Abstract
Background
Urinary tract infection (UTI) in children may be associated with long-term complications that could be prevented by prompt treatment.
Aim
To determine the prevalence of UTI in acutely ill children ≤ 5 years presenting in general practice and to explore patterns of presenting symptoms and urine sampling strategies.
Design and setting
Prospective observational study with systematic urine sampling, in general practices in Wales, UK.
Method
In total, 1003 children were recruited from 13 general practices between March 2008 and July 2010. The prevalence of UTI was determined and multivariable analysis performed to determine the probability of UTI.
Result
Out of 597 (60.0%) children who provided urine samples within 2 days, the prevalence of UTI was 5.9% (95% confidence interval [CI] = 4.3% to 8.0%) overall, 7.3% in those < 3 years and 3.2% in 3–5 year olds. Neither a history of fever nor the absence of an alternative source of infection was associated with UTI (P = 0.64; P = 0.69, respectively). The probability of UTI in children aged ≥3 years without increased urinary frequency or dysuria was 2%. The probability of UTI was ≥5% in all other groups. Urine sampling based purely on GP suspicion would have missed 80% of UTIs, while a sampling strategy based on current guidelines would have missed 50%.
Conclusion
Approximately 6% of acutely unwell children presenting to UK general practice met the criteria for a laboratory diagnosis of UTI. This higher than previously recognised prior probability of UTI warrants raised awareness of the condition and suggests clinicians should lower their threshold for urine sampling in young children. The absence of fever or presence of an alternative source of infection, as emphasised in current guidelines, may not rule out UTI in young children with adequate certainty.
Keywords: children, general practice, prevalence, symptoms, urinary tract infections
INTRODUCTION
The prevalence of urinary tract infection (UTI) in acutely ill children in general practice is unknown. UTI in young children is difficult to diagnose and many cases are probably missed.1–3 The challenge is that young children with UTI often present with non-specific symptoms that are also present in non-specific illness and in many other common conditions. Clinicians may therefore not consider the diagnosis or obtain a urine sample.
Childhood UTI has been associated with renal scarring and serious long-term complications, including hypertension, pre-eclampsia, and renal failure.4–7 A systematic review found renal scarring was present in approximately 15% of children following a first UTI.8 It remains unclear exactly what causes renal scarring to develop in some children, or which groups of children are most at risk.8 There is some evidence that even children without fever or those with a self-limiting UTI may nevertheless be at risk of renal scarring.9
Guidelines highlight the importance of prompt diagnosis and early treatment of UTI in children, to prevent renal scarring.2 However, urine is infrequently sampled from young children in primary care.10 GPs indicated that they would normally sample urine from only a small proportion of children presenting with acute illness, even when awareness of UTI and the non-specific presenting symptoms had been raised.11 The UK National Institute for Health and Clinical Excellence (NICE) guideline promotes increased urine sampling.2 However, clinicians may not respond to this recommendation unless there is evidence that the prevalence of UTI in children warrants increased testing.
Studies reporting the incidence and prevalence of UTI in children have varied by population, sampling method, and diagnostic criteria. Rates therefore vary widely, from 0.25% in a small UK GP study12 to 13.5% in a hospital-based study of febrile infants.13 A meta-analysis of the prevalence of UTI included 18 studies and a total of 22 919 children.14 It found a pooled prevalence of 7% in febrile children < 24 months old, and 7.8% in children < 19 years old with urinary symptoms.14 The applicability of these findings to general practice is limited because most studies were based in emergency departments with narrow inclusion criteria and age range, many excluded children without fever, and many sampled urine using invasive methods unsuitable for general practice. For children over 12 months old, all but one of the studies relied on urine samples obtained only if the treating clinician suspected a UTI. One study systematically obtained urine samples, but excluded boys older than 12 months and girls without a fever of ≥38.5°C.15
Recent UK guidelines state that UTI is unlikely if there are symptoms or signs suggestive of an alternative diagnosis, but there is evidence that UTI cannot be excluded on this basis.11,15,16 It is also unclear how important fever is as a diagnostic sign, as most prevalence studies exclude afebrile children.14 Data on the pretest probability of UTI in an acutely unwell child presenting in primary care would help clinicians manage these children. Systematically sampling urine from sequential, unselected children presenting in primary care with a wide range of non-specific symptoms is required to determine the prevalence of UTI. Potential risk factors and subsets of children can then be analysed to determine whether urine sampling can be more effectively targeted to those children most likely to have UTI.
How this fits in
Urinary tract infection (UTI) in children may be associated with long-term complications that could be prevented by prompt treatment. The current strategy of suspicion-led urine sampling is likely to miss the majority of cases of UTI in young children. The absence of fever or presence of an alternative source of infection does not necessarily rule out the possibility of UTI. The probability of UTI in children under the age of 3 years is reasonably high, irrespective of the presenting symptoms and signs. Larger studies are needed but a lower threshold for urine sampling in young children appears to be indicated.
The aims of this study were to identify the prevalence of UTI in acutely ill children under the age of 5 years presenting in UK primary care, and to explore patterns of presentations in terms of symptoms, signs, and previous history, in relation to clinical suspicion and clinical guideline-based sampling practice.
METHOD
General practices and patients
Following a pilot study of feasibility, general practices in South Wales were invited to participate in this study.11 Thirteen practices and five NHS microbiology laboratories across Wales participated between March 2008 and July 2010. Not all practices joined the study at the same time, so the duration of patient recruitment varied by practice, and not all clinicians in participating practices recruited. Children were eligible if they were aged <5 years and had an acute illness of <28 days’ duration. Children were excluded if they were on immunosuppressant treatment (chemotherapy or oral/intramuscular steroids for ≥2 weeks) or long-term antibiotic treatment (>28 days), or had previously taken part in the study.
Data collection
Carers of acutely unwell children presenting at participating general practices were provided with study information, and given an opportunity to discuss the study. Nurses or clinical studies officers recorded the details of presenting symptoms, medical and family history, temperature, pulse, and respiratory rate, and these were recorded on a case report form. Examination findings and a working diagnosis were recorded on the case report form by the responsible clinician. A urine sample was sought from all children. Clean catch was the preferred method but if this was not feasible, a nappy pad was used as recommended by current guidelines.2 If a urine sample was not obtained at the general practice, carers were given urine-sampling equipment to take home and asked to return the sample as soon as possible. The urine was sent to the routine NHS receiving laboratory and results sent to the responsible GP practice, following routine processes. Clinicians managed the child’s illness according to their standards of usual care. Laboratories also sent a copy of the urine result to the research team.
All urine samples were sent to NHS laboratories using the standard sample containers recommended by that laboratory. Three of the five laboratories required urine samples to be collected in containers containing boric acid (one of these laboratories provided special paediatric bottles containing boric acid). Practices stored urine samples in their fridges overnight if they received them after the daily specimen collection from the practice (usually midday). Laboratories processed and cultured the samples according to their standard operating procedures. There were some differences in standard operating procedures between laboratories. For example, one laboratory did not routinely culture urine that was negative on microscopy.
Definition of urinary tract infection
A positive culture was defined as pure or predominant bacterial growth of >105 colony-forming units (cfu)/ml on culture.2 All other results were considered negative for the main analyses. Additional sensitivity analyses defined a borderline culture as 104–105 cfu/ml of a single organism (in laboratories that recorded growth at this level), or >105 cfu/ml of two organisms. Urines with heavy mixed growths (>105 cfu/ml of >2 organisms) were considered contaminated. Only those urine samples received within 2 days of the initial consultation were included in the analysis.
Data entry
Data entry was double checked for 10% of all urine results and for 100% of those with positive and borderline cultures. Case report forms were collected from practices by research officers, and scanned using Cardiff Teleform (version 10.4.1). Data were combined, checked, anonymised, and analysed using SPSS (version 16).
Sample size requirement
To estimate the point prevalence of UTI in children under the age of 5 years presenting in general practice with an acute illness, with a 95% confidence interval (CI) of ±1% around a predicted prevalence of 3%, a sample size of 1100 was needed.17
Analysis
The prevalence and 95% CIs were calculated using methods appropriate for proportions close to zero.17
χ2 and Fisher’s exact tests were used to screen associations of symptoms, signs, and potential risk factors (for example, family history, previous medical history, age (categorised according to NICE < 3 months, ≥3 months to < 3 years, ≥3 years), sex, month of presentation, with the presence of UTI (Appendix 1).2
All symptoms and signs with a P-value of < 0.1 on univariate analysis were entered into a multivariable analysis, using forward stepwise logistic regression. All figures are presented to one decimal place except for P-values, which are presented to two decimal places.
Recruitment bias assessment
Clinicians were asked to keep ‘recruitment logs’ for eligible children who consulted, and to record the numbers of children invited and those consenting to participate. A check was made as to whether age and sex profiles were consistent with the practice population.
RESULTS
The recruitment rate varied between practices, from 1.5 per month to 11.7 per month. Four practices were not able to complete recruitment logs. The nine practices that did complete recruitment logs listed 63 eligible children who were approached but not recruited. The median age of non-recruited children was 1.6 years (interquartile range [IQR] = 1.0 to 3.1), and 34 (53.9%) were male.
Out of 1003 eligible children recruited (Figure 1), urine samples were obtained in 709 (70.7%). However, samples leaked in 23 (3.2%) cases and these were not analysed. Two hundred and ninety-four (41.5%) urine samples were received by laboratories on the day of recruitment. Only those urine samples received within 2 days of recruitment were used in this analysis (n = 597).
Figure 1.
Numbers of participants recruited and urine samples collected.
Five hundred and four 504 (50.2%) of the recruited children were male (Table 1) and their median age was 2.3 years (IQR = 1.00 to 3.49 years). Table 1 shows the characteristics of 597 children included in the analysis.
Table 1.
Participant characteristics for the 597 children included in the analysis and the 406 not included
| Characteristic | Those included in full analysis (n = 597) | Those not included in full analysis (n = 406) | P-value |
|---|---|---|---|
| Age | |||
| Median age, years (IQR) | 2.3 (1.0–3.5) | 1.6 (0.8–3.3) | < 0.01 |
| < 3 months, n (%) | 32 (5.4) | 28 (6.9) | |
| ≥3 months and < 3years, n (%) | 349 (58.5) | 289 (71.2) | |
| ≥3 years, n (%) | 216 (36.2) | 89 (21.9) | |
| Sex, n (%) | |||
| Male | 313 (52.4) | 191 (47.0) | 0.94 |
| Female | 284 (47.6) | 215 (53.0) | |
| GP working diagnosis, n (%) | |||
| Upper respiratory tract infection | 177 (29.6) | 121 (29.8) | 0.35 |
| Viral illness | 90 (15.1) | 55 (13.5) | |
| Lower respiratory tract infection | 48 (8.0) | 43 (10.6) | |
| UTI | 41 (6.9) | 13 (3.2) | |
| Tonsillitis | 32 (5.4) | 26 (6.4) | |
| Otitis | 32 (5.4) | 19 (4.7) | |
| Gastroenteritis | 26 (4.4) | 18 (4.4) | |
| Conjuctivitis | 15 (2.5) | 12 (3.0) | |
| Other | 100 (16.8) | 67 (16.5) | |
| No diagnosis | 36 (6.0) | 32 (7.9) | |
| Common presenting symptoms, n (%) | |||
| Runny nose | 423 (70.9) | 301 (74.1) | 0.20 |
| Cough | 413 (69.2) | 280 (69.0) | 0.94 |
| Clingy | 401 (67.2) | 272 (67.0) | 0.95 |
| Irritable | 383 (64.2) | 275 (67.7) | 0.24 |
| Hot/feverish | 355 (59.5) | 232 (57.1) | 0.46 |
| Poor feeding | 329 (55.1) | 223 (54.9) | 0.95 |
IQR = interquartile range.
There was no significant difference in sex (P = 0.94), but there was a difference in age between children included in the main analysis (those providing a urine sample within 2 days; n = 597) and those who were not included. Children who did not provide a urine sample within 2 days were approximately 8 months younger (median age 1.6 years) than those who did (median age 2.3 years). There was no statistical difference in GP diagnosis between those included and those not included in the main analysis. However, there was a greater proportion of those suspected of having a UTI among those included in the analysis, compared to those who were not.
In 431 (72.2%) cases the method of urine sampling used was indicated. Nappy pads were used in the majority of children less than 3 years old (100% aged < 3 months; 74.3% aged ≥3 months to < 3 years). Clean-catch collection was used in all children who were ≥3 years old. No difference was found in the prevalence of UTI according to urine-collection method when considering all children (P = 0.15), and there was no difference when considering the age range ≥3 months to < 3 years alone (UTI prevalence 6.6% in clean catch and 5.1% in nappy pad; P = 0.44).
Urine samples were obtained before the child left the surgery for 318 (53.2%) children included in the analysis. It was much less likely that the urine sample was received within 2 days of the consultation if the sample was not obtained before leaving the surgery (P < 0.01). Antibiotics were prescribed in 31.1% (99/318) of children who provided urine samples prior to leaving the surgery and in 25.1% (70/279) of those who did not.
Prevalence of urinary tract infection
The prevalence of UTI, defined as the growth of one organism >105 cfu/ml was 5.9% overall (Table 2). A further 2.9% had a borderline culture result. Almost half of the samples (48.4%) had mixed growths, presumed to be contaminants, and regarded as negative. Heavy mixed growths were more common in nappy-pad samples (61.7%) compared with clean-catch samples (13.2%; P > 0.01). Forty (6.7%) were not cultured, owing to negative microscopy results, and so were classified as negative.
Table 2.
Culture results for 597 children aged < 5 years presenting to primary care with an acute illness
| Culture result | n (%) | % (95% CI) |
|---|---|---|
| Positive | ||
| >105 cfu/ml single organism | 35 (5.9) | 5.9 (4.3 to 8.0) |
| Borderline | ||
| 105–105 cfu/ml single organism | 11 (1.8) | 2.8 (1.8 to 4.5) |
| >105 cfu/ml two organisms | 6 (1.0) | |
| Negative | ||
| Heavy mixed growth >105 cfu/ml | 208 (34.8) | 91.3 (88.8 to 93.3) |
| Mixed growth 105–105 cfu/ml | 81 (13.6) | |
| No growth or growth < 105 cfu/ml | 216 (36.2) | |
| Not cultured as microscopy negative | 40 (6.7) |
Risk factors and presenting symptoms and signs
Nineteen (6.7%) females and 16 (5.1%) males had UTI; this was not significantly different (P = 0.41). There was a trend towards a higher prevalence of UTI in the younger children (P = 0.05; Table 3).
Table 3.
Prevalence of urinary tract infection by age
| Age range (NICE) | Proportion with UTI | % with UTI | 95% CI |
|---|---|---|---|
| < 3 months | 4/32 | 12.5 | 5.0 to 28.1 |
| ≥3 months to < 3 years | 24/349 | 6.9 | 4.7 to 10.0 |
| ≥3 years | 7/216 | 3.2 | 1.6 to 6.5 |
NICE = National Institute of Health and Clinical Excellence.
Age category, symptoms, or signs that were associated with UTI with a P-value of < 0.1 on univariate analysis were entered into a logistic regression model (Table 4). Features not significantly associated with UTI included: history of being hot or feverish (P = 0.69), abdominal pain or vomiting, or a family history of UTI or kidney problems. An alternative site of infection (upper respiratory tract infection [URTI], lower respiratory tract infection [LRTI], tonsillitis, gastroenteritis, conjunctivitis, otitis) diagnosed by clinicians was not significantly associated with ruling out UTI (P = 0.64). Those with a UTI were less likely to have a history of asthma (P = 0.06), and more likely to have a history of kidney or bladder disease (P = 0.06) than those without.
Table 4.
Presenting symptoms and signs in children entered into the logistic regression model
| Symptom | Proportion (%) of those with UTI with symptom | Proportion (%) of those without UTI with symptom | Odds ratio | P-value univariate analysis |
|---|---|---|---|---|
| Increased urinary frequency | 11/35 (31.4) | 75/562 (13.3) | 3.0 | < 0.01 |
| Wetting when previously dry | 5/35 (14.3) | 32/562 (5.7) | 2.8 | 0.06 |
| Pain/crying when passing urine | 5/35 (14.3) | 26/562 (4.6) | 3.4 | 0.03 |
| Irritable/grouchy | 28/35 (80) | 355/562 (63.2) | 2.3 | 0.04 |
| Temperature measured in surgery ≥38°C | 15/35 (42.9) | 163/562 (29.0) | 1.8 | 0.08 |
| Muscle aches or pains | 0/35 (0) | 55/562(9.8) | 0.1 | 0.03 |
| Poor feeding/off food | 24/35 (68.6) | 305/562 (54.3) | 1.8 | 0.10 |
Multivariable analysis identified age range, pain or crying on passing urine, and increased urinary frequency or frequency of wet nappies as being associated with UTI (Table 5).
Table 5.
Multivariable analysis: variables included in the model
| Symptom/characteristic | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Urinary frequency | 2.6 | 1.2 to 5.7 | 0.02 |
| Pain on passing urine | 3.3 | 1.1 to 9.8 | 0.03 |
| NICE age range ≥3 years | 1.0 | Reference range | |
| NICE age range ≥3 months to < 3 years | 2.4 | 1.0 to 5.8 | 0.06 |
| NICE age range < 3 months | 5.5 | 1.5 to 21.0 | 0.01 |
P-value for model over the null model < 0.01. NICE = National Institute of Health and Clinical Excellence.
Based on the probability of UTI using the study model (Figure 2), an effective sampling strategy may therefore be to sample urine on all acutely ill children under the age of 5 years, except for those who are 3 years or older and have neither increased urinary frequency nor pain/crying on passing urine (Figure 2). Table 6 compares outcomes using the proposed sampling strategy (model) with either sampling based on GP suspicion of UTI or if current NICE guidelines had been followed, for the sample population. GP suspicion of UTI was based on the ‘working diagnosis’ question on the case report form, which was completed by GPs at the initial consultation and before urine culture results were available.
Figure 2.
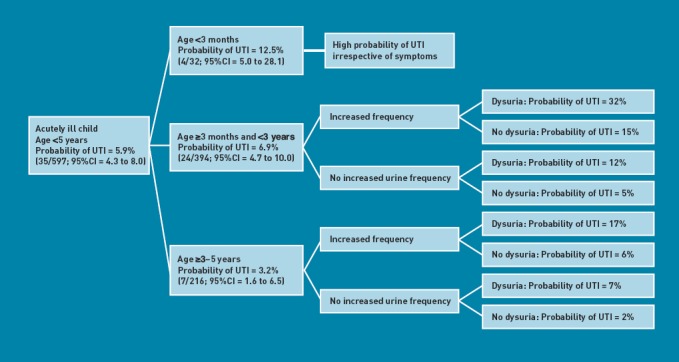
Probabilities of urinary tract infection based on the study model
Table 6.
Predicted outcomes of sampling based on GP suspicion, NICE guidance, and the proposed sampling strategy
| Age group | Urine sample | GP suspicion, n | If NICE guidelines had been applied, n | Proposed sampling strategy, n |
|---|---|---|---|---|
| < 3 months (n = 32) | Urine samples UTIs diagnosed UTIs missed |
0 0 4 |
9 1 3 |
32 4 0 |
| ≥3 months to < 3 years (n = 349) | Urine samples UTIs diagnosed UTIs missed |
19 3 21 |
150 10 4 |
349 24 0 |
| ≥3 years (n = 216) | Urine samples UTIs diagnosed UTIs missed |
22 4 3 |
77 6 1 |
33 6 1 |
| Total | Urine samples UTIs diagnosed UTIs missed |
41 (7%) 7 (20%) 28 (80%) |
236 (40%) 17 (49%) 18 (51%) |
414 (69%) 34 (97%) 1 (3%) |
NICE = National Institute of Health and Clinical Excellence.
DISCUSSION
Summary
A 5.9% (95% CI = 4.3% to 8.0%) prevalence of UTI was found among systematically sampled acutely ill children under the age of 5 years presenting in primary care. This was higher in those under the age of 3 years (7.3%). A multivariable logistic regression model that included age band, increased urinary frequency, and pain or crying when passing urine found that the probability of UTI in children aged 3 years or older without increased urinary frequency or pain when passing urine was 2%. The probability of UTI was higher in all other groups of children (≥5%).
Strengths and limitations
This was a prospective primary care-based prevalence study of UTI, in which acutely unwell children were well described clinically and had their urine systematically sampled. Urine samples were all analysed by the NHS laboratory local to the participating GP practice, keeping transport of samples and reporting of results consistent with normal practice.
The study did not recruit the target sample of 1100. Confidence intervals for the main estimate were wider (±2%) than had been hoped (1% around a prevalence of 3%). However, the prevalence of UTI was higher in the study sample than the 3% used for the sample size calculation, which mitigated loss of precision. The study was not powered to accurately determine the predictive value of symptoms and signs, and this resulted in large confidence intervals for the odds ratios and probabilities in the multivariable model. Many potentially predictive variables were tested, which increased the likelihood that some would be statistically significant purely by chance.
Not all eligible children presenting in practices were recruited, and not all recruited children provided a urine sample. There was a difference in age between those who were included in the main analysis and those who were not, with younger children less likely to provide a urine sample within 2 days (P < 0.01).
Nappy pads were commonly used for urine sampling. These were associated with an increased frequency of heavy mixed growth, implying contamination, compared to clean-catch specimens (P < 0.01). Some urine results categorised as ‘positive’ may have been caused by contaminating bacteria (false positives) and some of those with heavy mixed growth and categorised as ‘negative’ may have been a true UTI.18 It was found that UTI prevalence was higher in the clean-catch specimens, suggesting that false negatives may be more of a problem among nappy-pad specimens than false positives, but this difference was not statistically significant.
Antibiotic prescription in those children who did not provide a urine sample prior to leaving the surgery could potentially have interfered with subsequent urine results. This most likely would have resulted in additional false negatives and an underestimation of UTI prevalence.
The screening logs showed that recruited children were older than non-recruited children. This probably reflects the increased difficulties of obtaining urine samples in younger children.19 As there is a higher prevalence of UTI in younger children (in both this study and others), this may also indicate that the prevalence found in this study is an underestimation for the group overall.10 Clinicians suspected UTI more often in children included in the main analysis compared with those who were not. This may also be due to the non-specific nature of symptoms in younger children, or it may reflect greater encouragement given to obtain urine samples if the clinician suspected a UTI.
Comparison with existing literature
The 95% CI around the overall prevalence in this study (4.3% to 8.0%) excludes the prior estimate of 3% based on earlier studies.15,16 The prevalence is slightly lower than the 7% pooled prevalence found in a systematic review and meta-analysis of studies that were largely based in secondary care with narrow inclusion criteria (for example, aged < 2 years and fever >38°C).14 Both false-positive and false-negative urine results may have occurred. A large number of samples had heavy mixed growth on culture (classified as negative) and some of these may have masked a true UTI. Some of the positive cultures may represent ‘asymptomatic bacteriuria’.20 However, children were only eligible for the present study if they were acutely ill, and all met the laboratory diagnostic criteria for UTI as defined in UK NICE guidelines.2 Some authors have suggested two consecutive urine samples should be obtained to determine UTI.21 This would reduce the false-positive rate but could also increase the false-negative rate. Obtaining a single sample is already challenging in busy general practice. A requirement to obtain two consecutive samples for all children may reduce sampling in general practice. Both higher and lower cut-off values for bacterial counts have been suggested for achieving the ideal trade off between sensitivity and specificity, but for now >105 cfu/ml remains the standard for diagnosing UTI.2,18,21
The finding that fever was not associated with UTI within an acutely ill group of children is important, as most previous studies excluded children without fever. Coulthard et al also found that fever was not present in all children with UTI and that fever or other clinical symptoms or signs could not be used to predict renal scarring.9
NICE guidelines recommend that a urine sample is not necessary at first presentation of an ill child if there is evidence of an alternative source of infection.2 However, the presence or absence of an alternative source of infection was not associated with UTI in the present study. Other studies have also found that the presence of an alternative source of infection cannot reliably rule out UTI.11,15,16
Implications for research and practice
The findings of this study suggest that the probability of UTI in children under the age of 3 years is reasonably high, irrespective of the presenting symptoms and signs. There were no symptoms or signs that ruled in or ruled out UTI with adequate precision in this age group and setting. With a pre-test (pre-urine sample) probability of >5%, a urine sample appears justified in far more acutely unwell children under the age of 3 years presenting in primary care than previously thought or currently practised. Implementing such a recommendation would represent a considerable increase in urine sampling, with associated costs and inconvenience. It would also increase the possibility of false-positive results with associated further unnecessary, additional investigations. However, the current strategy of suspicion-led urine sampling is likely to miss the majority of cases of UTI, along with the opportunity to treat promptly, and hence minimise morbidity and possibly reduce the risk of future complications.
It was found that, in children older than 3 years, without urinary frequency or pain on passing urine, there was a low probability (2%) of UTI. It may be that routine urine sampling is not indicated in this group.
Using the proposed model would mean sampling urine in 10 times more children compared to sampling based on current practice/GP suspicion, and would mean sampling twice as many children as is currently recommended by NICE. However, only 3% of UTIs would be missed, compared with 51% when implementing NICE guidelines and 80% when sampling is based on GP suspicion alone.
The management of children has not been considered in this study, beyond whether or not a urine sample would need to be obtained to identify those at risk of UTI. A further study, powered to test the predictive value of symptoms, signs, and clinical history features, is needed to determine whether dipsticks or combinations of symptoms and signs can be used to guide treatment without relying on urinary culture. It is not clear whether antibiotics can safely be delayed until the culture result is available. Increasing urine sampling may lead to increases in unnecessary antibiotic prescribing while waiting for culture results. Current guidelines advise that dipsticks are unreliable in children under 3 years of age, but may be useful in older children.2 If clinicians wish to increase urine sampling in their own practices, it is advisable to obtain the appropriate equipment (large collection pots for clean catch, which can be put inside a potty, and nappy pads), and emphasise the importance of sampling irrespective of urinary symptoms, preferably by clean-catch specimens, and, wherever possible, obtaining the sample before the child leaves the surgery.
The proposed model should be validated in another large sample of children. The potential benefits of diagnosing more UTIs, but possibly not until culture results become available, need to be considered alongside the additional associated costs of increasing urine sampling. Currently, all children with UTI (acutely ill with bacteriuria on culture) are considered at risk of renal complications, but only a small proportion will develop them.2 It remains unclear whether serious long-term sequelae will be prevented by increasing the diagnosis and treatment of UTI in children, and whether the required substantial increase in urine sampling would be cost effective.
A larger study that systematically samples acutely unwell children and follows them up over the longer term is needed to identify which children are most likely to have a UTI and which are at greatest risk for complications.2
It was found that almost 6% of acutely unwell children presenting to UK general practice met the criteria for a laboratory diagnosis of UTI. This prior probability is higher than previously recognised and should increase GPs’ awareness of this condition. Current guidelines promote prompt diagnosis of UTI in children.2 Based on the findings of this study, clinicians should have a lower threshold for urine sampling in acutely ill children, and consider sampling urine in all acutely unwell children less than 3 years old, irrespective of symptoms, and in those aged 3–5 years with either dysuria or urinary frequency. In contrast to the NICE guideline, the present study suggests that neither the absence of fever nor the presence of an alternative site of infection satisfactorily rules out UTI in young children.
Acknowledgments
We thank the children, families, GP practices, and NHS laboratories who participated in the study. We are particularly indebted to Dr Robin Howe and Dr Mandy Wootton for their microbiological expertise.
Appendix 1. Table of the symptoms, signs, and risk factors screened for association with urinary tract infection
| Symptoms |
|
| Signs |
|
| Other potential risk factors |
|
Funding
This study was funded by a Welsh Government National Institute for Health and Social Care Research (NISCHR)/Medical Research Council Health Research Partnership Award. The South East Wales Trials Unit is funded by the National Institute for Social Care and Health Research. Further support was from the Wales School of Primary Care research, funded by NISCHR, and by the NISCHR Clincial Research Centre. The study was sponsored by Cardiff University.
Ethical approval
Ethical approval for the study was obtained from the South East Wales Local Research Ethics committee (ref no. 08/WSE03/11). Informed, written consent was obtained from all participants.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Coulthard MG, Vernon SJ, Lambert HJ, Matthews JN. A nurse led education and direct access service for the management of urinary tract infections in children: prospective controlled trial. BMJ. 2003;327(7416):656. doi: 10.1136/bmj.327.7416.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. Urinary tract infection in children: diagnosis, treatment and long-term management. CG54. London: NICE; 2007. [Google Scholar]
- 3.Hay AD, Whiting P, Butler CC. How best to diagnose urinary tract infection in preschool children in primary care? BMJ. 2011;343:d6316. doi: 10.1136/bmj.d6316. [DOI] [PubMed] [Google Scholar]
- 4.Vernon SJ, Coulthard MG, Lambert HJ, et al. New renal scarring in children who at age 3 and 4 years had had normal scans with dimercaptosuccinic acid: follow up study. BMJ. 1997;315:905–908. doi: 10.1136/bmj.315.7113.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks SH, Verrier Jones K, Roberts R, et al. Effect of symptomless bacteriuria in childhood on subsequent pregnancy. Lancet. 1987;2:991–994. doi: 10.1016/s0140-6736(87)92558-x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson SH, Eklof O, Eriksson CG, et al. Development of hypertesion and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ. 1989;299(6701):703–706. doi: 10.1136/bmj.299.6701.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennerstrom M, Hansson S, Hedner T, et al. Ambulatory blood pressure 16–26 years after the first urinary tract infection in childhood. J Hypertens. 2000;18(4):485–491. doi: 10.1097/00004872-200018040-00019. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh N, Ewing A, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126(6):1084–1091. doi: 10.1542/peds.2010-0685. [DOI] [PubMed] [Google Scholar]
- 9.Coulthard MG, Lambert HJ, Kier MJ. Do systemic symptoms predict the risk of kidney scarring after urinary tract infection? Arch Dis Child. 2009;94(4):278–281. doi: 10.1136/adc.2007.132290. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham AM, Edwards A, Jones KV, et al. Evaluation of a service development to increase detection of urinary tract infections in children. J Eval Clin Pract. 2005;11(1):73–76. doi: 10.1111/j.1365-2753.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien K, Stanton N, Edwards A, et al. Prevalence of urinary tract infection (UTI) in sequential acutely unwell children presenting in primary care: exploratory study. Scand J Prim Health Care. 2011;29(1):19–22. doi: 10.3109/02813432.2011.554268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson J. Incidence and outcome of symptomatic UTI in children. BMJ. 1979;1(6174):1330–1332. doi: 10.1136/bmj.1.6174.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DS, Huang SH, Lin CC, et al. Urinary tract infection in febrile infants younger than eight weeks of age. Pediatrics. 2000;105(2):E20. doi: 10.1542/peds.105.2.e20. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302–308. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 15.Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102(2):e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 16.Hoberman A, Chao HP, Keller DM, et al. Prevalence of urinary tract infection in febrile infants. J Pediatr. 1993;123(1):17–23. doi: 10.1016/s0022-3476(05)81531-8. [DOI] [PubMed] [Google Scholar]
- 17.Newcombe R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Lau AY, Wong S-N, Yip K-T, et al. A comparative study on bacterial cultures of urine samples obtained by clean-void technique versus urethral catheterization. Acta Paediatr. 2007;96(3):432–436. doi: 10.1111/j.1651-2227.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Voort J, Edwards A, Roberts R, Verrier Jones K. The struggle to diagnose UTI in children under two in primary care. Fam Pract. 1997;14(1):44–48. doi: 10.1093/fampra/14.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925–933. doi: 10.1111/j.1651-2227.1985.tb10059.x. [DOI] [PubMed] [Google Scholar]
- 21.Coulthard MG, Kalra M, Lambert HJ, et al. Redefining urinary tract infections by bacterial colony counts. Pediatrics. 2010;125(2):335–441. doi: 10.1542/peds.2008-1455. [DOI] [PubMed] [Google Scholar]



