Abstract
Despite improvements in early detection and treatment, cancer remains a major cause of mortality. Death from cancer is largely due to metastasis, which results in spreading of tumor cells to other parts of the body. The metastatic process is poorly understood, is often unpredictable, and usually results in incurable disease. There are no therapies specifically designed to target metastases or to block the metastatic process. Development and pre-clinical testing of new cancer therapies is limited by the scarcity of in vivo models that authentically reproduce human tumor growth and metastatic progression. Here, we report development of novel models for breast tumor growth and metastasis, which exist in the form of transplantable tumors derived directly from patients. These tumor grafts not only represent the diversity of human breast cancer, but also maintain essential features of the original patients’ tumors, including histopathology, clinical markers, hormone responsiveness, and metastasis to specific sites. Genomic features, such as gene expression profiles and DNA copy number variants, are also well maintained between the original specimens and the tumor grafts. We found that co-engraftment of primary human mesenchymal stem cells with tumor grafts helps to maintain the phenotypic stability of the tumors, and increases tumor growth by promoting angiogenesis and reducing necrosis. Remarkably, tumor engraftment is also a prognostic indicator of disease outcome: newly diagnosed women whose primary breast tumor successfully engrafted in mouse mammary glands had significantly reduced survival compared to patients whose tumors did not engraft. Thus, orthotopic breast tumor grafting marks a first step toward personalized medicine by replicating the diversity of human breast cancer through patient-centric models for tumor growth, metastasis, drug efficacy, and prognosis.
INTRODUCTION
Breast cancer remains a serious healthcare problem and, despite improvements in early detection and treatment, kills more than 40,000 people per year in the U.S. alone (www.seer.cancer.gov). Current targeted therapies for breast cancer are only effective for particular tumor types: examples include various endocrine blockade therapies (e.g. tamoxifen or aromatase inhibitors) for estrogen receptor-positive (ER+) tumors, and trastuzumab or lapatinib for HER2-positive tumors. There are currently no targeted therapies approved for patients with so-called ‘triple negative’ or ‘basal-like’ breast tumors (tumors that are usually ER−, progesterone receptor negative (PR−), and HER2−), which remain the most deadly forms of breast cancer1. So, despite marked progress in our understanding of cancer biology, the translation of research findings into new therapies for cancer is still an enormous barrier to progress: recent data suggests a 90% failure rate for new oncology drugs in the clinic2.
Development of new therapies is limited by the scarcity of authentic in vivo models of human breast cancer with which to examine the biology of tumors and how they metastasize, and to use for validation of the efficacy of potential new drugs. Such models currently rely on cell line xenografts, which only partially recapitulate the genetic features3,4 and metastatic potential of tumors in patients, resulting in poor predictions of how drugs will perform in a clinical setting2,5,6. The divergence of cell lines from actual human tumors is likely due to selective pressures resulting from in vitro propagation: growth on tissue culture plastic and in other artificial culture conditions, and maintenance in the absence of critical components of the tissue microenvironment. Nevertheless, efforts toward developing cancer cell lines and sub-lines as models for breast tumor progression7, site-specific metastasis8 and/or response to experimental therapeutics9 have proved to be very informative.
Engraftment of actual tumor tissues into immunodeficient mice (termed ‘tumor grafts’) provides improvement over implantation of cancer cell lines, in terms of phenocopying human tumors and predicting drug responses in patients10–13. However, tumor graft strategies for hormone-driven cancers such as breast or prostate cancer have had very limited success, making cell line xenografts the ‘gold standard’ for modeling these common types of human cancer, despite the disadvantages5. In particular, the scarcity of models that exhibit spontaneous, clinically relevant metastasis from breast tumors is concerning, given that the vast majority of deaths from breast cancer are due to metastasis (www.seer.cancer.gov). As a result, metastasis is very difficult to study, and there are currently no drugs designed specifically to prevent metastasis, or to specifically target metastatic lesions based on their unique characteristics.
We developed a technique for engraftment of breast tumors directly from breast cancer patients into the mammary glands of mice and report generation of a publicly available collection of highly characterized orthotopic breast tumor grafts. Importantly, the tumor grafts comprise all major types of breast cancer, and represent several of the known molecular subtypes. The tumor grafts were propagated by serial transplantation, without any in vitro culture steps, thus eliminating the problem of selective adaptation to culture conditions. Tumor grafts maintained critical features of the parental tumors including histolopathology, sites of metastasis, clinical markers, gene expression profiles, DNA copy number, and estrogen dependence for ER+ tumors. We also discovered that co-engraftment of primary human mesenchymal stem cells with patient tumors promoted angiogenesis, increased tumor growth, and facilitated maintenance of ER protein levels during serial propagation.
A clinically significant feature of these tumor grafts is their ability to recapitulate spontaneous metastasis with patterns similar to those observed in the original patients. Remarkably, women whose tumors engrafted in mouse mammary glands had significantly reduced survival compared to patients whose tumors did not successfully engraft. To our knowledge, these are the first data to reveal that engraftment of human breast tumors into mice immediately following surgical resection can provide prognostic information for new patients. In sum, our data support the finding that tumor grafts are improved, clinically relevant models for breast cancer research and provide an important step in the direction of personalized medicine.
RESULTS
Generation of a tumor graft bank representing all major clinical breast cancer subtypes
We transplanted 49 fresh primary tumors or metastatic breast cancer cell samples, collected immediately following surgery or fluid drainage from 42 different patients, into cleared mammary fat pads of NOD/SCID mice. Tumors grew from 18/49 samples (37%), and 13 tumor lines from 10 patients were successfully maintained through multiple rounds of serial transplantation (27% of total). For this report, we have fully characterized all of the lines that were validated for stable passage up to the end of 2010 (12 lines from 10 patients). Five of these tumor lines were ER+/PR+, 7 were ER−/PR−, and 5 were HER2+ (Table 1 and Supplementary Table 1). Four were from primary tumors and 8 were from metastatic effusions. Tumors that did not grow, or grew and subsequently receded, comprised 20 primary tumors, 2 lymph node metastases, 2 bone metastases, and 7 malignant effusions. Therefore, the source of the tumor (primary versus metastasis) did not significantly predict successful engraftment (Fisher’s exact test; p=0.09), nor did ER/PR status (p=0.99) or HER2 status (p=0.25). Of note, all primary tumors were from patients that had not received chemotherapy prior to tissue collection.
Table 1.
Abbreviated data for each tumor and corresponding tumorgraft line
| Patient Information1 | Xenograft Information1,2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Source | Primary Diagnosis3 | ER status4 | PR status4 | HER2 status4 | Clinical metastasis5 | ER status6 | PR status6 | HER2 status6 | Estrogen Dependence7 | Metastasis8 |
| HCI-001 | 1° breast tumor | IDC | neg | neg | neg | Lung | neg | neg | neg | n/a | Lung, LN |
| HCI-002 | 1° breast tumor | IDC | neg | neg | neg | LN | neg | neg | neg | n/a | LN |
| HCI-003 | 1° breast tumor | IDC | pos | pos | neg | LN | pos | pos | neg | Yes | Lung, LN |
| HCI-004 | 1° breast tumor | IDC | neg | neg | neg | Not detected | neg | neg | neg | n/a | Not detected |
| HCI-0059 | Pleural Effusion | mixed IDC and ILC | pos | pos | pos | Lung, bone | pos | pos | pos | Yes | Lung, LN, peritoneum |
| HCI-0069 | Pleural Effusion | pos | pos | not tested | not tested | Lung, peritoneum LN, | |||||
| HCI-0079 | Pleural Effusion | pos | pos | not tested | not tested | Lung, LN, bone | |||||
| HCI-008 | Pleural Effusion | IBC | neg | neg | pos | Skin, lung | neg | neg | pos | n/a | Lung, LN |
| HCI-009 | Ascites | Poorly differentiated adenocarcinoma | neg | neg | neg | LN, pancreas bone, peritoneum | neg | neg | neg | n/a | Lung, peritoneum LN |
| HCI-010 | Pleural Effusion | IDC | neg | neg | neg | Lung | neg | borderline | neg | n/a | Lung, LN |
| HCI-011 | Pleural Effusion | IDC | pos | pos | neg | LN, pleura | pos | pos | neg | no, but estrogen stimulated | Lung, LN, bone |
| HCI-012 | Pleural Effusion | IDC | neg | neg | pos | LN, pericardium | neg | neg | pos | n/a | LN, thymus |
See Supplementary Table for additional information
Based on at least 3 generations of transplantation
IDC: infiltrating ductal carcinoma; ILC: infiltrating lobular carcinoma; IBC: inflammatory breast cancer
Based on clinical diagnosis
At last follow-up. LN: lymph node
See Supplementary Table 2. n/a: not applicable
At necropsy upon visual inspection. LN: lymph node
From the same patient, collected at different times
To determine whether successful versus unsuccessful engraftment correlated with the amount of tumor in the fragments or tumor-to-stroma ratios, we embedded tumor fragments from primary tumors that had been preserved in parallel to transplantation (5 that successfully engrafted and 5 that did not engraft). Hematoxylin and eosin staining of 4–6 different fragments from each of the 10 tumors revealed no obvious differences in tumor or stromal contribution to the fragments (Supplementary Figure 1 and data not shown).
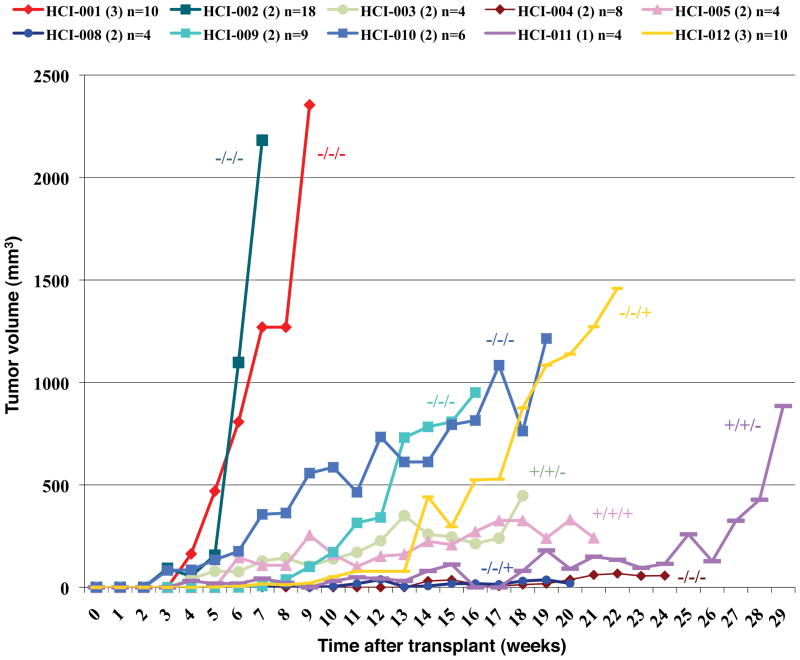
Tumors that were ER−, progesterone receptor negative (PR−), and HER2− (triple negative breast cancers) grew fastest as tumor grafts (Figure 1), a phenomenon often observed clinically 14. Tumor growth rates for all subtypes tended to increase with serial passage, although the differences were not statistically significant (Supplementary Figures 2–4).
Figure 1. ER−PR−HER2− (triple negative) tumors grew more quickly than ER+ or HER2+ tumors as tumor grafts.
Growth of primary tumor grafts is represented as tumor volume versus time after engraftment. Tumors were classified by ER, PR, and HER2 status as noted (e.g. −/−/−, in that order). Samples are color coded as shown in Supplementary Table 1 and Figure 5a, and the number in parentheses indicates the passage number depicted on this graph.
Tumor grafts resemble the patient tumors from which they are derived
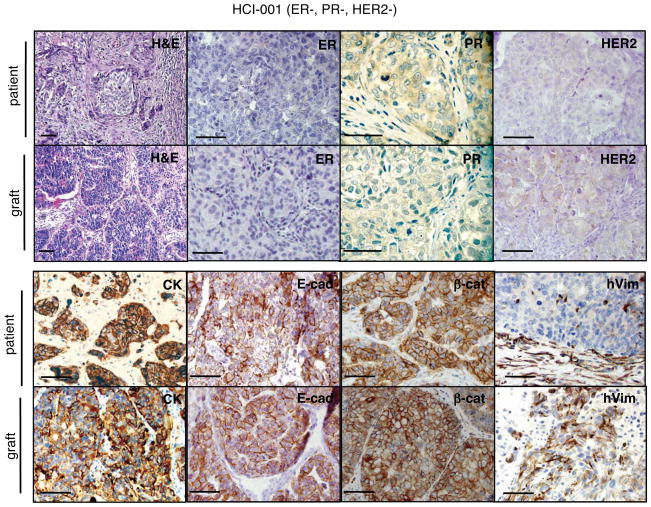
All tumors were fixed, sectioned and stained, then evaluated by a board-certified clinical breast pathologist who was blinded to the sample identities. Tumor graft derivatives of primary specimens were remarkably similar to the parental tumors. Pathologic analysis for each tumor and its tumor graft derivative(s) is shown in Supplementary Table 1, and representative histology and immunostaining is shown in Figure 2 and Supplementary Figures 5–13. Each tumor was stained with antibodies specific for wide spectrum cytokeratins, E-cadherin, β-catenin, and human vimentin to validate the epithelial nature of the tumors. The pattern of expression of these markers was well maintained between the original tumors and the tumor grafts, as were the molecular markers for breast cancer that are tested clinically (expression of ER, PR and HER2 proteins) (Table 1; Figure 2; Supplementary Table 1; Supplementary Figures 5–13).
Figure 2. Tumor grafts resembled the original tumors from which they were derived.
A representative ER−PR−HER2− tumor graft (HCI-001) is shown in comparison to the original patient sample. The tumor ID and the original clinical diagnosis for ER, PR, and HER2 are shown at the top. Sections from the patient’s primary breast tumor (patient), and from representative tumor grafts from the same patient (graft). Stains shown are hematoxylin and eosin (H&E) as well as antibody stains for ER, PR, HER2, cytokeratin (CK), E-cadherin (E-cad), β-catenin (β-cat), and human specific vimentin (hVim). Positive antibody signals are brown in color, with hematoxylin (blue) counterstain. Some images are shown at higher magnification to visualize nuclear staining. All scale bars correspond to 100 microns.
Components of the human-derived stroma were largely lost after engraftment, as judged by loss of human vimentin protein in vimentin-negative tumors (Supplementary Figure 6). This resulted in an overall higher proportion of human tumor cells within the grafts, as shown by enrichment in cytokeratin staining (Figure 2 and Supplementary Figures 5–7 and 9–10). To examine the contribution of mouse stroma to tumor grafts, we performed staining for three stromal cell types commonly found in tumors: inflammatory leukocytes, fibroblasts, and endothelial cells. Staining with antibodies specific for rodent CD45 (a pan-leukocyte marker) revealed prominent murine leukocytic infiltration into the xenografts (Supplementary Figure 14), while tumor grafts were negative for human-specific CD45 staining (see below). To discern human-derived versus mouse-derived fibroblast contribution to the tumor grafts, we utilized two available antibodies: human-specific anti-vimentin, and an antibody that recognizes both human and mouse vimentin. We found that the dominant fibroblast population was derived from the mouse (Supplementary Figure 14). The same strategy was used to determine the species origin of endothelial cells that formed the tumor vasculature using anti-CD31 antibodies; mouse-derived endothelium was clearly detected, while human-derived endothelial cells were not detected (see below). Thus, tumor associated stroma from the human samples was largely replaced by mouse-derived stroma in tumor grafts.
The presence of ER in breast tumors is predictive of favorable response to hormone-modulating therapies due to dependence of tumor growth on estrogen. Although the majority (~70%) of newly diagnosed breast cancers are ER+, this tumor type is under-represented in mouse models due to loss of ER expression or lack of estrogen dependence15. We tested whether the ER+ tumor grafts (see Supplementary Figures 6, 8, and 12) retained estrogen dependence by attempting to grow them without estrogen supplementation and/or in mice that had undergone surgical ovariectomy. The ER+ tumor grafts remained dependent on estrogen for tumor growth and/or were stimulated by estrogen, mimicking a key physiological characteristic of ER+ breast tumors in patients (Supplementary Table 2).
Spontaneous metastasis from breast tumor grafts emulates metastatic behavior occurring in patients
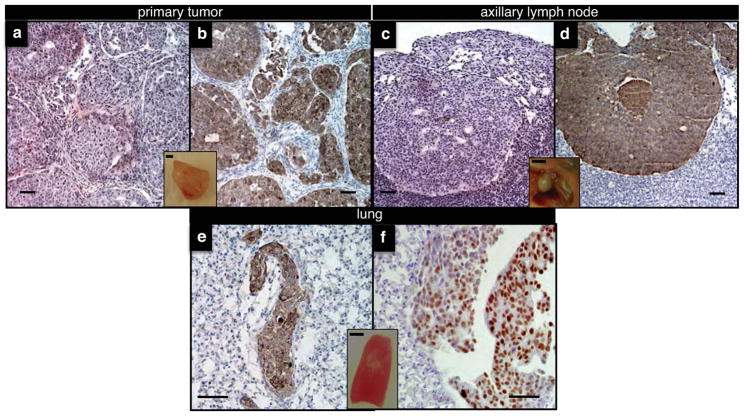
Xenografts derived from human breast cancer cell lines are often poorly metastatic from the orthotopic site; thus, there is heavy reliance on experimental metastasis models (injection of cancer cell lines directly into the circulation of mice). It was striking that the majority of tumor grafts from patient specimens were metastatic, and generated patterns of metastasis similar to that seen in the original patients. The most common site of metastasis in both patients and tumor grafts was lymph nodes. In the mice, the axillary nodes were most commonly involved (inguinal nodes were removed with mammary fat pad clearing upon transplantation of tumor grafts), but metastases were also found in the thoracic and mesenteric lymph nodes. Spontaneous metastasis was also detected in other lymphatic organs such as thymus, as well as in lungs, bone, and peritoneum of mice carrying grafted tumors (Supplementary Table 1). Metastases could be detected grossly or by using either hematoxylin-eosin staining or immunohistochemical staining with antibodies such as those specific for cytokeratin or ER, depending on the characteristics of each primary tumor (Figure 3 and Supplementary Figures 15–16).
Figure 3. Tumor grafts spontaneously metastasized to clinically relevant sites.
Representative examples of a mammary tumor graft (primary tumor) and spontaneous metastases from HCI-011, as detected in sections of axillary lymph nodes and lungs of mice at the time of necropsy. Metastases were easily identifiable by routine histology (H&E; a,c) or by staining with antibodies specific for cytokeratin (b,d,e) or ER (f). Insets are representative pictures of each organ taken prior to fixing/embedding. Scale bars in the main panels correspond to 100 microns, whereas inset scale bars represent 3 millimeters.
In order to approximate the frequency of metastasis as accurately as possible in several lines, we performed hematoxylin-eosin staining and immunohistochemistry on sections from entire organs (lungs and lymph nodes) upon necropsy, systematically staining 3 serial sections every 25 microns. We chose three lines with different clinical marker profiles: HCI-011 (ER+/PR+/HER2−), HCI-005 (ER+/PR+/HER2+), and HCI-009 (ER−/PR−/HER2−), and examined at least 3 tumors from each of 3 different serial passages per line. The percent of mice exhibiting regional and distant metastases varied between the tumor graft lines. A summary of these data as well as representative images for comparative histology between primary tumors and metastases, immunohistochemical stains, and information on the known sites of metastasis in the corresponding patients are included in Table 2 and Figure 3 (HCI-011), Supplementary Figure 15 (HCI-009), and Supplementary Figure 16 (HCI-005). These data reflect frequencies of metastasis at necropsy, which corresponds to an average tumor growth time of 4 months for HCI-009 and HCI-011 (to achieve 2 cm primary tumors), and 5 months for HCI-005 (to achieve 1 cm primary tumors) (Supplemental Table 1). No primary tumor resection was required to achieve these frequencies of metastasis. The minimum latency required for metastases to be detectable has not been determined.
Table 2.
Metastasis frequencies from line HCI-011.
| HCI-011 | # mice from which organs sectioned | Patient information | ||
|---|---|---|---|---|
| Generation | lymph nodes | lung | metastasis | vital status |
| 1° graft | 2/3 | 2/3 | Lymphatics, pleura | Dead |
| 2° graft | 2/3 | 2/3 | ||
| 3° graft | 3/3 | 3/3 | ||
| total | 7/9 | 7/9 | ||
| frequency | 78% | 78% | ||
| frequency of both LN1 and lung involvement | 78% | |||
lymph node
We also examined a small number of mice from these 3 lines (HCI-005, HCI-009, and HCI-011) and one sub-line of HCI-005 (HCI-007) in more detail for evidence of bone metastasis. We chose HCI-005/HCI-007 and HCI-009 because they are the only two tumor grafts in our collection that were derived from patients that were known to have developed bone metastasis. We chose HCI-011 because it was derived from a patient with ER+ metastatic cancer that was refractory to hormone therapy. This type of tumor often metastasizes to bone, although at the time of her death the patient was not known to have developed symptoms of bone metastasis. The hind limbs of tumor-bearing mice were fixed, decalcified, sectioned, and stained to perform histology and immunohistochemistry, which revealed ER+, cytokeratine-positive bone micrometastasis in one of these lines: HCI-007 (Supplementary Figure 17). Thus, at least one tumor graft line is capable of metastasizing to bone; this line is derived from a patient that experienced bone metastasis (Supplementary Table 1). Future studies to determine the frequency of bone metastasis in this line, and whether bone metastasis occurs in other lines with or without resection of the primary tumor and/or with labeled tumor cells for increased sensitivity are underway.
Tumor graft growth and phenotypic stability is improved by co-implantation of primary human mesenchymal stem cells
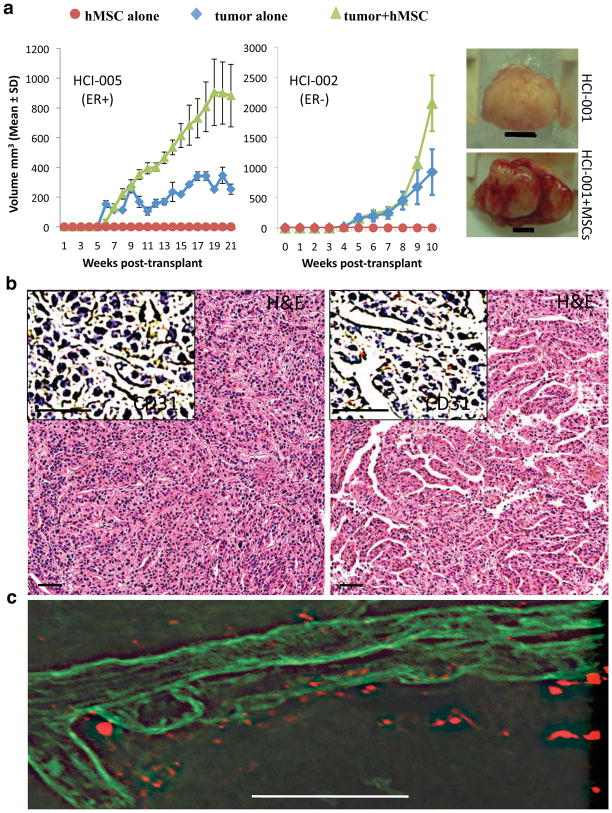
Growth of some tumor graft lines appeared to be limited by severe necrosis resulting in poor growth after initial engraftment, and we wondered whether addition of human-derived stromal cells might overcome this problem. Xenografts derived from human breast cancer cell lines have been reported to recruit bone marrow-derived mesenchymal stem cells (MSCs), which facilitate tumor growth, progression and metastasis16. To determine whether addition of human MSCs might help facilitate tumor propagation in our models, we co-implanted established tumor grafts with primary human MSCs. We found that, in all three of the different tumor graft lines we tested (two ER− and one ER+), addition of MSCs increased tumor growth (Figure 4a). However, no significant differences in tumor cell proliferation or apoptosis were detected by immunostaining for Ki67, phosphorylated histone H3 protein, and cleaved caspase 3, respectively (Supplementary Figure 18). In contrast, examination of microvessels in the tumors showed that the presence of MSCs increased vascularity (Figure 4b) through recruitment of murine endothelial cells (Supplementary Figure 19), which was consistent with the observation that MSC-containing tumors appeared bloodier than control tumors (Figure 4a). We next asked whether the MSCs were directly involved with formation of the blood vessels. Fluorescent labeling of MSCs prior to injection revealed that the mouse-derived microvessels were not actually comprised of human MSCs, but MSCs were located adjacent to vessels (Figure 4c). These data suggest that MSCs enhance tumor growth rates by supporting vascularization of tumors, resulting in less necrosis and increased blood volume, the combination of which would contribute to the observed increase in tumor growth.
Figure 4. Co-engraftment of human mesenchymal stem cells (hMSCs) promotes vascularization and growth of tumor grafts.<.
br>a. Left and middle: Growth rates are shown for cohorts of tumor grafts (derived from either the ER+ tumor HCI-005 or the ER− tumor HCI-002) implanted either alone (blue diamonds), or with MSCs (green triangles). Mice injected with MSCs alone are indicated by red circles. Right: Photograph of representative tumors (derived from the ER− tumor HCI-001) grown with (bottom) or without (top) MSCs, isolated 59 days after transplantation. Tumors grown with MSCs were both bloodier and larger. Scale bar represents 5 millimeters. b. H&E staining, and antibody staining for CD31 (inset), identified elaborate vascular networks in tumor grafts in the presence of hMSCs (right panel) compared to the same tumor graft line growing in the absence of hMSCs (left panel). c. Confocal microscopy on thick frozen tumor graft sections showed that blood vessels (identified by lectin staining; green) are in close proximity to, but not comprised of hMSCs (identified by diI label; red).
To determine whether the initial addition of MSCs provided reversible or irreversible effects on tumor growth rates, we carried out another round of transplantation of tumor grafts that had been grown in the presence or absence of MSCs in the previous generation (MSCs were not added again in next-generation transplants). The next-generation tumors showed no growth advantage due to presence of MSCs in the first transplant (Supplementary Figure 20a). These data indicate that, as previously noted17, MSCs do not simply promote the selection of a pre-existing, aggressive cell population within the tumor. Our data are consistent with the idea that MSCs sustain tumor growth through their effects on the host vasculature.
In the course of our studies, we noted that the presence of MSCs seemed to support the retention of strong ER positivity within ER+ tumors. Although ER protein is clearly retained with serial passage of ER+ tumor grafts without experimental exposure to MSCs, higher levels of ER staining were sustained upon serial transplantation in tumors that had previously been grown with MSCs (Supplementary Figure 20b). This result was validated by quantification of ER protein by semi-quantitative Western analysis, which revealed that the presence of MSCs resulted in maintenance of approximately 5.4-fold higher ER protein levels upon subsequent transplantation (Supplementary Figure 20c). Together, these data suggest that MSCs can have multiple, positive effects on human breast tumor grafts, including enhancement of vascularity and maintenance of ER protein expression. This is particularly noteworthy since loss of ER protein with tumor progression or serial transplantation is a common problem with models of ER+ breast cancer18.
Breast cancer molecular subtypes are preserved in orthotopic tumor grafts
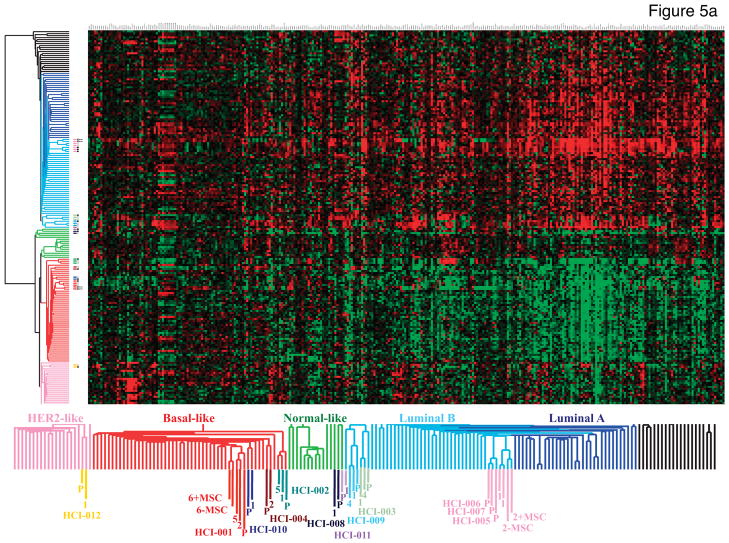
Treatment decisions for breast cancer patients are currently determined by anatomic staging (tumor size, lymph node status, distant metastasis) and the presence or absence of molecular markers (ER, PR, and HER2). However, the clinical behavior of tumors is better predicted by gene expression profiling19–21. A particularly successful strategy has been to risk-stratify patients based on the biologic/intrinsic molecular subtype of their tumor21–25. Using an expanded set of these “intrinsic” genes21, we assessed the molecular similarities between the original human tumors and their tumor grafts. The dendrogram derived from unsupervised hierarchical clustering shows the overall relatedness of the tumors based on their gene expression profiles (Figure 5a). All tumor and tumor graft pairs clustered adjacent to each other, and within a node containing other primary tumors of their subtype.
Figure 5. Gene expression and copy number variations found in the original tumors are well maintained in tumor grafts.
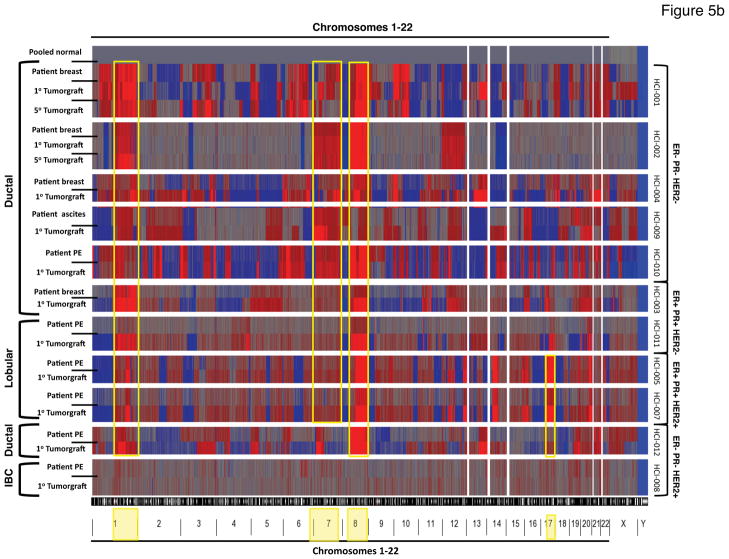
a. Hierarchical unsupervised clustering of microarray data (Whole Human Genome Agilent 44k and 24k) from invasive breast cancers and tumor grafts using a 1291 “intrinsic” gene set. The dendrogram shows “normal-like” breast tissue (light green) and the common cancer subtypes referred to as Luminal A (dark blue), Luminal B (light blue), HER2− enriched (pink), and Basal-like (red). Sample that do not clearly associate with any molecular subtype are shown in black. Each matched tumor/tumor graft case is color-coded (see Figure 1 and Supplementary Table 1) followed by a P or a number in the same color to distinguish the parent tumor from the tumor graft(s), respectively (the numbers correspond to the passage number in mice). The designation of +MSC indicates array data from tumors co-injected with MSCs, or the control tumors from the same experiments (−MSC). The parent tumors and tumor graft(s) (whether or not MSCs were co-injected) clustered next to each other on the terminal ends of the dendrogram, and were thus more closely related in their overall gene expression profiles to each other than to other tumors, even of the same subtype. An enlarged view of the sample clusters is shown on the bottom for clarity. b. Genome-wide single nucleotide polymorphism (SNP) arrays were used to discern DNA copy number changes relative to normal DNA (isolated from blood donated by five individual disease-free females and then pooled; top row). Each tumor and the corresponding tumor graft (and, in some cases tumor grafts that were serially passaged five times; 1° or 5°, respectively) are indicated on the left, along with the clinical type of breast cancer represented. Sample identities are shown on the right, along with the status of ER, PR, and HER2. A copy number of 2 (normal) is indicated by gray; copy number greater than 2 (chromosomal gain or amplification) is shown in red; and copy number less than 2 (chromosomal loss or deletion) is shown in blue. The position of the copy number variants across the 22 autosomal chromosomes and 2 sex chromosomes is depicted at the bottom. Note the common amplicons on chromosomes 1q, 7, 8, and 17q (yellow boxes), and the common low copy number of the Y chromosome (all patients and normal donors were female). PE: pleural effusion; IBC: inflammatory breast cancer.
In addition to the unsupervised analysis, we also classified the primary tumors and tumor grafts using the PAM50 supervised subtype predictor21, with similar results (Supplementary Table 1). We noted that HCI-008 gave discrepant results between two pleural effusions isolated directly from this patient, switching from Basal-like in the first pleural effusion sample to Luminal B in the second sample. According to gene expression microarray data, the two samples from this patient did not represent each other or other subtypes. This unusual scenario might reflect the heterogeneity of the original pleural effusion sample and/or effects of treatment. We favor the latter scenario because, in fact, this sample yielded two different tumor types upon transplantation into multiple mice: breast cancer and CD45+ human lymphoma (Supplementary Figure 21). Serial propagation of the breast cancer resulted in a homogeneous ER−PR−HER2+ breast tumor line (Supplementary Figure 9); the lymphoma line was not further propagated. Unfortunately, this patient died shortly after obtaining the samples that gave rise to the tumor grafts, and lymphoma was not diagnosed by the time of her death. We therefore concluded that the “switch” in molecular subtype classification is most likely a result of heterogeneity in the tumor sample, potentially confounded by a lymphoma.
The only other discrepancy observed between original human tumors and tumor grafts was in case HCI-009: a patient whose ascites fluid contained tumor cells that were initially classified as HER2-enriched, but subsequently classified as Luminal B from the tumor graft. Three serial transplants from this patient all classified as Luminal B, suggesting that the transplants remained stable. All samples from HCI-009 appeared to be Luminal B by unsupervised clustering analysis as well. Conversely, for case HCI-012 the clustering showed a HER2-enriched tumor subtype for both the original human tumor and tumor graft using unsupervised clustering, but both were classified as Luminal B by the supervised PAM50 algorithm. Together, these data indicate that while there were occasional “borderline” cases in which the subtype was difficult to determine even from primary patient specimens, the molecular features of tumor grafts strongly reflected those of the original tumors.
Maintenance of DNA copy number variations between primary samples and tumor grafts
As a second approach to gauge changes that occurred as a result of expanding patient tumors in mice as tumor grafts, we performed genome-wide single nucleotide polymorphism (SNP) microarray analysis on all of the samples and used this information to glean DNA copy number across the entire human genome. As with the gene expression data, the patterns of copy number variations found in the original tumors were typically maintained in tumor grafts (Figure 5b; Supplementary Figures 22–31). The most obvious changes were the enhancement of existing aberrancies in tumor grafts. This is presumably due to the increased contribution of human tumor cells to the sample after grafting, since the human stroma is lost and replaced by mouse stroma (Figure 2 and Supplementary Figures 5–14). The largest changes appeared in the ER+ tumor grafts (e.g. HCI-003 and HCI-011; see Supplementary Figures 24 and 29). However, we did not find changes common to all tumor grafts relative to their parent tumors, suggesting that copy number changes are not solely due to growth in the mouse host.
There were copy number variations in certain regions that were consistent across most tumors and tumor grafts (Figure 5b). Examples include large amplifications on chromosomes 8 and 1q, which were found in all samples except HCI-008 (HER2+ inflammatory breast cancer). Large gains on chromosome 7 were seen in all except the two ER−PR−HER2+ tumors, and the expected gains in chromosome 17q were seen in all of the HER2+ samples except HCI-012, which was from a relapsed tumor refractory to herceptin (Supplementary Table 1). Taken together, the gene expression and DNA copy number data provide evidence that tumor grafts maintain the prominent genomic and gene expression characteristics of the original tumors. Raw data from the gene expression microarray experiments and SNP array experiments are available for download at http://www.ncbi.nlm.nih.gov/geo/ under the accession number
Prognostic value of orthotopic tumor grafts
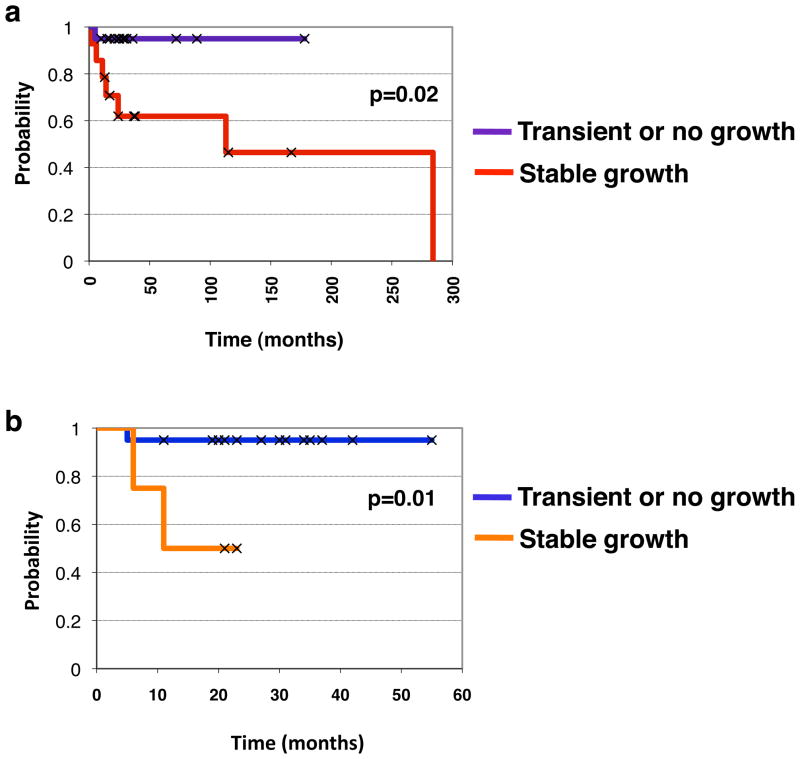
As described above, various breast tumor specimens derived from patients displayed differential ability to grow as tumor grafts in the mammary glands of NOD/SCID mice. Some specimens did not grow at all over an 8–12 month observational period; some specimens grew well and were maintained through serial transplantation; and some specimens grew large enough to be palpable but then spontaneously receded, and were therefore unable to be maintained. Since successful engraftment of the tumors did not correlate with the status of clinical markers (ER, PR, or HER2) or with the tissue source (breast versus a metastatic site), we postulated that the ability of a tumor to survive and grow in a foreign host might reflect a more aggressive phenotype that is independent of known clinical variables. Positive engraftment (vs. negative or transient engraftment) of tumor samples correlated with shorter survival across all patients studied (p=0.02, log-rank statistics; Figure 6a), indicating that the tumor grafts represent the most aggressive disease. To determine whether the ability of a tumor to generate a xenograft might serve as a potential indicator of patient prognosis, we examined graft data and clinical outcome information from all new breast cancer patients in our study who did not have prior cancer treatment or detectable metastasis at the time of surgery. This comprised 24 patients, whose median follow-up time to date is/was 28 months. Tumor grafts were successfully maintained from 4 of those patients (HCI-001, HCI-002, HCI-003, and HCI-004). Transient growth that subsequently receded was obtained from an additional 4 patients. No growth was achieved from tumor tissue derived from the remaining 16 patients. Kaplan-Meier analysis and log-rank statistics showed that the ability of a primary breast tumor to successfully graft into the mouse mammary fat pad significantly correlated with reduced overall survival (p=0.01; Figure 6b). It was notable that tumors that initially grew but were not able to be maintained with serial passage were not significantly associated with poor outcome (data not shown). Thus, the ability to generate stable orthotopic tumor grafts provided retrospective prognostic information about the course of the disease in patients, and therefore has potential to be used as a surrogate indicator of risk for disease progression. These data also serve as additional evidence to bolster our findings that tumor grafts represent authentic models for the most aggressive tumor types, along with the fact that tumor grafts exhibited prominent metastasis in mice.
Figure 6. Successful growth of clinical breast cancer primary tumor specimens as tumor grafts significantly predicts shorter survival times.
a. Kaplan-Meier survival analysis showing probability of survival for all breast cancer patients examined. The patients were stratified by whether their tumors did not grow or were not able to be maintained in mice (dark blue line) versus those that did grow in mice (red line); p=0.02 by log-rank statistics. b. Kaplan-Meier survival analysis showing probability of survival for new breast cancer patients whose primary tumors either did not grow or were not able to be maintained in mice (dark blue line) versus those that did grow in mice (orange line); p=0.01 by log-rank statistics.
DISCUSSION
We have established a unique bank of serially transplantable, orthotopic breast tumor grafts that retain critical characteristics of the original tumor specimens from living breast cancer patients. This work revealed that (1) our current bank of tumor grafts, which is still growing, already comprises all major clinical types of breast cancer, and multiple molecular subtypes; (2) tumor grafts maintain critical features of the patient tumors including histology and pathology, clinical markers, gene expression profiles, copy number variants and, for estrogen receptor positive tumors, estrogen dependence and/or responsiveness; (3) addition of MSCs stimulates tumor graft growth and reduces necrosis, presumably by increasing vascular density within tumors; (4) tumor grafts spontaneously metastasize in mice to many of the same organs that were affected in the original patient; and (5) tumor engraftment is a prognostic factor for survival time even in new breast cancer patients without known metastatic disease. Together, these findings indicate that tumor grafts are excellent models for human breast cancer. Because of their high potential for clinical relevance, and their ease of use once established, tumor grafts should provide ample opportunities to significantly impact breast cancer research and therapy.
Breast cancer is a heterogenous disease with respect to pathology, histology, mutations, gene expression, metastasis profiles, and response to therapy. As a result, breast cancer may be more accurately classified as a collection of related diseases, rather than as one disease. High-throughput methods, such as global gene expression analysis, have succeeded in classifying breast cancers into several intrinsic subtypes with clinical utility, based on the molecular features of tumors21. Thus, an important feature of the tumor grafts described herein is the retention of gene expression profiles and DNA copy number variants similar to those of the original tumor samples. This manifests in maintenance of the molecular subtypes of the breast cancers, which will be important for modeling the biology of various types of breast cancer or for drug development toward specific breast tumor subtypes.
Our approach was to use genomic information, in the form of gene expression profiles and DNA copy number variants, to assess the relative similarities and differences between the original tumor specimens and tumor grafts. In several cases, we were also able to glean information about how stable the tumor grafts remained with serial passage in mice, or after a relapse of disease (Supplementary Figure 31). Although our approach was not an attempt to thoroughly characterize the tumor graft bank, which is beyond the scope of this report, these data will contribute to the utility of these tumor grafts as a technical resource for future research.
We found that co-engraftment of MSCs with human breast cancers can facilitate blood vessel development, reduce necrosis, and increase tumor growth. We also found that MSCs promote maintenance of ER expression upon serial propagation of ER+ tumors. While the mechanisms by which these cells support tumor progression and phenotypic stability are beyond the scope of this technical report, it is plausible that they do so by enriching the microenvironment of the mouse mammary gland with human growth factors, pro-angiogenic factors, and/or chemokines that favor tumor growth26. This effect may be akin to that previously described when mouse mammary fat pads were “humanized” with irradiated human fibroblasts27. Another possibility is that the stem cell properties of MSCs allow them to aid tumor growth, potentially by differentiating into specialized supporting cells within the tumor. MSCs do not appear to directly form blood vessels within the tumor however, despite the increased vascular density observed in their presence.
Co-injection of hMSCs did not significantly affect the number or size of metastases in any of the multiple lines we studied, possibly due to the already high frequency of metastasis observed in the parent tumor grafts. It is also noteworthy that although we consistently saw that MSCs improved the growth of existing grafts, the addition of hMSCs did not improve the “take rate” of 3 different tumors that failed to engraft in NOD/SCID mice without the addition of hMSCs. The fact that MSCs only promoted growth of existing tumor grafts, and only transiently, is consistent with their effects on the host vasculature, and suggests that there is cross-talk between tumor cells, endothelial cells, and MSCs. Such cross-talk between tumor cells and MSCs has previously been reported to support metastasis of breast cancer cell lines17. Gene expression profiling revealed that tumors co-injected with MSCs still cluster with their parent tumor and their related grafts grown in the absence of MSCs, indicating that the presence of MSCs does not drastically change the tumor characteristics.
Together, these data suggest that the main effect of hMSCs in tumor grafts is in supporting tumor angiogenesis once engraftment occurs and growth ensues. In the future, it will be interesting to determine whether hMSCs uniquely provide this function in patient tumor grafts, or whether other stromal cells such as fibroblasts, macrophages, or other bone-marrow derived cells may serve redundant functions.
One of the most promising parallels between the human breast cancers and their corresponding tumor grafts is their ability to spontaneously metastasize with high frequency. A previous report detailing transplantation of human breast tumors used a subcutaneous, rather than orthotopic, approach and reported “frequent” metastasis to lungs in only 3/17 lines. In addition, the subcutaneous approach resulted in a lower take rate of primary breast tumors compared with metastastic breast tumors18. In contrast, our take rate was not significantly different between primary and metastatic specimens, and all but one orthotopic tumor graft line spontaneously metastasized. Metastasis to lymphatics, pleura, peritoneum, and lungs were commonly seen in both patients and mice, to varying degrees. Evidence for bone metastasis was also noted from 1 tumor graft line. In the mice, the development of metastases from tumor grafts occurred spontaneously within a very feasible time frame for study: it did not require resection of the primary tumor or any other manipulation, and metastases were often large enough to see upon gross examination.
Our data indicate that implantation of freshly isolated human breast tumor specimens into the orthotopic site of mice facilitates development of models that closely resemble progression of the human disease. Although metastasis commonly occurred to the lymphatics and lungs, we did not note overt signs of metastasis to either liver or brain, which are other common sites of metastasis in breast cancer patients. Several possibilities may explain this conundrum. First, it is possible that development of liver and brain metastasis is a slower process, yielding only micro-metastases at the time of necropsy for these studies. In this case, primary tumor resection with longer mouse follow-up time, or specific labeling of tumor cells may be required to detect lesions in liver or brain. Second, it is possible that the tumor grafts we derived from our patients do not have the capacity to metastasize to liver or brain; none of the patients in our study developed clinical metastasis to these organs. This possibility can be addressed in the future with implantation of tumors from patients that developed more widespread metastatic disease. Although we have not yet performed a thorough characterization of frequencies and sites of metastasis for all of the lines, we have been able to detect some evidence of bone metastasis in at least one line from a patient known to have bone metastasis. Future work will be geared toward examining more mice from each line for site-specific metastasis.
Our understanding of the mechanisms of breast cancer metastasis, and development of strategies that stem from blocking those mechanisms, has been stymied by lack of models in which metastasis occurs and mirrors that seen in the clinic. We surmise that the increased metastatic potential of our tumor grafts is directly related to lack of in vitro manipulation, although this remains to be tested. Direct implantation of tumors into mice may preserve the ability of the cells to interact with supporting cells within the tumor microenvironment; such features may be lost in the in vitro setting. Another possibility is that tumor cells with self-renewal properties, known as tumor-initiating cells28, may be better preserved by direct implantation rather than culturing. Since these cells are also thought to be important for initiation of metastases at distant sites29, retention of tumor-initiating cells could also contribute to the increased metastatic potential of the tumor grafts. In fact, our data suggest that the ability of cancer cells to grow as tumors that mimic human breast cancer in vivo and the ability to grow in tissue culture in vitro are not always compatible: several of the tumor graft lines do not grow, or even die, under standard culture conditions (YSD and YCL, unpublished observations). Together, our data give weight to the notion that, while certainly useful, cell lines are not always ideal for modeling breast cancer in vivo.
Multiple groups30,31 have noted the importance of using mice that are more severely immuno-compromised than NOD/SCID mice for engraftment of primary human tumors (i.e. the double mutant NOD/SCID;IL2Rγ−/− (NSG) mice, which lack NK cells as well as mature lymphocytes32). Although we began our study when NSG mice were not as readily available, and have continued using NOD/SCID mice for the sake of consistency, we have tested the growth of several lines in NSG mice. We have found that certain tumors (i.e. HCI-004 and HCI-008) grow faster in NSG mice than in NOD/SCID, and others grow equally well in both (i.e. HCI-012) (Supplementary Table 1); no significant differences in metastasis have been noted thus far. It is important to note, however, that our studies were not carried out at limiting dilution, and that larger differences in tumor behavior in different mouse strains might be apparent under more stringent conditions. These data do suggest that, as previously reported33, tumor progression depends on interactions between the immune system and tumor, which can significantly affect tumor behavior.
Our data also showed that the ability of a patient’s tumor to grow as a tumor graft was a significant indicator of shorter survival time and therefore a poor prognosis. Not only does this suggest a potential functional assay for assessing outcomes (albeit not yet practical for clinical use), but also further reinforces the notion that the tumor grafts accurately model the cancers from which they are derived. Thus, tumor grafts provide an increasingly valid context in which to significantly advance research in the fields of tumor biology: tumor-host interactions, angiogenesis, tumor progression and metastasis, and pre-clinical testing of promising new drugs. Expansion of these and upcoming tumor grafts is underway, as is the generation of sub-lines containing molecular markers with which to more easily follow intravital tumor growth and metastasis. These models will be made publicly available along with the corresponding clinical, gene expression, and SNP array data for use in the research community.
METHODS
Methods and their associated references can be found in the Supplementary Information with the online version of the paper.
Supplementary Material
Footnotes
PUBLIC DATA ACCESS
Raw data from the gene expression microarray experiments and SNP array experiments are available for download at http://www.ncbi.nlm.nih.gov/geo/ under the accession number Gene expression data for the pre-clustered “intrinsic” gene set, after merging with data from Parker et al. can be downloaded as Supplementary Table 3 with the online version of the paper.
References
- 1.Carey LA. Through a glass darkly: advances in understanding breast cancer biology, 2000–2010. Clin Breast Cancer. 2010;10:188–195. doi: 10.3816/CBC.2010.n.026. [DOI] [PubMed] [Google Scholar]
- 2.Hait WN. Anticancer drug development: the grand challenges. Nat Rev Drug Discov. 2010;9:253–254. doi: 10.1038/nrd3144. [DOI] [PubMed] [Google Scholar]
- 3.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R. The role of preclinical animal models in breast cancer drug development. Breast Cancer Res. 2009;11 (Suppl 3):S22. doi: 10.1186/bcr2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 7.Ethier SP. Human breast cancer cell lines as models of growth regulation and disease progression. J Mammary Gland Biol Neoplasia. 1996;1:111–121. doi: 10.1007/BF02096306. [DOI] [PubMed] [Google Scholar]
- 8.Bos PD, Nguyen DX, Massague J. Modeling metastasis in the mouse. Curr Opin Pharmacol. 2010;10:571–577. doi: 10.1016/j.coph.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Press JZ, et al. Xenografts of primary human gynecological tumors grown under the renal capsule of NOD/SCID mice show genetic stability during serial transplantation and respond to cytotoxic chemotherapy. Gynecol Oncol. 2008;110:256–264. doi: 10.1016/j.ygyno.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim MP, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel VC, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 15.Wagner KU. Models of breast cancer: quo vadis, animal modeling? Breast Cancer Res. 2004;6:31–38. doi: 10.1186/bcr723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Haibi CP, Karnoub AE. Mesenchymal stem cells in the pathogenesis and therapy of breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:399–409. doi: 10.1007/s10911-010-9196-7. [DOI] [PubMed] [Google Scholar]
- 17.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 18.Marangoni E, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13:3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 19.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 20.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 21.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 23.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Dissecting a Discrepancy in the Literature: Do Mesenchymal Stem Cells Support or Suppress Tumor Growth? Stem Cells. 2010 doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 30.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agliano A, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 33.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.