Abstract
During the many idle moments that comprise daily life, the human brain increases its activity across a set of midline and lateral cortical brain regions known as the “default network.” Despite the robustness with which the brain defaults to this pattern of activity, surprisingly little is known about the network’s precise anatomical organization and adaptive functions. To provide insight into these questions, this article synthesizes recent literature from structural and functional imaging with a growing behavioral literature on mind wandering. Results characterize the default network as a set of interacting hubs and subsystems that play an important role in “internal mentation” – the introspective and adaptive mental activities in which humans spontaneously and deliberately engage in everyday. .
Keywords: default network, default mode, memory, social cognition, self, emotion, valuation, mind wandering, spontaneous thought, resting state functional connectivity
INTRODUCTION
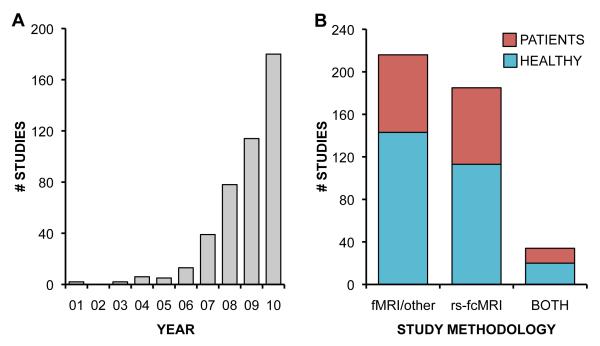
The brain’s “default mode network” is among the most rapidly growing neuroscientific topics of the new millennium (Fig. 1A). Since the appointment of its name a decade ago (Raichle and others 2001), the default network has garnered considerable interest for its high level of resting metabolic activity, which decreases in the face of externally-directed attention (Minoshima and others 1997; Gusnard and Raichle 2001). Additionally, the robust resting-state activity correlations within the default network have been thoroughly cited in recent years (Fig. 1B; Fox and Raichle 2007; Buckner and others 2008), and their disruption represents a hallmark of numerous psychiatric and neurological diseases (Fig. 1b; Box 1; Buckner and others 2008; Broyd and others 2009; Zhang and Raichle 2010).
Fig 1.
Prevalence of default network studies in the literature. A. Total number of studies relating to the default network separated by year published. Data was obtained from a PubMed search on the phrases “Default Mode” OR “Default Network” filtered to discard unrelated studies. Published dates reflect the date in which studies were printed rather the advanced E-Publication date. B. Studies are further classified based on their methodology and participant cohort to highlight the prevalence of resting-state functional connectivity techniques and the relevance of the default network to psychiatric and neurological disease. fMRI or other = Task-evoked fMRI and all other experimental techniques (i.e. event-related potentials, diffusion tensor imaging, etc.); rs-fcMRI = resting-state functional connectivity MRI; both = studies employing rs-fcMRI and at least one other neuroimaging technique.
Box 1. Disruption of the default network in disease.
A mysterious finding in neuroimaging research is that the default network is altered in a wide range of psychiatric and neurological diseases, including major depression, generalized anxiety disorder, social phobia, post-traumatic stress disorder, schizophrenia, attention-deficit hyperactivity disorder (ADHD), autism, Tourette’s syndrome, Alzheimer’s disease, stroke, chronic pain, cirrhosis, amytrophic lateral sclerosis, progressive multiple sclerosis, persistent vegetative state, and so on (Buckner and others 2008; Bryod and others 2009; Zhang and Raichle 2010). While the default network and its disruption in disease has predominantly been explored using rs-fcMRI techniques (given their feasibility and amenableness to patient populations), the network has also been shown to exhibit alterations in task-related activations and deactivations, changes in underlying brain structure, and the development of molecular pathology (i.e. Buckner and others 2005; Sperling and others 2009; Sheline and others 2009).
Possible explanations for the ubiquitous disruption of the default network across varied diseases are few and far between. An intriguing possibility put forth by Buckner and colleagues in the context of Alzheimer’s disease is that the default network’s high resting metabolic activity and widespread connectivity may initiate a series of activity-dependent molecular cascades that accelerate pathological insult (Buckner and others 2005 2008 2009). Disease may also target the default network disproportionately because of its relatively recent evolution compared to more phylogenetically stable sensory brain systems (Hill and others 2010). Finally, other possibilities include a common environmental or genetic underpinning, perhaps manifested through altered neurotransmitter systems (e.g. Broyd and others 2009). Whatever the underlying reason for the consistent disruption, it is clear the default network and its alteration in disease deserve further exploration.
Given the surge of neuroscientific discussion concerning the default network, one might expect that the network’s precise anatomical organization and adaptive functions have already been established. Yet discrepant views reveal the functional-anatomic properties of the default network are far from being fully understood. On the one hand, the strong association between default network activity and passive states fosters a sentiment that the network lacks apparent function, existing only as a background idling state when the mind simply “shuts off.” A different set of views attribute the default network to active mental processes, but these views differ remarkably in their claims. For example, whereas default network activity during passive states is sometimes attributed to exploratory monitoring of the external environment, other times its activity is attributed to internal, introspective thoughts. To complicate matters still, the link between default network activity and mind wandering might suggest the network is maladaptive to goal-directed cognition, yet these ideas stand in opposition to recent data highlighting its adaptive role in certain types of goal-directed thought.
To help resolve these discrepancies and gain insight into the functions of the default network, the present article reviews recent results from anatomical connectivity, task-evoked neuroimaging, and resting state functional connectivity MRI (rs-fcMRI) with a growing behavioral literature on spontaneous thought, or “mind wandering,” to propose that the default network plays an adaptive role in internal mentation. To set the stage for this proposal, the article first defines the regions within the default network using converging approaches including task-induced deactivations, connectional anatomy, diffusion tensor imaging (DTI), and rs-fcMRI. Next, recent evidence is reviewed suggesting that the network is best described as a heterogeneous brain system comprising distinct subsystems that converge on core hubs. The article then explores the overarching functions of the default network and its relation to spontaneous thought, followed by a more detailed discussion of the possible functional contributions of each subsystem.1
A LARGE-SCALE BRAIN SYSTEM
The appreciation that the brain is organized into a set of large-scale brain systems is reflected in recent years by the increasing popularity of correlational, connectivity, and graph analytical techniques (Fox and Raichle 2007; Bressler and Menon 2010). Such an appreciation is perhaps most universally held for systems supporting sensory and attentional functions. These “extrinsic” systems are amenable to scientific investigation in non-human animals, having evolved relatively early in phylogenetic history and comprising functions that are readily experimentally observable.
In contrast, understanding the functions of “intrinsic” systems such as the brain’s “default mode network” often require the use of introspective techniques that may be less experimentally tractable and are not as amenable to investigation in non-human animals. Such challenges likely contributed to the relatively recent and somewhat accidental discovery of the default mode network, where in 1997, neuroscientists at Washington University in St. Louis quantified patterns of blood flow using Positron Emission Tomography (PET) across nine different externally-oriented, goal-directed tasks. When these patterns of blood flow were compared to those elicited during the experimental control conditions of fixation or passive viewing of task-related stimuli, the researchers noted a striking observation. A set of medial and lateral brain regions exhibited consistently greater blood flow during the passive “resting” conditions compared to the active, experimentally-directed tasks (Shulman and others 1997). These relative reductions in brain activity during experimentally-directed tasks were often referred to as “task-induced deactivations2” (TIDs). Similar patterns were later replicated in a separate meta-analysis of 9 PET studies (Mazoyer and others 2001).
In a seminal article published in 2001, Marcus Raichle and colleagues called for neuroscientific attention to these regions, highlighting their role as a large-scale brain system and associating their engagement during passive states with a “default mode of brain function” (Raichle and others 2001; see also Gusnard and Raichle 2001). In the past decade, the “default mode network,” or simply the “default network,” has been cited over 550 times3.
Similar to other large-scale brain systems, the default network can be defined both in terms of its anatomical connectivity and functional coherence during extended periods of rest and experimentally-directed tasks. To provide insight into the regions that define the default network, the present manuscript reviews data yielded by such methods, beginning with the original means by which the default network was defined: task-induced deactivations.
TASK-INDUCED DEACTIVATIONS AND THE DEFAULT NETWORK
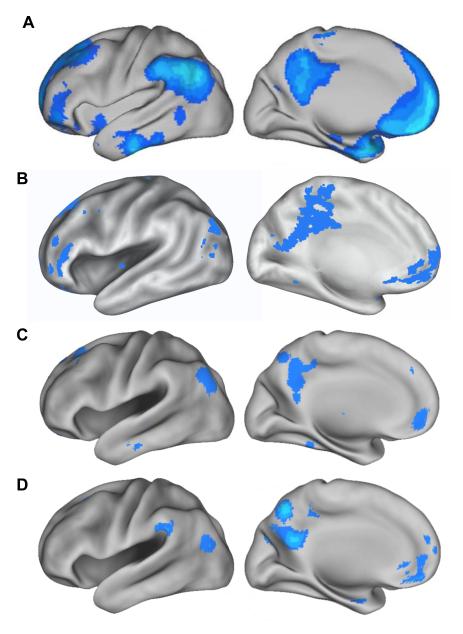
Regions that exhibit TIDs across studies are shown in Fig. 2, which projects cortical data from four previously published meta-analyses onto inflated brain surfaces for ease of comparison (Fig. 2A: Shulman and others 1997; Fig. 2B: Mazoyer and others 2001; Fig. 2C: Spreng and others 2009; Fig. 2D: Laird and others 2009). Although the studies employ different imaging techniques, statistical analyses and thresholds, convergence across the maps highlights the most robust components of the default network. The results agree remarkably well with other studies reporting TIDs (e.g. Binder and others 1999; Schilbach and others 2008; Buckner and others 2009), including those that demonstrate convergence across blocked and event-related task paradigms (Bucker and others 2008).
Fig. 2.
Cortical distribution of task-induced deactivations. Data from four previously-published meta-analyses of task-induced deactivations is re-plotted on inflated brain surfaces (Caret software, Van Essen 2005) for ease of visual comparison. Permission was obtained from the authors of these studies. A. Task-induced deactivations averaged over 9 distinct PET studies from Shulman and others (1997; see also Buckner and others 2005 2008), thresholded at a 0.5% PET count signal change.1 B. A conjunction image reflecting task-induced deactivations from 9 distinct PET studies from Mazoyer and others (2001, Figure 1; thresholded at p < 0.001 uncorrected). C. Data from Spreng and others (2009; Figure 1), which represents an activation likelihood estimate (ALE) meta-analysis of 16 studies, plotted using an FDR-corrected threshold of p < 0.05. D. Task-induced deactivations from the ALE meta-analysis of Laird and others (2009, Figure 1), plotted using an FDR-corrected threshold of p < 0.005. 1Figure adapted with permission from John Wiley and Sons ©; see Buckner and others (2008; Figure 2).
On the medial wall, the default network includes a large portion of the medial prefrontal cortex (MPFC) extending dorsally and ventrally. Activation encompasses all or part of areas 9, 10 (10m, 10r, 10p), 24, 32 (32ac, 32pl), 11m, and 14, according to the cytoarchitectonic maps of Ongur and others (2003). Medial parietal cortex is also prominent, comprising the posterior cingulate cortex (PCC; areas 23 and 31) and retrosplenial cortex (Rsp; areas 29 and 30). Note that the precuneus (area 7m) is noticeable in two of the four meta-analyses, yet as will be discussed later, the precuneus does not converge with other default network regions in terms of its anatomical connectivity or rs-fcMRI (Buckner and others 2008; Margulies and others 2009; Andrews-Hanna and others 2010a; Pfefferbaum and others 2011).
TIDs also suggest less consistent involvement of the medial temporal lobe (MTL), including the hippocampal formation (HF) and the parahippocampal cortex (PHC). However, the MTL appears more prominent at lower thresholds (Fig. 2a) and it is possible that distortion and signal loss commonly associated with this structure might obscure its underlying activity (see also Greicius and others 2004). Furthermore, as discussed later, regions within the MTL are anatomically and functionally connected with several other components of the default network, and it is activated when individuals engage in mnemonic processes that are common during passive states. Additionally, further work will be necessary to investigate the possible role of the nearby amygdala in the default network, as it appears to demonstrate TIDs in two of the four meta-analyses.
On the lateral surface, TIDs are robust in parietal cortex, prominently ventral to the intraparietal sulcus and encompassing the posterior inferior parietal lobule (pIPL) and angular gyrus (at or near area 39). The nearby supramarginal gyrus (near area 40) and temporoparietal junction (TPJ; near areas 39/22) is also noticeable in some maps, as is involvement of the lateral temporal lobe, particularly near the middle and inferior temporal gyri. Finally, in the lateral frontal lobe, task-induced deactivations span the inferior, middle and superior frontal gyri near Brodmann areas 47, 45, 8, 9, and 10.
It is important to note that since functional imaging paradigms measure relative differences between experimental tasks of interest and periods of passive rest or fixation, the pattern of TIDs will depend heavily on the experimental task of interest. Note that many of the goal-directed tasks surveyed in the meta-analyses are externally-oriented in nature, requiring focused attention to visual or other sensory stimuli. Such focused external attention is notably attenuated during passive control states, yielding robust TIDs in the default network. In contrast, goal-directed tasks that are more of an internal or introspective nature exhibit less TIDs, and oftentimes, task-evoked activity in the default network even increases compared to passive baseline (Buckner and Carroll 2007; Buckner and others 2008; Spreng and others 2009; see Spreng and others 2010 for further discussion). Thus, to claim that the default network decreases its activity during all goal-directed tasks isn’t strictly accurate. The presence or absence of brain regions exhibiting TIDs should always be interpreted in the context of the experimentally-directed task and should not be used as the sole definition of the regions that comprise the default network.
ANATOMICAL CONNECTIVITY WITHIN THE DEFAULT NETWORK
Neuroanatomical tracing studies in non-human primates
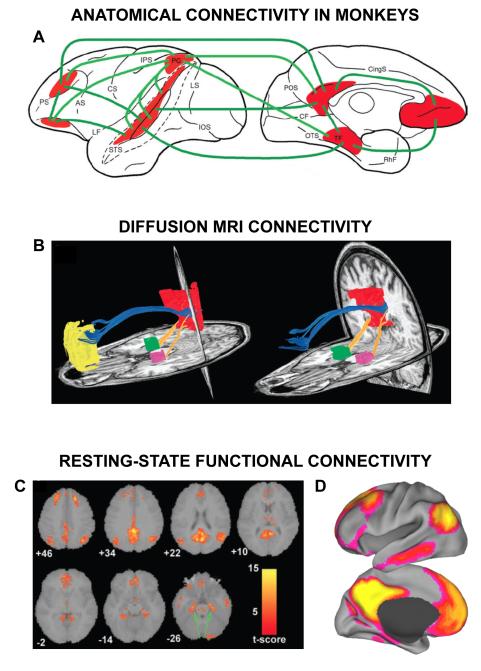
Neuroanatomical tracing techniques, including retrograde and anterograde tracing, reveal long-range monosynaptic connections and provide important insights into the connectional anatomy of the default network. Unfortunately, such techniques are only applicable to non-human animals, and human cortical expansion makes direct comparison between species somewhat difficult. Nevertheless, recent neuroimaging studies in macaques and chimpanzees reveal a homologous set of default network-like regions that exhibit properties similar to those in humans (Vincent and others 2007; Rilling and others 2007; Kojima and others 2009; Margulies and others 2009). Given these similarities, it is worthwhile to briefly summarize the major anatomical connections between homologous default network regions in non-human primates. These connections are illustrated in Fig. 3A in a diagram compiled by Binder and others (2009) and are only briefly described here (see also Buckner and others 2008).
Fig. 3.
Converging approaches highlight the anatomical organization of the default network. A. This diagram, from Binder and others (2009, Figure 7B), outlines the major white matter connections between default network regions as revealed from anatomical tracing studies in macaques.1 B. Diffusion tractography from a single human participant highlights white matter tracts connecting the medial prefrontal, medial parietal, and medial temporal cortices within the default network. Figure from Greicius and others (2009; Figure 1B)1 C. Co-activation of default network regions (including the hippocampal formation) at rest as revealed by independent component analysis. Figure from Greicius and others (2004, Figure 2A)2. D. Using a seed-based temporal correlation rs-fcMRI approach, Fox and others (2005) revealed robust spontaneous BOLD correlations between default network regions3. Figures were reproduced or adapted with permission from 1Oxford University Press ©, 2© 2004, National Academy of Sciences, U.S.A., and 3Elsevier Limited © (adapted from Raichle and Snyder 2007, Figure 1C).
The medial prefrontal cortex includes several cytoarchitectonically-distinct regions that have undergone considerable reorganization and cortical expansion in humans (e.g. Ongur and others 2003). These regions connect to many of the major default network regions including the PCC, lateral temporal lobe (superior temporal sulcus and temporal pole), and the MTL (Barbas and others 1999; Koybayashi and Amaral 2003; Morecraft and others 2004; Parvizi and others 2006).
The medial parietal cortex, another structure exhibiting widespread anatomical connectivity, includes PCC (areas 23 and 31), the nearby Rsp (area 29/30), and the precuneus (area 7m). The PCC is anatomically connected to Rsp and 7m, as well as many other default network regions including MPFC, inferior parietal lobule (near area PG and 7a), lateral temporal lobe (along the superior temporal sulcus) and the MTL (including the HF and PHC; Barbas and others 1999; Kobayashi and Amaral 2003; Morecraft and others 2004; Parvizi and others 2006). Most of the reciprocal connections between the medial parietal cortex and the MTL pass through the Rsp, a region that also exhibits strong reciprocal projections to the lateral and medial PFC (e.g. Koybayashi and Amaral 2003). In contrast, the connectivity pattern of the precuneus diverges from that of the PCC and Rsp, with distinct subdivisions being strongly anatomically and functionally connected to visual, sensorimotor, and attention areas (Buckner and others 2008; Margulies and others 2009). Finally, the monkey inferior parietal lobule (near areas PG and 7a) is anatomically connected with the regions outlined above, in addition to the anterior and posterior superior temporal sulcus and the MTL (e.g. Cavada and Goldman-Rakic 1989).
Thus, many of the established default network regions in humans share reciprocal connections even in non-human primates. Of relevance for later sections, regions exhibiting particularly widespread connectivity include MPFC and PCC. These midline structures are candidate hubs within the default network.
Diffusion MRI Imaging
Recent diffusion imaging techniques permit non-invasive, in vivo measurement of white matter tracts in humans and have made considerable headway toward providing additional clues to the anatomical organization of the default network. Using such techniques, the cingulum bundle has been shown to connect the PCC to the MPFC (Figure 3B; Greicius and others 2009; van den Heuvel 2009), and distinct white matter tracts link the MPFC to the inferior parietal lobe (van den Heuvel 2009). In turn, the inferior parietal lobe connects to the lateral temporal lobe via the middle longitudinal fasciculus (Makris and others 2009), and to the MTL via the inferior longitudinal fasciculus and cingulum bundle (Uddin and others 2010). Completing the circuit, the MTL connects back to the PCC/Rsp (Fig. 3B; Greicius and others 2009).
THE DEFAULT NETWORK AND RESTING-STATE FUNCTIONAL CONNECTIVITY
The increasing popularity of rs-fcMRI techniques, including seed-based correlation approaches and independent component analysis (ICA), has significantly advanced our understanding of the default network (Fig. 1B). Though the underlying source of resting-state signals is still debated, a robust finding in neuroimaging is the presence of low-frequency, spontaneous fluctuations across the brain that organize into distinct, tightly-correlated functional-anatomic networks often mirroring task-evoked patterns of brain activity (Fox and Raichle 2007; Smith and others 2009). In 2003, the major application of rs-fcMRI to the default network by Greicius and colleagues (2003) sparked a rapid progression of studies characterizing the network in healthy young adults (reviewed in Buckner and others 2008), development (reviewed in Power and others 2010), aging (e.g. Damoiseaux and others 2008; see also Andrews-Hanna and others 2007), clinical populations (Box 1, Fig. 1B; reviewed in Broyd and others 2009; Zhang and Raichle 2010), and non-human primates (Vincent and others 2007; Margulies and others 2009). In young adults, both seed-based and ICA techniques reveal functional coherence between default network regions, including the MPFC, PCC, lateral parietal lobe, lateral temporal cortex extending to temporal pole, and the MTL (Fig. 3C,D; e.g. Greicius and others 2003 2004; Fox and others 2005; Vincent and others 2006; Golland and others 2008). These studies confirm and extend the prior literature on task-induced deactivations and anatomical connectivity, and also hint at its heterogeneous organization.
Interestingly, whereas regions comprising the default network are positively correlated with one another, often being referred to as a “task-negative” or “intrinsic” system, they appear to be intrinsically distinct from (and oftentimes negatively correlated with) regions comprising the task-positive or “extrinsic” network (e.g. Fox and others 2005; Golland and others 2008). While ongoing debate surrounds the source of the negative correlations, it is widely established that the default network is anatomically and functionally distinct from networks involved in sensory and external attentional functions.
THE DEFAULT NETWORK COMPRISES HUBS AND SUBSYSTEMS
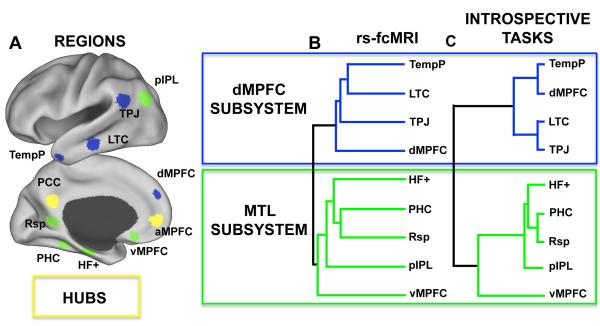
Results from anatomical and functional connectivity studies suggest the default network is intrinsically organized into distinct subsystems that converge on core hubs (Buckner and others 2008). Consistent with its widespread pattern of connectivity, the PCC appears to play a key integrative role, being significantly functionally-correlated with all other default network regions even after taking their pair-wise regional correlations into account (e.g. Fransson and Marrelec 2008). In addition, graph analysis techniques applied to both rs-fcMRI and diffusion imaging data reveal the PCC (and to a large extent, the MPFC) exhibits hub-like properties throughout the brain, reflected by its role as a heteromodal association area (e.g. Hagmann and others 2008; Buckner and others 2009). The intrinsic functional organization of the default network was directly explored by Andrews-Hanna and others (2010a), who used rs-fcMRI, graph analysis, and hierarchical clustering techniques to examine the clustering properties of eleven a priori regions within the default network (Fig. 4A). Consistent with prior findings outlined above, both the PCC and anterior MPFC (near areas 10/32) exhibited the highest formal graph analysis measure of network hubs, being significantly functionally correlated with all other regions within the default network. Importantly, when hierarchical clustering was applied to the remaining regions, the HF and PHC clustered together with the Rsp, vMPFC, and pIPL into a “MTL subsystem,” whereas a distinct “dorsal MPFC subsystem” included the dorsal MPFC (dMPFC), TPJ, lateral temporal cortex (LTC) and temporal pole (Fig. 4B). Thus, these results suggest that the default network can be described within an architectural framework highlighting both patterns of convergence and divergence.
Fig. 4.
The default network comprises hubs and subsystems. A. The network properties of eleven regions of interest within the default network were explored using graph analysis. Results revealed the aMPFC and PCC (yellow regions) qualified as hubs within the network. B. The rs-fcMRI clustering properties of all remaining regions were next explored with hierarchical clustering. Distinct clusters are color-coded to highlight the dMPFC subsystem (blue) and the medial temporal lobe subsystem (green). C. Similar clustering patterns emerged in task-evoked analyses when participants engaged in introspective tasks including reflecting on their present mental states and envisioning their future. Panels adapted with permission from Elsevier Limited © (see Andrews-Hanna and others 2010a, Figures 1B,D and 5B).
OVERARCHING FUNCTIONS OF THE DEFAULT NETWORK
In summary thus far, a growing body of research highlights the default network as a system of anatomically connected and functionally correlated brain regions exhibiting elevated activity during undirected passive tasks. While these observations offer some initial clues about the overarching functions of the default network, the network’s precise functional properties are challenging to establish because passive states are unconstrained (e.g. Morcom and Fletcher 2007).
Internal mentation or external attention?
In a recent review article, Buckner and others (2008) outlined two plausible hypotheses regarding the engagement of the default network during passive states (see Figure 11 of Buckner and others 2008). The “sentinel hypothesis” holds that activity within all or part of the default network reflects attention to the external environment, particularly when broadly monitoring the environment for upcoming stimuli or other significant, unpredictable events (Shulman and others 1997; Raichle and others 2001; Gusnard and Raichle 2001; Gilbert and others 2007). Providing support for the role of the default network in broad external attention, Shulman and colleagues (1997) noted that TIDs were more pronounced when tasks involved foveal stimuli. Additionally, activity within the default network increases during tasks when participants anticipate targets that occur in unpredictable compared to predictable locations (Hahn and others 2007; but see Small and others 2003), as well as during certain stimulus-oriented tasks compared with stimulus-independent versions in which participants mentally carry out complex tasks in their head (e.g. Gilbert and others 2005, 2006, 2007). Importantly, these studies reveal negative trial-by-trial relationships between default network activity and response time, such that trials associated with greater activity were linked to faster performance speed (Hahn and others 2007; Gilbert and others 2006).
However, in addition to broadly monitoring the external environment during awake passive states, participants commonly engage in internally-directed cognitive processes including “generation and manipulation of mental images, reminiscence of past experiences based on episodic memory, and making plans” (Mazoyer and others 2001, p295; see also Ingvar 1979; Andreasen and others 1995; Binder and others 1999). The “internal mentation hypothesis” proposes that these spontaneous introspective processes, sometimes referred to as stimulus-independent thought, task-unrelated thought, mind wandering, daydreaming, or zoning-out, give rise to default network activity (e.g. Binder and others 1999 2009; Gusnard and Raichle 2001; Mazoyer and others 2001; Buckner and others 2008; Spreng and others 2009). These observations lead to Andreasen’s ironic acronym for passive states: “REST” (Random Episodic Silent Thought; Andreasen and others 1995).
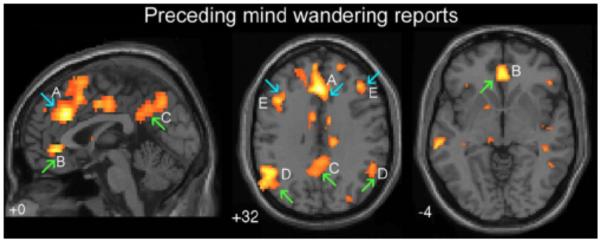
Within the past decade, a wealth of neuroimaging and lesion data has provided support for the role of the default network in spontaneous and goal-directed internal mentation. For example, recent neuroimaging studies have reported positive relationships between default network activity and spontaneous internal mentation, both within and between subjects (e.g. McKiernan and others 2006; Mason and others 2007; Wang and others 2009; Vanhaudenhuyse and others 2010). Importantly, Christoff and colleagues (2009) observed that task trials preceding reports of mind wandering during sustained attention tasks were associated with elevated default network activity, particularly when participants are unaware that their mind has wandered (Figure 5). Additionally, both default network activity and frequency of spontaneous thoughts inversely correlate with task difficulty, with easier and more practiced tasks often yielding a greater percentage of spontaneous thoughts (reviewed in Smallwood and Schooler 2006; McVay and Kane 2010), as well as more pronounced default network activity (e.g. McKiernan and others 2006; Mason and others 2007). Finally, both spontaneous thoughts and default network activity often predict poor performance on a wide range of tasks (reviewed in Smallwood and Schooler 2006; McVay and Kane 2010; Buckner and others 2008; see also Christoff and others 2009; Reichle and others 2010). Supporting the internal mentation hypothesis, increased default network activity (with the curious exception of the medial temporal lobe) is only detrimental to successful memory encoding, while its activation is beneficial for episodic memory retrieval (e.g. Daselaar and others 2009; Kim and others 2010; Vannini and others 2011).
Fig. 5.
Default network activation is linked to spontaneous thought. A combined fMRI / thought sampling study revealed activation of several default network regions prior to reports of mind wandering when participants were behaviorally probed during a sustained attention task. Interestingly, participants also activated frontoparietal control regions when experiencing spontaneous thoughts, a finding which is currently a matter of debate (McVay and Kane 2010; Smallwood 2010). Figure reproduced with permission from Christoff and others (2009, Figure 2).
Thus, existing data links default network activity during passive states to two plausible, but opposing functions: broad attention to the external environment and spontaneous internal mentation. To disambiguate between these distinct hypotheses, Andrews-Hanna and others (2010b) compared default network activity across three conditions experimentally-manipulated with respect to the direction (external vs. internal) and scope (broad vs. focal) of participants’ attention. Providing support for the internal mentation hypothesis, regions within the default network increased their activity during the passive condition for which behavioral thought probes revealed the highest frequency of spontaneous thoughts, and exhibited minimal difference in activity between the broad and focal external attention conditions. Consistent with lesion and connectivity studies, the precuneus was preferentially engaged when participants broadly monitored their external environment, but rs-fcMRI connectivity analyses revealed the precuneus was positively correlated with regions outside of the default network. Thus, these data are most consistent with the idea that default network activity during passive states primarily reflects spontaneous internal thoughts.4
THE ADAPTIVE ROLE OF THE DEFAULT NETWORK IN SPONTANEOUS INTERNAL MENTATION
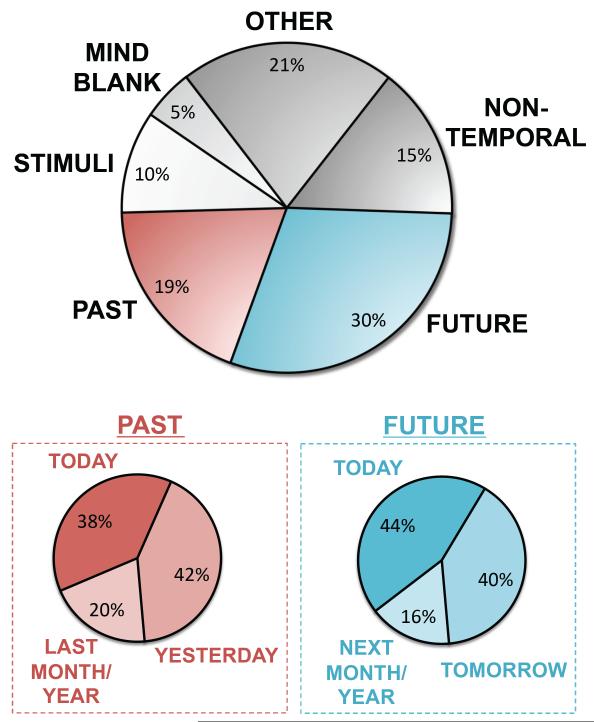
Though the studies above provide strong support for the role of the default network in internal mentation, many of them highlight the maladaptive consequences of default network activity and spontaneous thoughts for goal-directed cognition. However, multiple lines of evidence suggest the default network and spontaneous thought may serve a broader adaptive purpose (Singer 1966; Klinger 1971; Buckner and others 2008; Christoff 2009; Baars 2010; Wamsley and Stickgold 2010). First, humans engage in spontaneous thought with an astoundingly high frequency. Daily experience sampling techniques estimate that humans spend between 30-50% of daily life engaged in thoughts unrelated to the immediate task at hand (e.g. Klinger and Cox 1987; Killingsworth and Gilbert 2010). Second, insight into the nature of participants’ thoughts also speaks to their possible adaptive significance. Consistent with Klinger’s “current concern hypothesis” (Klinger 1971), studies conducted in real-world or laboratory/MRI settings reveal that participants engage in highly personally-significant and goal-directed thoughts prominently about one’s past and future (Fig. 6; Klinger and Cox 1987; Binder and others 1999; Mazoyer and others 2001; Klinger 2009; Andrews-Hanna and others 2010b; Delamillieure and others 2010). Importantly, these thoughts can be experimentally manipulated by altering the motivational value, or “payoff,” associated with attention to such thoughts or to the task at hand, with results supporting a model whereby processing of internal and external information compete for a limited set of attentional resources (Antrobus and others 1966). For example, when participants are primed with distressing personally-relevant information prior to a task, participants maximize their spontaneous thoughts about this information and perform poorly on the task. Conversely, when the “payoff” for successful task performance is boosted with monetary incentives, participants minimize their spontaneous thoughts and enhance their task performance.
Fig. 6.
Awake resting states are associated with spontaneous internal mentation. Andrews-Hanna and others (2010b) administered retrospective thought sampling questionnaires to 139 participants after staring at a fixation crosshair between 2-4 consecutive resting runs in the MRI scanner. Participants reported spending nearly half of their time engaged in episodic past or future thought, with a preference towards thinking about the recent past and immediate future. Figure modified from Andrews-Hanna and others (2010b, Figure 8).
An intriguing possibility is that engaging in spontaneous thought may allow individuals to construct and simulate alternative scenarios, mentally-organize their plans, and prepare for what may lie ahead. Additionally, spontaneous thoughts may facilitate the organization and structuring of daily events, promoting consolidation of the most personally-salient information into long-term memory. These simulative processes might be supported by a MTL that dynamically interacts with cortical regions within and outside the default network. Supporting this possibility, recent studies have linked resting MTL activity or MTL-cortical functional connectivity to individual differences in spontaneous episodic thoughts (Andrews-Hanna and others 2010b), memory ability (Wig and others 2008), and consolidation of recent experiences (Tambini and others 2010). Additionally, reactivation of hippocampal neurons during off-task “rest” periods in rats reflect the animal’s prior experience and predict its future choices (reviewed in Buckner 2010). Interestingly, similar ideas are echoed in the literature on sleep and dreaming, whereby a growing body of work has begun to link memory consolidation in sleep and night dreams to the default network (reviewed in Wamsley and Stickgold 2010; Nir and Tononi 2010; Domhoff in press).
THE ROLE OF THE DEFAULT NETWORK IN GOAL-DIRECTED INTERNAL MENTATION
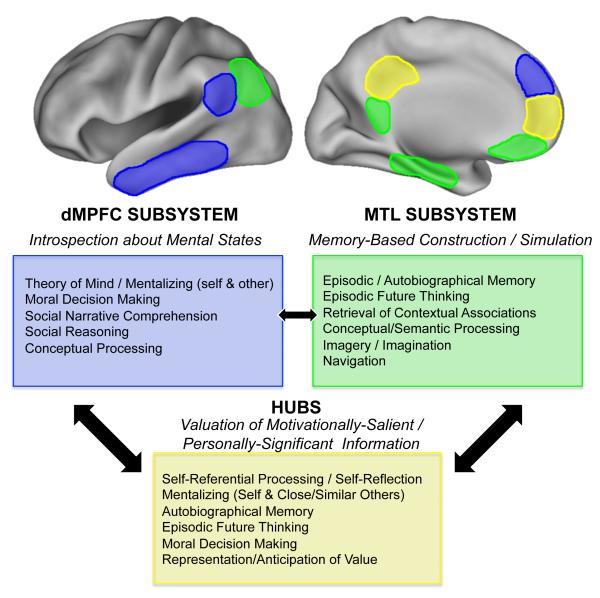
Further support for the adaptive role of the default network in internal mentation is provided by the wide range of goal-directed tasks that activate the network5 (Fig. 7). Consistent with prior theories dissociating externally-oriented from internally-oriented attention (Golland and others 2008; Buckner and Carroll 2007; Buckner and others 2008; Spreng and others 2009; Binder and others 2009), these tasks share in common the process of directing one’s attention inwards, as when retrieving a mnemonic representation or when introspecting about one’s own mental states. However, despite the overarching similarities among such tasks, they differ substantially in the default network regions that they activate, exhibiting patterns that align well with the hubs and subsystems architecture.
Fig. 7.
Proposed functional-anatomic organization of the major default network components. A schematic drawing of the default network hubs (yellow) and subsystems (blue = dMPFC subsystem; green = MTL subsystem) is highlighted along with each components’ hypothesized functions and the tasks that frequently activate them. Arrows reflect approximate strength of connectivity between default network components. See text for references. Note regional anatomic boundaries are approximate.
To investigate the functional contributions of the distinct default network components to internal mentation, Andrews-Hanna and colleagues (2010a) applied item and clustering analyses to task-evoked functional MRI data while participants completed a variety of introspective tasks. Consistent with the proposed architectural framework, a pattern of results emerged that mirrored the clustering properties between regions previously observed using rs-fcMRI (Fig. 4C). Whereas regions within the dMPFC subsystem exhibited similar activation profiles in response to the four task conditions – being preferentially active when participants reflected on their present mental states – the functional profiles of these regions were distinct from those comprising the MTL subsystem. In contrast, the MTL subsystem preferentially activated when participants made episodic predictions about their future. Consistent with their hub-like properties, the aMPFC and PCC shared functional properties of both subsystems, activating preferentially for both self-relevant conditions. Consistent with prior theories (Hassabis and Maguire 2007; Schacter and others 2008; Szpunar and others 2009), more than one third of the variance in trial-by-trial activity within the MTL subsystem was predicted by task strategies involving retrieval of past information and mental construction of a novel scene. In contrast, activity within the hubs, and to a lesser but significant degree within the dMPFC subsystem, was predicted by strategies involving introspection about affective, personally-significant information. To provide further insight into the functions of these components, the next section synthesizes relevant findings from prior literature pertaining to memory, social cognition, and emotion.
The MTL subsystem and its role in memory-based construction
Though the critical role of the medial temporal lobe for long-term declarative memory retrieval has been established for quite some time (Scoville and Milner 1957), more recent findings have highlighted the additional involvement of a wider cortical network, including the Rsp, PCC, pIPL, and MPFC. These regions comprise the MTL subsystem and are consistently activated during autobiographical memory and other recollection-based laboratory memory tasks (Fig. 7; Svoboda and others 2006; Cabeza and St. Jacques 2007; McDermott and others 2008; Spreng and others 2009). Interestingly, many of the regions within the MTL subsystem are also reliably engaged when individuals retrieve contextual associations (Bar 2007), semantic and conceptual knowledge (Binder and others 2009), and spatial information (i.e. when navigating a familiar environment; Spiers and Maguire 2006; Vann and others 2009).
An interesting possibility echoed by other researchers is that an adaptive function of memory retrieval may be to facilitate mental construction of novel episodes to help individuals prepare for the immediate and distant future (Buckner and Carroll 2007; Bar 2007; Gilbert and Wilson 2007; Schacter and others 2008; Buckner 2010). Humans have the unique ability to rapidly retrieve stored episodic, conceptual and contextual representations and use this information to construct or “simulate” future outcomes before they happen. Additionally, episodic future simulation can yield long-term adaptive benefits, including augmenting the subjective value of future rewards (Peters and Buchel 2010) and improving the likelihood of achieving long-term goals (e.g. Gollwitzer and Brandstatter 1997).
Supporting the possibility that memory aids constructive simulation, numerous studies have observed striking parallels between the phenomenological characteristics and neural underpinnings associated with retrieval of past information and prospective thought (reviewed in Schacter and others 2008; Szpunar 2010; see also Addis and others 2007; Szpunar and others 2007; D’Argembeau and others 2010; Spreng and Grady 2010). Such parallels also extend to imagination void of a temporal context (i.e. in the present), where the MTL subsystem becomes engaged when individuals imagine objects and sounds (Daselaar and others 2010; Summerfield and others 2010) and integrate these items into coherent simulated episodes (Hassabis and others 2007; Addis and others 2009; Summerfield and others 2010). Collectively, these findings suggest the MTL subsystem may play a particular role in memory-based construction or simulation.
The dMPFC subsystem and its role in introspection about mental states
In contrast to the constructive functions of the MTL subsystem, the dMPFC subsystem may play an important role in introspecting about the mental states of social agents. A rich body of work within the field of social neuroscience has consistently pointed to a network of brain regions, including the dMPFC, TPJ, Lateral Temporal Cortex, and Temporal Pole, that become engaged when individuals reflect upon, evaluate, or appraise social information (Fig. 7; Frith and Frith 2003; Lieberman 2007; Van Overwalle 2009). These introspective social processes can be directed toward one’s own thoughts, feelings, and desires (i.e. “mental states”; reviewed in Ochsner and others 2004; Van Overwalle 2009; van der Meer and others 2010; see also Johnson and others 2005; Lombardo and others 2010; Moran and others in press), as well as the mental states of others – a process often referred to as “theory of mind” or “mentalizing” (reviewed in Frith and Frith 2003, Ochsner and others 2004 2005; Lieberman 2007; Van Overwalle 2009; see also Lombardo and others 2010; Spreng and Grady 2010; Rabin and others 2010; Dodell-Feder and others in press). Interestingly, mentalizing may account for the involvement of the dMPFC subsystem when participants engage in interpersonal interactions (Rilling and others 2004), view animated shapes (Tavares and others 2008), read social narratives (Yarkoni and others 2008), or reason about social problems (Van Overwalle 2011) and moral dilemmas (Moll and others 2005). Collectively, these findings suggest the dMPFC subsystem plays a broad role in introspecting about mental states. However, it will be up to future studies to determine how specific the dMPFC subsystem is for these processes or whether it plays a more general role in non-social processes such as reasoning, metacognition, emotion, or language comprehension.
The aMPFC and PCC hubs and their role in valuation of personally-significant affective information
The hubs of the default network – aMPFC and PCC – activate across a diverse range of mnemonic, social, and emotional tasks that involve personally-significant and other motivationally-salient information (Fig. 7). In the memory domain, these midline regions become engaged when individuals recollect episodic and autobiographical information (reviewed in Svoboda and others 2006; Schacter and others 2008; Spreng and others 2009; McDermott and others 2009; see also Kim and others 2010; St. Jacques and others in press), consistent with the idea that such tasks evoke a sense of “autonoetic consciousness,” or the subjective feeling of re-experiencing the past (Tulving and others 1985; Cabeza and St. Jacques 2007). Activity within the midline cores is most pronounced when individuals remember real autobiographical events compared to previously-imagined fictitious events (Hassabis and others 2007; Summerfield and others 2009). Similarly, the aMPFC and PCC robustly activate when individuals simulate hypothetical personal future events (reviewed in Schacter and others 2008; Spreng and others 2009; see also Abraham and others 2008; D’Argembeau and others 2010; Spreng and Grady 2010; Andrews-Hanna and others 2010a; Gerlach and others in press), particularly during realistic contexts for which participants have past experience (Szpunar and others 2009).
Within the domain of social cognition, the aMPFC and PCC become engaged when individuals reference information to themselves or reflect on personal preferences, beliefs, values, feelings, abilities, and physical attributes (reviewed in Amodio and Frith 2006; Ochsner and others 2004 2005; Northoff and others 2006; Schmitz and Johnson 2007; Mitchell 2009; Van Overwalle 2009; van der Meer and others 2010; see also Jenkins and Mitchell 2010; Andrews-Hanna and others 2010a; Benoit and others 2010; Krienen and others 2010; Sajonz and others 2010; Whitfield-Gabrieli and others 2011) as well as engage in personal moral dilemmas (reviewed in Moll and others 2005). Additionally, the aMPFC (and to some extent the PCC) may mediate the self-reference effect in memory (Macrae and others 2004) by preferentially activating in response to self-descriptive or self-relevant information in a manner that increases linearly with stimulus self-relevancy (Macrae and others 2004; Moran and others 2006, 2009; Benoit and others 2010). Importantly, a growing number of studies suggest that the midline cores also increase their activity when individuals process information about similar (reviewed in Mitchell 2009) or close others (reviewed in Schmitz and Johnson 2007; van Overwalle 2009; see also Grigg and Grady 2010), including friends (Ochsner and others 2005; Krienen and others 2010; Benoit and others 2010), family members (Bartels and Zeki 2004; Moran and others in press), and romantic partners (Bartels and Zeki 2004).
Consistent with the idea that personally-significant information is intrinsically affective and motivationally-salient in nature (Olsson and Ochsner 2008), both the aMPFC and the PCC activate when individuals experience or anticipate affect (reviewed in Maddock 1999; Kober and others 2008). Though discussed most prevalently in regards to the MPFC, both midline regions have repeatedly been shown to increase their activity in response to a wide range of rewarding stimuli, particularly in a manner that tracks the anticipated or experienced value associated with the stimuli (e.g. reviewed in Montague and others 2006; see also Kable and Glimcher 2007; Chib and others 2009; Ballard and Knutson 2009; Hare and others 2009). Often value is disproportionately weighted towards the immediate rather than the delayed future, and activity within the aMPFC and PCC reflect this immediacy bias (McClure and others 2004; Ersner-Hershfield and others 2008; Mitchell and others 2010). In addition to rewarding stimuli, there is some evidence that aMPFC and PCC activate when participants experience or anticipate personally-salient negative affect, including social threat (Wager and others 2009), pain (Atlas and others 2010), and other aversive stimuli (Maddock 1999). Based on the functional convergence across the domains of memory, social cognition, and emotion, an intriguing possibility is that the aMPFC and PCC cores play a prominent role in the valuation of personally-significant and other highly salient information.
Once information is attributed a high personal value, it can then be used to guide and motivate behavior, perhaps through the interaction of the midline cores with subcortical regions or by further internal processing carried out by the distinct subsystems. For example, the dMPFC subsystem may allow individuals to reflect on the mental states elicited by the stimulus and the MTL subsystem may allow individuals to integrate this introspective information into a goal-directed plan.
FUTURE DIRECTIONS
Though the past decade of research has led to significant advancements in our understanding of the default network, several questions remain unanswered (Box 2). To satisfy a fully coherent functional account of the default network, it will be important to characterize how the subsystems interact with each other when individuals engage in real-world decisions that involve integrating information from distinct subsystems. Given their widespread connectivity to both subsystems, the hubs are candidate regions for facilitating efficient crosstalk between subsystems. Additionally, though the present article focused on the functions of the distinct default network components, it is likely that the regions within each component (i.e. the vMPFC compared to the HF, or the TPJ compared to the dMPFC), as well as any corresponding subregions, may have functionally-distinct roles. It will be important to consider the finer-scale organization of the default network as we move forward.
Box 2. Questions for Future Research.
What is the role of the default network in more basic and automatic functions such as affective processing (i.e. Olsson and Ochsner 2008), semantic retrieval (Binder and others 2009), or associations (Bar 2007)? How might these functions modulate activity in the default network during more complex tasks?
What are the distinct functional roles of each region within the default network? How do other structures not discussed in this review contribute to the default network (i.e. cerebellum, basal ganglia, thalamus, amygdala, and lateral frontal cortex)?
How do the MTL and dMPFC subsystems interact during passive states and active tasks? Do the aMPFC and PCC hubs mediate this interaction?
How does the default network interact with the external attention system (i.e. when participants rapidly switch between internal and external modes of cognition, or when a task requires integration of external attention with an internal representation)?
What is the absolute baseline level of neural activity within the default network? How does it change during externally-directed and internally-directed tasks?
What is the relationship between awake passive states and sleep, in terms of their neural underpinnings, spontaneous thoughts, and adaptive functions?
What are the electrophysiological underpinnings of the “resting state”?
How “uniquely-human” is the ability to introspect, remember the past, and predict the future? What are the neural protoforms of the default network in non-human animals?
Another challenge for future research will be to investigate how the default network interacts with other large-scale brain systems in the service of goal-directed behavior. How do we integrate sensory information from the external world with our internal motivational goals? Recent research highlights the possible involvement of a frontoparietal control system in the executive control of external and internal attention (Vincent and others 2008; Spreng and others 2010; Gerlach and others in press). This brain system is anatomically positioned between the default network and the external attention system and dynamically couples with either system depending on the task context. Within this executive control network, the anterior insula is thought to play a unique role in rapidly switching between external and internal modes of cognition and is uniquely positioned to initiate action through its connectivity to the anterior cingulate and pre-supplementary motor area (Menon and Uddin 2010). A more detailed account of system interactions will be necessary to fully establish the role of the default network in goal-directed behavior.
SUMMARY
The main objective of this article has been to bridge together seemingly disparate literatures from cognitive neuroscience and psychology to provide insight into the anatomical organization and adaptive functions of the brain’s default network. This cross-discipline endeavor has highlighted strong support for the overarching function of the brain’s “default network” in internal mentation. Evidence from task-induced deactivations, anatomical connectivity and functional coherence suggest that within this broad framework, the default network’s finer organizational structure is characterized by distinct subsystems that converge on core hubs. These components may contribute differently to internal mentation, allowing individuals to simulate the past and future, reflect on the mental states of social agents, and place high value on that which is personally-significant.
Instead of being psychologically constrained to the here-and-now, humans have the unique ability to disengage from the external world and turn our thoughts inwards, to that which we find personally-significant. Through mental simulation of our past, future, and the minds of others, we travel far beyond the observable; “surely so prominent a set of activities cannot be functionless” (Klinger 1971 p. 347). As evidenced by the rapid progress since the default network was discovered nearly a decade ago, the quickening pace of research and discovery holds great promise for our continued insight into our ever-wandering minds.
ACKNOWLEDGEMENTS
The preparation of this manuscript was supported by an NIMH-funded Interdisciplinary Behavioral Science Center grant for the University of Colorado Boulder (PI: Marie Banich; P50 MH079485) and an NIMH-funded Ruth L. Kirschstein National Research Service Award (F32 MH093985). I would like to thank Randy Buckner, Tor Wager, Donna Rose Addis, Brian Knutson, Andrew Reineberg, Tal Yarkoni, and Lauren Atlas for their valuable discussion, as well as Marie Banich and Nathan Spreng, who provided particularly constructive feedback on a prior draft of the manuscript.
Abbreviations of anatomical regions
- dMPFC
dorsal medial prefrontal cortex
- aMPFC
anterior medial prefrontal cortex
- vMPFC
ventral medial prefrontal cortex
- PCC
posterior cingulate cortex
- Rsp
retrosplenial cortex
- MTL
medial temporal lobe
- PHC
parahippocampal cortex
- HF
hippocampal formation
- pIPL
posterior inferior parietal lobule
- TPJ
temporoparietal junction
- LTC
lateral temporal cortex
- TempP
temporal pole
Footnotes
Note that since space limitations permit only brief discussion and selective citations, the reader is referenced to prior review articles for more in depth discussion pertaining to the topics. When possible, an emphasis is placed on recent findings that are not discussed in the cited reviews.
A current matter of debate is whether default network activity during resting states reflects the network’s absolute baseline level of neural activity or whether the default network increases its activity above absolute baseline during rest because individuals engage in mental activity when attention to the external environment is minimized (Binder and others 1999; Raichle and others 2001; Gusnard and Raichle 2001; Morcom and Fletcher 2007; Raichle and Snyder 2007; Buckner and others 2008).
This PubMed search was performed February, 2011 using the search criteria “default mode” OR “default network.”
It should be noted that although this study provides initial support for the role of the default network in internal mentation, it does not rule out the network’s possible role in more diffuse background attention or attention to non-visual sensory modalities, as when attending to the noise of the MRI scanner. It will therefore be important for future studies to examine these alternate possibilities.
The use of the term “goal-directed” here refers to active engagement in experimentally-directed tasks. These tasks require attention to intrinsically-oriented/introspective processes and oftentimes also require an overt motor or verbal response. Note however that tasks vary widely in their requirement for executive control of attention, with some being more automatic (i.e. contextual associations) and others being more controlled (i.e. autobiographical planning). Interestingly, recent studies suggest that in addition to regions within the default network, introspective tasks requiring higher-order executive processes engage additional regions that comprise a “frontoparietal control network” (e.g. Spreng and others 2010; Gerlach and others in press).
REFERENCES
- Abraham A, Schubotzb RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Res. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M-A, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D, Frith C. Meeting of minds: The medial frontal cortex and social cognition. Nature Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadllo T, Arndt S, Rezai K, Watkins G. Remembering the past: Two facets of episodic memory explored with positron emission tomography. J Am Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. others. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner R. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010b;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner R. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010a;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Percept Motor Skills. 1966;23:399–417. [Google Scholar]
- Atlas L, Bolger N, Lindquist K, Wager T. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars B. Spontaneous repetitive thoughts can be adaptive: Postscript on “mind wandering”. Psych Bull. 2010;136:208–210. doi: 10.1037/a0018726. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knuston B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cog Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski S, Rempel-Clower N. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Benoit R, Gilbert S, Volle E, Burgess P. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50:1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai R, Graves W, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state: A functional MRI study. J Cog Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bressler S, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cog Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Broyd S, Bemanuele C, Debener S, Helps S, James C, Sonuga-Barke S. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. others. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual Rev Psych. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function and relevance to disease. Annals NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cog Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos A. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St. Jacques P. Functional neuroimaging of autobiographical memory. Trends Cog Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic P. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chib V, Rangel A, Shimojo S, O’Doherty J. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon A, Smallwood J, Smith R, Schooler J. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Nat Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon A, Smith R. The role of spontaneous thought in human cognition. In: Vartanian O, Mandel D, editors. Neuroscience of decision making. Psychology Press; In press. [Google Scholar]
- D’Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D. The neural basis of personal goal processing when envisioning future events. J Cog Neurosci. 2010;22(8):1701–1703. doi: 10.1162/jocn.2009.21314. others. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita E, Barkhof F, Scheltens P, Stam CJ. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. others. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Porat Y, Huijbers W, Pennartz C. Modality-specific and modality-independent components of the human imagery system. Neuroimage. 2010;52:677–685. doi: 10.1016/j.neuroimage.2010.04.239. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Doucet G, Mazoyer P, Turbelin M-R, Delcroix N, Mellet E. The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res Bull. 2010;81:565–573. doi: 10.1016/j.brainresbull.2009.11.014. others. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. Neuromage. doi: 10.1016/j.neuroimage.2010.12.040. In press. [DOI] [PubMed] [Google Scholar]
- Domhoff GW. The neural substrate for dreaming: Is it a subsystem of the default network? Consc Cog. doi: 10.1016/j.concog.2011.03.001. In Press. [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H, Elliott Wimmer G, Knutson B. Saving for the future self: Neural measures of future self-continuity predict temporal discounting. Soc Cog Aff Neurosci. 2008;4:85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate plays a pivotal role in the default mode network: Evidence from partial correlation analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. Development and neurophysiology of mentalizing. Phil Trans Royal Soc London. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach K, Spreng RN, Gilmore A, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. Neuroimage. doi: 10.1016/j.neuroimage.2011.01.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Wilson T. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: The default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Euro J Neurosci. 2005;21(5):1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–553. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer P, Brandstatter V. Implementation intentions and effective goal pursuit. J Pers Soc Psych. 1997;73:186–199. [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty R. Resting state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Nat Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Nat Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. The default network and processing of personally relevant information: Converging evidence from task-related modulations and functional connectivity. Neuropsychologia. 2010;48(13):3815–3823. doi: 10.1016/j.neuropsychologia.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Giagandet X, Meuli R, Honey C, Wedeen V. Mapping the structural core of human cerebral cortex. PLOS One. 2008;6:1479–1493. doi: 10.1371/journal.pbio.0060159. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cerebral Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer C, Rangel A. Self-control in decision-making involves modulation of the vMPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cog Sci. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Nat Acad Sci USA. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar D. “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness: On the functional anatomy of the conscious state. Acta Neurology Scand. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Soc Neurosci. 2010;12:1–8. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Johnson S, Schmitz T, Kawahara-Baccus T, Rowley H, Alexander A, Lee J. The cerebral response during subjective choice with and without self-reference. J Cog Neurosci. 2005;17:1897–1906. doi: 10.1162/089892905775008607. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J, Glimcher P. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth M, Gilbert D. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task-positive and task-negative networks. Neuroimage. 2010;49:1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E. Structure and functions of fantasy. John Wiley & Sons, Inc; New York: 1971. [Google Scholar]
- Klinger E, Cox W. Dimensions of thought flow in everyday life. Imag Cog Person. 1987;7:105–128. [Google Scholar]
- Klinger E. Daydreaming and fantasizing: Thought flow and motivation. In: Markman KD, Klein WMP, Suhr JA, editors. Handbook of imagination and mental simulation. Psychology Press; 2009. [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kober H, Barret L, Joseph J, Bliss-Moreau E, Lindquist K, Wager T. Functional grouping and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Onoe H, Hikosaka K, Tsutsui K, Tsukada H, Watanabe K. Default mode of brain activity demonstrated by positron emission tomography imaging in awake monkeys: Higher rest-related than working memory-related activity in medial cortical areas. J Neurosci. 2009;29(46):14463–14471. doi: 10.1523/JNEUROSCI.1786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F, Tu P, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A, Eickhoff S, Li K, Robin D, Glahn D, Fox P. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. Social cognitive neuroscience: A review of core processes. Ann Rev Psych. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lombardo M, Chakrabarti B, Bullmore E, Wheelwright S, Sadek S, Suckling J. Shared neural circuits for mentalizing about the self and others. J Cog Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. others. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield J, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maddock R. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou G, Kaiser J, Sorg S, Kennedy D, Pandya D. Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2009;19:777–785. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D, Vincent JL, Kelley C, Lohmann G, Uddin L, Biswal B. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Nat Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;215:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer P, Zago L, Mellet E, Bricogne S, Etard O, Houde F. Cortical networks for working memory and executive function systain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. others. [DOI] [PubMed] [Google Scholar]
- McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ S. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay J, Kane M. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psych Bull. 2010;136:188–197. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey K, Foster N, Kuhl D. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Phil Trans Royal Soc London. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Schirmer J, Ames D, Gilbert D. Medial prefrontal cortex predicts intertemporal choice. J Cog Neurosci. 2011;23:857–866. doi: 10.1162/jocn.2010.21479. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveria-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Montague P, King-Casas B, Cohen J. Imaging valuation models in human choice. Ann Rev Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Soc Neurosci. 2009;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Lee S, Gabrieli JDE. Dissociable neural systems supporting knowledge about human character and appearance in ourselves and others. J Cog Neurosci. doi: 10.1162/jocn.2010.21580. In press. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cog Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morcom A, Fletcher P. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Sitlwell-Morecrft KS, Gedney MT, Pandya DN. Cytoarchictecture and cortical connections of the posterior cingulate and adjacent somatosensorty fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Nir Y, Tononi G. Dreaming and the brain: From phenomenology to neurophysiology. Trends Cog Sci. 2009;14:88–100. doi: 10.1016/j.tics.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl G, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Beer J, Robertson E, Cooper J, Gabrieli JDE, Kihlstrom J. The neural correlates of directed and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. others. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Glover G. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cog Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. others. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner K. The role of social cognition in emotion. Trends Cog Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry A, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen G, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Nat Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel A-L, Muller-Oehring E, Shankaranarayanan A, Alsop D. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cerebral Cortex. 2011;21(1):233–44. doi: 10.1093/cercor/bhq090. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J, Fair D, Schlaggar B, Petersen S. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard D. A default mode of brain function. Proc Nat Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and unique neural correlates of autobiographical memory and theory of mind. J Cog Neurosci. 2010;22(6):1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Reichle E, Reineberg A, Schooler J. Eye movements during mindless reading. Psych Sci. 2010;21:1300–1310. doi: 10.1177/0956797610378686. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Barks S, Parr L, Preuss T, Faber T, Pagnoni G. A comparison of resting-state brain activity in humans and chimpanzees. Proc Nat Acad Sci USA. 2007;104:17146–17151. doi: 10.1073/pnas.0705132104. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind within interpersonal interactions. Neuroimage. 2004;22:1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sajnoz B, Kahnt T, Margulies D, Park S, Whittmann A, Stoy M. Delineating self-referential processing from episodic memory retrieval: Common and dissociable networks. Neuroimage. 2010;50:1606–1617. doi: 10.1016/j.neuroimage.2010.01.087. others. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Annals NY Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consc Cog. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Johnson S. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci and Biobehav Rev. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psych. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle M, Vaishnavi S, Snyder AZ. The default mode network and self-referential processes in depression. Proc Nat Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezen FM, Raichle ME. Common blood flow changes across visual tasks: II: Decreases in cerebral cortex. J Cog Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. others. [DOI] [PubMed] [Google Scholar]
- Singer JL. Daydreaming: An introduction to the experimental study of inner experience. Random House, Inc; New York: p. 1066. [Google Scholar]
- Small D, Gitelman D, Gregory M, Nobre A, Parrish T, Mesulam M-M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psych Bulletin. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C. Correspondence of the brain’s functional architecture during activation and rest. Proc Nat Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Laviolette P, O’Keefe K, O’Brian J, Rentz D, Pihlajamaki M. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers H, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cog Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cog Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng NR, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques P, Conway M, Lowder M, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J Cog Neurosci. doi: 10.1162/jocn.2010.21518. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia. 2010;48:1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon M, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK. Episodic Future Thought: An Emerging Concept. Perspectives Psych Sci. 2010;5(2):142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JC, McDermott KB. Contextual processing in episodic future thought. Cerebral Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Nat Acad Sci USA. 2007;104(2):642–7. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares P, Lawrence A, Banard P. Paying attention to social meaning: An fMRI study. Cerebral Cortex. 2008;18:1876–1885. doi: 10.1093/cercor/bhm212. [DOI] [PubMed] [Google Scholar]
- Tulving E. How many memory systems are there? Am Psych. 1985;40:385–398. [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen D, Greicius MD. Dissociable connectivity within human angular gyrus and intraparietal sulcus: Evidence from functional and structural connectivity. Cerebral Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Kahn R, Pol H. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Map. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David A. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vannini P, O’Brien J, O’Keefe K, Pihlajamäki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cerebral Cortex. 2011;21(1):22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Hum Brain Map. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54:1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M. Two distinct neuronal networks mediate the awareness of environment and of self. J Cog Neurosci. 2010;23:570–578. doi: 10.1162/jocn.2010.21488. others. [DOI] [PubMed] [Google Scholar]
- Vann S, Aggleton J, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;20:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel G, Fox MD, Snyder AZ, Baker J, Van Essen DC. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. others. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. others. [DOI] [PubMed] [Google Scholar]
- Wager T, Waugh C, Lindquist M, Noll D, Fredrickson B, Taylor S. Brain mediators of cardiovascular responses to social threat part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley E, Stickgold R. Dreaming and offline memory processing. Current Bio. 2010;20:R1010. doi: 10.1016/j.cub.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yu C, Xu L, Qin W, Li K, Xu L. Offline memory reprocessing: Involvement of the brain’s default network in spontaneous thought processes. PLOS One. 2009;4:e4867. doi: 10.1371/journal.pone.0004867. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Wig G, Grafton S, Demos K, Wolford G, Petersen S, Kelley W. Medial temporal lobe bold activity at rest predicts individual differences in memory ability in healthy young adults. Proc Nat Acad Sci USA. 2008;105:18555–18560. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Speer N, Zacks J. Neural substrates of narrative comprehension and memory. Neuroimage. 2008;41:1408–1425. doi: 10.1016/j.neuroimage.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]