokeratin 8) and macrophage (F4/80) markers. As shown in Figure 5A, addition of B/B led to larger MEC structures, consistent with previously published studies (21). While co-culture of MECs with BMDM did not significantly affect size of the structures (data not shown), a significant increase in the number of invasive structures was observed (Figure 5A,B). With the addition of the CXCR2 inhibitor, macrophages were still recruited to the MECs (Figure 5A), but the percentage of invasive structures was significantly inhibited (Figure 5A,B). These data suggest that Cxcr2 ligands secreted from primary macrophages induce invasion of primary MECs.
Figure 5. Macrophages induce invasion of primary mammary epithelial cells grown in 3D culture.
A) Primary MECs from iFGFR1 mice were grown in Matrigel in the absence or presence of BMDM. Every 2-3 days fresh media was added with the treatments: -/+ 30 nM B/B and -/+ 200 nM CXCR2 inhibitor. After 10 days of treatment, cells were fixed and immunostained with cytokeratin 8 (green) and F4/80 (red). DAPI (blue) labeled nuclei. Arrows indicate invasive structures. Left images light microscopy Scale bars=50 μm. B) For each treatment, approximately 80 structures were examined for invasion from 3 independent experiments. Error bars represent SEM. *P<0.05, **P<0.01
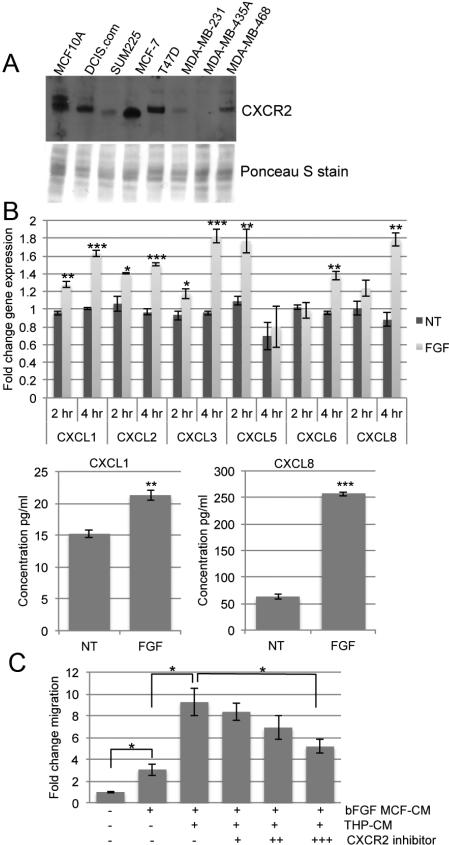
Human macrophages secrete CXCR2 ligands that promote migration of human breast cancer cells
To verify the results of the mouse iFGFR1 system, we used human breast cancer cell lines. We first examined the expression of CXCR2 in different breast cell lines: normal (MCF10A), pre-invasive (MCF10DCIS.com and SUM225), estrogen receptor positive (MCF-7 and T47D) and triple negative (MDA-MB-231, 435A, and 468) (Figure 6A). Since MCF-7 cells express CXCR2 and they have previously been shown to respond to FGF treatment (26), we chose to use these cells to determine the ability of bFGF stimulation to promote CXCR2 ligand induction in macrophages. MCF-7 cells were treated with or without bFGF and the MCF-7 conditioned media was added to the human monocyte cell line THP-1 that had been differentiated to macrophages with PMA as described (27). After 2 and 4 hours, THP-1 cells were collected for gene expression analysis. In addition to the Cxcr2 ligands in mice, human cells also express CXCL8. Gene expression of all CXCR2 ligands, with the exception of CXCL7 (data not shown), increased following exposure to conditioned media from MCF-7 cells treated with bFGF (Figure 6B). Further analysis of protein expression demonstrated significant increases in secretion of both CXCL1 and CXCL8 (Figure 6B), while the other ligands were not expressed at detectable levels by ELISA (data not shown). bFGF treatment of THP-1 cells alone did not induce expression of these chemokines, suggesting that these results are not due to the direct action of bFGF on macrophages (data not shown). The MCF-7/THP-1 conditioned media was used as the chemoattractant in a transwell migration assay to examine the migration of MCF-7 cells. Media from MCF-7 cells exposed to bFGF increased migration compared to no treatment and there was an additional increase when the media was also exposed to THP-1 cells (Figure 6C). Finally, migration was inhibited with a CXCR2 inhibitor (Figure 6C), demonstrating that the CXCR2 ligands are important for macrophage-induced breast cancer cell migration.
Figure 6. The migratory ability of MCF-7 cells increases with macrophage secreted CXCR2 ligands.
A) Whole cell lysates were collected from the different cell lines and equal amounts were used for immunoblot analysis. The expression of CXCR2 was determined and the membrane was stained with Ponceau S for a loading control. B) THP-1 cells were exposed to conditioned media from MCF-7 cells treated with 50 ng/ml bFGF or no treatment (NT). Cells were collected after 2 or 4 hours, and RNA was isolated for qRT-PCR for the given genes that were normalized to cyclophilin (upper panel). For protein expression, THP-1 cells were incubated with MCF-CM for 2 hours and then serum free media for 24 hours. The conditioned media was analyzed using ELISAs (lower panel). C) MCF-CM, -/+ bFGF, was exposed to THP-1 cells for 24 hours, and the conditioned media, MCF/THP-CM was used as the chemoattractant for a transwell migration assay. MCF-7 cells were plated on top of the insert with ethanol (solvent control) or CXCR2 inhibitor (+ 100 nM, ++ 200 nM, and +++ 400 nM). After 18 hours, the migrated cells on the bottom of the insert were stained with hematoxylin and counted. Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Recent studies have demonstrated that FGFR signaling contributes to breast cancer growth and resistance to conventional therapies (12, 13). Our studies focus on understanding how activation of FGFR1 in tumor cells leads to pro-tumorigenic alterations in the tumor microenvironment. We describe here a novel mechanism of paracrine interaction between tumor cells and macrophages that is driven by FGFR1 activation in the tumor cells and chemokine expression in the macrophages. We have previously demonstrated that activation of iFGFR1 in the HC-11/R1 cells results in the induction of a number of secreted factors that can influence macrophage recruitment (16) and cytokine production (17). The studies described here further analyze the mechanisms through which macrophages contribute to early stage tumorigenesis. While performing co-culture studies, we found a number of genes to be regulated in an expected manner based on published studies of gene expression in tumor associated macrophages, including increased ArgI and IL-10 and decreased IL-12 (28, 29). Interestingly, however, decreased expression of TGFβ gene expression was consistently observed in the macrophages both in vivo and in vitro, which is not consistent with the phenotype of macrophages associated with late stage tumors (30). Although levels of TGFβ protein were not detectable by ELISA in the in vitro co-culture model (data not shown), a decrease in Smad3 activity was observed in the macrophages, consistent with the decrease in pSmad3 observed in vivo. Together these studies demonstrate an overall decrease in the TGFβ/Smad3 signaling pathway in macrophages in response to factors from FGFR1-driven mammary tumor cells. The specific factors and mechanisms responsible for this decrease are likely complex and remain to be determined. Early in mammary tumor formation, TGFβ is known to be growth suppressive (8), so it is possible that decreased TGFβ in the microenvironment, either from epithelial cells, infiltrating macrophages or other stromal cells, is critical for the development of early stage lesions. Consistent with this, we found that adding exogenous TGFβ to the media of primary MECs in 3D culture completely inhibited iFGFR1-induced acinar growth (Supplemental Figure 2). These results suggest that in the early stages of tumor formation, the presence of TGFβ producing macrophages, such as those associated with the promotion of late-stage tumors, might actually result in tumor inhibition. Therefore, the association of macrophages with decreased levels of TGFβ expression and pathway activity, as observed in our studies, may be important for successful early-stage tumor formation.
Our results demonstrate that decreased expression of genes associated with the TGFβ pathway correlates with increased expression of inflammatory chemokines. Furthermore, restoration of TGFβ signaling with exogenous TGFβ inhibits the expression of these chemokines. An inverse correlation between the TGFβ pathway and expression of inflammatory chemokines has been observed in other studies. For example, decreased TGFβ responsiveness in prostatic fibroblasts caused an upregulation of chemokines including CXCL1, leading to the adhesion of prostate cells to the bone matrix (31). Also, loss of TGFβ signaling in mammary fibroblasts resulted in increased production of the proinflammatory chemokine Ccl2, which contributed to growth and metastasis of 4T1 mammary tumors (32). In addition, recent studies using a mouse model of pancreatic ductal adenocarcinoma also demonstrated an increase in Cxcr2 ligands associated with loss of Tgfbr2 in the pancreas (24). Interestingly, these studies demonstrated that induction of the Cxcr2 ligands was dependent on the NFκB pathway, whereas induction in macrophages in our model is dependent upon the ERK signaling pathway. These results possibly represent cell-type specific differences in CXCR2 ligand regulation. Taken together, our results, along with published studies, demonstrate that decreased TGFβ signaling is associated with increased expression of inflammatory chemokines, and that these chemokines are capable of contributing to tumor formation and progression.
Interactions between chemokines and chemokine receptors are known to contribute to breast cancer progression (33). Recent studies have implicated CXCR2 and its various ligands in breast cancer. A number of studies have focused on CXCL8, also known as IL-8, which binds to both CXCR1 and CXCR2 and appears to be the primary CXCR2 binding chemokine induced in response to bFGF-treated breast cancer cells (Figure 6B). Expression of CXCL8 has been linked to increased tumor grade and experimental studies have demonstrated its ability to regulate angiogenesis and tumor progression (33-35). CXCR2 expression itself has been detected on breast cancer cells (34) and inhibition of CXCR2 decreased mammary tumor cell invasion in vitro (36). Furthermore, knock-down of CXCR2 in metastatic mammary tumor cells led to decreased metastasis in orthotopic transplantation models (36). The CXCR2 ligand CXCL3 was identified in a screen for genes associated with basal-like breast cancers and a CXCR2 inhibitor was shown to decrease viability of basal-like breast cancer cell lines in vitro (37). Furthermore, polymorphisms in the CXCR2 gene have been identified in breast cancer patients and these polymorphisms correlated with larger tumor size, higher tumor grade and increased lymph node metastasis (38). However, the functional consequences of this polymorphism on CXCR2 expression and/or activity remain to be determined. Our studies demonstrate that activation of epithelial cell-specific CXCR2 by chemokine-producing cells within the tumor stroma may play a role in early stages of tumorigenesis. Taken together, these studies suggest that the CXCR2 axis may represent a viable pathway to target during both early and late stage tumor development.
Our studies have focused on the use of mammary epithelial cells expressing an iFGFR1 construct. To demonstrate that the results were not specific to the iFGFR1 model, we validated our results using the MCF-7 cell line, which has been previously used to study endogenous FGF signaling (26, 39, 40). Similar to the results obtained with the iFGFR1 model, activation of the FGF signaling pathway in MCF-7 cells also led to an increase in CXCR2 ligand gene expression in macrophages. Interestingly, FGFR1 activity has been shown to promote resistance of estrogen receptor positive breast cancer cells to endocrine-based therapies (13). Although it has been reported that estrogen receptor positive cells, including MCF-7, express lower levels of CXCR2 than more invasive breast cancer cell lines (34), our studies as well as another report (41) suggest that estrogen receptor positive cells express the same or even more CXCR2 than more invasive breast cancer cell lines. Furthermore, our studies demonstrate that MCF-7 cells are capable of responding to CXCR2 ligands in chemotaxis assays and that inhibition of CXCR2 is sufficient to inhibit migration towards the stimulated THP-1 cells. These results suggest that activation of the FGF pathway may contribute to enhancement of tumor progression by regulating the expression of tumor-promoting chemokines in infiltrating immune and other stromal cells. In addition, it is possible that FGF signaling in tumor cells may contribute to breast cancer growth and therapeutic resistance by regulating both the tumor cells and the microenvironment.
While some studies have demonstrated that breast cancer cells produce CXCR2 ligands, which feed back to regulate tumor cell activity in an autocrine manner, our studies demonstrate that cells within the stroma might also represent an important source of CXCR2 ligands. Similarly, studies by Halpern et al. demonstrated that mesenchymal stem cells are also a source of CXCR2 ligands, which are capable of promoting migration of cells in vitro and suggest that CXCR2 ligands may be important for homing of tumor cells to bone (42). Our results demonstrate that the production of CXCR2 ligands is enhanced in macrophages in response to soluble factors released from transformed mammary epithelial cells and that these factors are then capable of promoting migration and invasion of non-invasive cells. These results offer a novel mechanism by which macrophages associated with the invasive edges of developing tumors might promote invasion through the basement membrane. Together, these results suggest that targeting CXCR2 in breast cancer patients may be effective at both early and late stages of tumor formation and progression.
In summary, our studies have focused on the effects of tumor cell-specific FGFR1 activation on alterations within the tumor microenvironment. We have previously demonstrated a rapid inflammatory response characterized by recruitment of macrophages to the epithelial structures (16). We have also demonstrated that anti-inflammatory drugs are capable of inhibiting the initiation of FGFR1-driven epithelial lesions, demonstrating that inflammation is an important promoter of FGFR1-driven tumorigenesis (17). Using the iFGFR1 model, we have developed novel co-culture models to identify specifically how FGFR1-driven tumor cells communicate with the microenvironment. We have identified a paracrine mechanism in which soluble factors from the FGFR1-activated cells lead to increased production of CXCR2 ligands, which then feed back to promote migration and invasion of the tumor cells. Importantly, these findings were validated using an FGF-responsive breast cancer cell line, demonstrating that activation of endogenous FGF signaling leads to similar results as the iFGFR1 model. These results suggest that in FGF-driven breast cancers, targeting CXCR2 activity, possibly in conjunction with specifically targeting FGFR activity, may lead to an effective therapeutic strategy. Further studies are required to determine which patients might benefit from this type of therapy.
Supplementary Material
Acknowledgements
We would like to thank Dr. Jeff Rosen for providing reagents and advice for these studies and Dr. Fariba Behbod for providing reagents used in this study. Also, we would like to thank Johanna Reed and Lindsey Bade for critical reading of the manuscript. In addition, we would like to acknowledge the use of the confocal microscope at the Masonic Cancer Center made available through an NCRR Shared Instrumentation Grant (#1 S10 RR16851).
Grant support
Funding for these studies was provided by grants from the American Cancer Society (RSG-09-192-01-LIB) and NCI (1R01CA132827) to KLS.
Footnotes
Competing interests. The authors declare that they have no competing interests.
Authors’ contributions
LRB performed the cell culture 2D and 3D co-culture assays and contributed to drafting of the manuscript. KLS conceived of the study, directed the research, and drafted the manuscript. She also performed the in vivo macrophage isolation and expression studies.
References
- 1.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 2.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 3.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 4.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107(5):2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield LM, Piek E, Bottinger EP. TGF-beta signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6(1):67–82. doi: 10.1023/a:1009568532177. [DOI] [PubMed] [Google Scholar]
- 9.Lanigan F, O'Connor D, Martin F, Gallagher WM. Molecular links between mammary gland development and breast cancer. Cell Mol Life Sci. 2007;64(24):3159–84. doi: 10.1007/s00018-007-7386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe R, Pearson A, Herrera-Abreu MT, Johnson D, Mackay A, Welti JC, et al. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2011;17(16):5275–86. doi: 10.1158/1078-0432.CCR-10-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70(5):2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letessier A, Sircoulomb F, Ginestier C, Cervera N, Monville F, Gelsi-Boyer V, et al. Frequency, prognostic impact, and subtype association of 8p12, 8q24, 11q13, 12p13, 17q12, and 20q13 amplifications in breast cancers. BMC Cancer. 2006;6:245. doi: 10.1186/1471-2407-6-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welm BE, Freeman KW, Chen M, Contreras A, Spencer DM, Rosen JM. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J Cell Biol. 2002;157(4):703–14. doi: 10.1083/jcb.200107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwertfeger KL, Xian W, Kaplan AM, Burnett SH, Cohen DA, Rosen JM. A critical role for the inflammatory response in a mouse model of preneoplastic progression. Cancer Res. 2006;66(11):5676–85. doi: 10.1158/0008-5472.CAN-05-3781. [DOI] [PubMed] [Google Scholar]
- 17.Reed JR, Leon RP, Hall MK, Schwertfeger KL. Interleukin-1beta and fibroblast growth factor receptor 1 cooperate to induce cyclooxygenase-2 during early mammary tumourigenesis. Breast Cancer Res. 2009;11(2):R21. doi: 10.1186/bcr2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xian W, Schwertfeger KL, Vargo-Gogola T, Rosen JM. Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J Cell Biol. 2005;171(4):663–73. doi: 10.1083/jcb.200505098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009;11(5):R66. doi: 10.1186/bcr2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Xian W, Schwertfeger KL, Rosen JM. Distinct roles of fibroblast growth factor receptor 1 and 2 in regulating cell survival and epithelial-mesenchymal transition. Mol Endocrinol. 2007;21(4):987–1000. doi: 10.1210/me.2006-0518. [DOI] [PubMed] [Google Scholar]
- 22.Maggio-Price L, Treuting P, Bielefeldt-Ohmann H, Seamons A, Drivdahl R, Zeng W, et al. Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor-beta dysregulation as a model for studying inflammation-associated colon cancer. Am J Pathol. 2009;174(1):317–29. doi: 10.2353/ajpath.2009.080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, et al. Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem. 2000;275(47):36653–8. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- 24.Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121(10):4106–17. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa R, Xie S, Lee KY, Stanbridge RD, Bhasvar P, Sukkar MB, et al. GRO-alpha regulation in airway smooth muscle by IL-1beta and TNF-alpha: role of NF-kappaB and MAP kinases. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L66–74. doi: 10.1152/ajplung.00384.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bade LK, Goldberg JE, Dehut HA, Hall MK, Schwertfeger KL. Mammary tumorigenesis induced by fibroblast growth factor receptor 1 requires activation of the epidermal growth factor receptor. J Cell Sci. 2011;124(Pt 18):3106–17. doi: 10.1242/jcs.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129(4):1016–25. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 28.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180(4):2011–7. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Sterling JA, Fan KH, Vessella RL, Shyr Y, Hayward SW, et al. Loss of TGF-beta Responsiveness in Prostate Stromal Cells Alters Chemokine Levels and Facilitates the Development of Mixed Osteoblastic/Osteolytic Bone Lesions. Mol Cancer Res. 2012;10(4):494–503. doi: 10.1158/1541-7786.MCR-11-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hembruff SL, Jokar I, Yang L, Cheng N. Loss of transforming growth factor-beta signaling in mammary fibroblasts enhances CCL2 secretion to promote mammary tumor progression through macrophage-dependent and -independent mechanisms. Neoplasia. 2010;12(5):425–33. doi: 10.1593/neo.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali S, Lazennec G. Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26(3-4):401–20. doi: 10.1007/s10555-007-9073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22(2):256–65. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, et al. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109(4):507–15. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- 36.Nannuru KC, Sharma B, Varney ML, Singh RK. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog. 2010;10:40. doi: 10.4103/1477-3163.92308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44CD24 stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, et al. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer. 2010;10:283. doi: 10.1186/1471-2407-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JF, Crepin M, Liu JM, Barritault D, Ledoux D. FGF-2 and TPA induce matrix metalloproteinase-9 secretion in MCF-7 cells through PKC activation of the Ras/ERK pathway. Biochem Biophys Res Commun. 2002;293(4):1174–82. doi: 10.1016/S0006-291X(02)00350-9. [DOI] [PubMed] [Google Scholar]
- 40.Pond AC, Herschkowitz JI, Schwertfeger KL, Welm B, Zhang Y, York B, et al. Fibroblast growth factor receptor signaling dramatically accelerates tumorigenesis and enhances oncoprotein translation in the mouse mammary tumor virus-Wnt-1 mouse model of breast cancer. Cancer Res. 2010;70(12):4868–79. doi: 10.1158/0008-5472.CAN-09-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 42.Halpern JL, Kilbarger A, Lynch CC. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011;308(1):91–9. doi: 10.1016/j.canlet.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.