Abstract
Background and objectives
Chronic obstructive pulmonary disease (COPD) is responsible for significant morbidity and mortality worldwide. We evaluated the characteristics of stable COPD patients in the pulmonology clinics of seven Asian cities and also evaluated whether the exposure to biomass fuels and dusty jobs were related to respiratory symptoms, airflow limitation, and quality of life in the COPD patients.
Methods
This cross-sectional observational study recruited 922 COPD patients from seven cities of Asia. The patients underwent spirometry and were administered questionnaires about their exposure to cigarette smoking, biomass fuels, and dusty jobs in addition to respiratory symptoms and health related quality of life.
Results
Of the patients, there appeared to be variations from city to city in the history of exposure to biomass fuels and dusty jobs and also in respiratory symptoms of cough, phlegm, wheeze, and dyspnea. These symptoms were more frequent in those COPD patients with a history of exposure to biomass fuels than without and those with a history of exposure to dusty jobs than without (P < 0.01 for all comparisons). Airflow limitation was more severe in those COPD patients with a history of exposure to biomass fuels than without (52.2% predicted versus 55.9% of post-bronchodilator forced expiratory volume in 1 second [FEV1], P = 0.009); quality of life was poorer in those with exposure to biomass fuels than without (40.4 versus 36.2 of the St George’s Respiratory Questionnaire [SGRQ] total score, P = 0.001). Airflow limitation was more severe in those COPD patients with a history of exposure to dusty jobs than without (51.2% predicted versus 57.3% of post-bronchodilator FEV1, P < 0.001); quality of life was poorer in those with dusty jobs than without (41.0 versus 34.6 of SGRQ score, P = 0.006).
Conclusion
In Asian cities, the characteristics of COPD patients vary and the history of exposure to biomass fuels or dusty jobs was related to frequency of symptoms, severe airflow limitation, and poor quality of life.
Keywords: COPD, Asia, biomass, dust
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide.1 Its high prevalence and associated medical expenses impose large socioeconomic burdens.
This is especially true in Asian cities, due to high rates of smoking, outdoor or indoor air pollution including biomass fuel combustion, and exposure to occupational dusts and noxious gases.2,3 COPD shows heterogeneous features that are demonstrated with the variability in symptoms, comorbidities, and exacerbations.4,5 The heterogeneity of COPD may be due to the variations in genetic influences and environmental exposures among which exposures to biomass fuels and dusty jobs were reported to be related with the development of COPD in Asia.6,7 Therefore, to assess the heterogeneity of COPD, it would be reasonable to compare the exposure history of biomass fuels and dusty jobs, demographic characteristics, and clinical characteristics of COPD patients from different cities or regions.
The Asian Network for Obstructive Lung Disease (ANOLD)8 is a group of collaborative researchers studying the heterogeneous characteristics of Asian COPD patients. We have evaluated the history of exposure to biomass fuels and dusty jobs, respiratory symptoms, health-related quality of life, comorbidities, and respiratory medications in COPD patients from seven cities in Asia. We also examined the relations of respiratory symptoms, severe airflow limitation, and poor quality of life to the exposure to biomass fuels and dusty jobs. We hypothesized that biomass fuel exposure or dusty job exposure would be related to increased risks of respiratory symptoms, severe airflow limitation, and poor quality of life in COPD patients.
Methods
Study design
This was a cross-sectional observational study involving COPD patients from seven Asian cities. Patients underwent simple chest radiography and pre- and post-bronchodilator spirometry and were administered questionnaires about respiratory symptoms; exposure to tobacco smoking, biomass fuels, and dusty jobs; health-related quality of life; comorbidities; and respiratory medication during a single visit. This study was approved by the Institutional Review Board at every participating center, and all study participants provided written informed consent.
Study subjects
COPD patients were recruited between September 2009 and September 2010 at the pulmonary clinics in seven Asian cities: Beijing, China; Colombo, Sri Lanka; Penang, Malaysia; Quezon City, Philippines; Sapporo, Japan; Seoul, Korea; and Taipei, Taiwan. The Japanese data were obtained from the Hokkaido COPD study;9 the Korean data were obtained from a Korean cohort of obstructive lung disease named the KOLD cohort.10 Ten clinics or hospitals in or near Sapporo, eleven in or near Seoul, and only one in other Asian cities participated in the recruitment of patients. The above seven cities were chosen as they were the location of the authors’ study group, the Asian Network for Obstructive Lung Disease.
All COPD patients were of Asian ethnicity, aged ≥40 years and had post-salbutamol forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio < 0.7. Smokers were defined as individuals with a history of cigarette smoking for at least 10 pack-years, whereas nonsmokers were defined as individuals with a lifetime history of <100 cigarettes.
Patients were excluded if they had a history of asthma, bronchiectasis, tuberculosis, or other concomitant respiratory diseases. Patients with old healed tuberculosis with sequelae not associated with cavities or destroyed lung, but associated both with the extent of radiological lesion not extending beyond one lobe territory and with the above defining criteria of COPD were included. Patients were also excluded if they were in an exacerbated state but they were allowed to participate one month after an exacerbation. Patients were also excluded if they had a history of prior lung surgery including lung volume reduction, uncontrolled cancer, or radiation therapy to the chest, mediastinum, or breast. Patients who refused salbutamol administration and pregnant women were excluded.
Questionnaires
The standardized questionnaires assessed respiratory history and symptoms, smoking history, family history, health-related quality of life, comorbidities, and current respiratory medications. These questionnaires included a modified version of the American Thoracic Society Division of Lung Diseases Respiratory Epidemiology Questionnaire, Charlson comorbidity conditions and the St George’s Respiratory Questionnaire (SGRQ). Questionnaires were administered by interviewers.11–13
Cigarette smoking was evaluated using the question, “Have you ever smoked cigarettes? (No means less than 100 cigarettes in your life).” Biomass fuel exposure was evaluated using the question, “For cooking and/or heating, have you ever been exposed to biomass fuels such as wood, agricultural crop residues, animal dung, charcoal, and others?” Exposure to a dusty job was evaluated using the question, “Have you ever worked for 1 year or more at any dusty job?” Paternal (or maternal) smoking was evaluated with the question, “Was your father (or mother) ever a cigarette smoker?”
Cough was evaluated using the question, “Do you usually have a cough? (excluding clearing of the throat),” and phlegm was evaluated with the question, “Do you usually bring up phlegm from your chest?” Chronic bronchitis was defined as both cough and phlegm for over 3 consecutive months for a period of more than 2 years.14 Wheeze was evaluated with the question, “Have you ever had wheezing or whistling in your chest?” Dyspnea was evaluated using the modified Medical Research Council Dyspnea grade.15
Current medications were evaluated using the question, “At present, do you use medications to treat your breathing problems?” If the subject answered yes, he or she was asked to choose specific medication(s).
Spirometry and body mass index
Spirometry was performed as proposed by the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force.16 For post-bronchodilator spirometry we used 200 μg of salbutamol with a spacer. We used various spirometric machines (Chestac; Chest MI Inc, Tokyo, Japan; MasterScreen pulmonary function test [PFT]; CareFusion Corporation, San Diego, CA; Vmax Encore 22; CareFusion Corporation; KoKo Spirometry; Pulmonary Data Services Inc, Louisville, CO; Quark PFT 4; Cosmed, Rome, Italy), each of which was calibrated daily. Quality control of spirometry was performed at the center of each city and also monitored at the Coordination Center at Asan Medical Center, Seoul, Korea. At each center the same personnel performed spirometry using the same spirometry machine which was calibrated daily with syringe. The graphs of spirometry were delivered to and monitored by the Coordination Center. The reference equations for spirometry were developed from corresponding local populations for Japan and Korea, or adopted from European populations for the other countries.17–20
Body mass index (BMI) was calculated as the patient’s weight in kilograms divided by the square of the height in meters. Underweight was defined as BMI < 18.5 kg/m2 and overweight as BMI ≥ 25 kg/m2.21
Data analysis
The Chi-square or Kruskal–Wallis test was performed for the evaluation of variation in patients’ characteristics among the seven Asian cities. Multiple logistic regression analysis was performed to evaluate whether the risk factor of biomass fuel (or dusty job) exposure was associated with respiratory symptoms after the adjustment for age, sex, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, cigarette smoking, exposure to dusty jobs (or biomass fuels), and city.
P-values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS version 18.0 software (IBM Corporation, Armonk, NY).
Results
Characteristics of subjects
Between September 2009 and September 2010, we recruited 922 COPD patients from pulmonology clinics of seven cities in Asia: 70 in Beijing, China; 268 in Sapporo, Japan; 173 in Seoul, Korea; 92 in Penang, Malaysia; 109 in Quezon City, Philippines; 110 in Colombo, Sri Lanka; and 100 in Taipei, Taiwan.
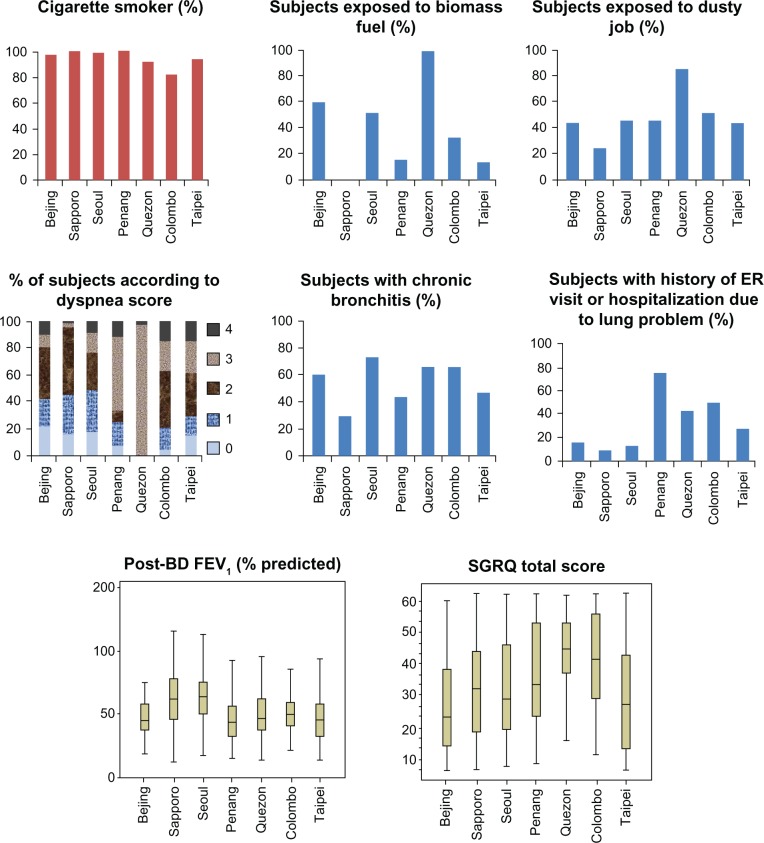
We observed a variation of characteristics of the patients with COPD collected from the seven Asian cities in terms of possible exogenous causes, lung function, clinical symptoms (dyspnea score, presence of chronic bronchitis), health-related quality of life, comorbidities, and history of lung problems including exacerbations (Figure 1 and Table 1; P value <0.001 for all analyses).
Figure 1.
Characteristics of subjects according to Asian cities.
Abbreviations: BD, bronchodilator; FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire.
Table 1.
Characteristics of subjects in seven Asian countries
| Total | Beijing, China | Sapporo, Japan | Seoul, Korea | Penang, Malaysia | Quezon, Philippines | Colombo, Sri Lanka | Taipei, Taiwan | |
|---|---|---|---|---|---|---|---|---|
| Subjects, number | 922* (100%) | 70 | 268 | 173 | 92 | 109 | 110 | 100 |
| Mean age, years (SD) | 68.2 (8.5) | 68.9 (7.1) | 69.4 (8.1) | 69.0 (7.4) | 68.3 (8.8) | 64.1 (8.8) | 63.8 (8.1) | 72.3 (8.4) |
| Male | 864 (93.7%) | 69 (98.6%) | 252 (94.0%) | 164 (94.8%) | 83 (90.2%) | 100 (91.7%) | 103 (93.6%) | 93 (93.0%) |
| Cigarette smoker | 879 (95.3%) | 68 (97.1%) | 267 (99.6%) | 170 (98.3%) | 92 (100%) | 100 (91.7%) | 89 (80.9%) | 93 (93.0%) |
| Biomass exposure | 296 (32.1%) | 41 (58.6%) | 0 (0%) | 89 (51.4%) | 13 (14.1%) | 106 (97.2%) | 34 (30.9%) | 13 (13.0%) |
| Dusty job | 412 (44.7%) | 31 (44.3%) | 63 (23.5%) | 80 (46.2%) | 42 (45.7%) | 94 (86.2%) | 58 (52.7%) | 44 (44.0%) |
| Cough | 457 (49.6%) | 32 (45.7%) | 46 (17.2%) | 103 (59.5%) | 60 (65.2%) | 90 (82.6%) | 98 (89.1%) | 28 (28.0%) |
| Phlegm | 540 (58.6%) | 51 (72.9%) | 68 (25.4%) | 142 (82.1%) | 82 (89.1%) | 92 (84.4%) | 77 (70.0%) | 28 (28.0%) |
| Chronic bronchitis | 201 (21.8%) | 16 (22.9%) | 28 (10.4%) | 59 (34.3%) | 18 (19.6%) | 30 (27.5%) | 29 (26.4%) | 21 (21.0%) |
| Wheeze | 693 (75.2%) | 63 (90.0%) | 171 (63.8%) | 112 (64.7%) | 74 (80.4%) | 96 (88.1%) | 102 (92.7%) | 75 (75.0%) |
| MMRC dyspnea | ||||||||
| Grade 0 | 118 (12.8%) | 16 (22.9%) | 44 (16.4%) | 31 (17.9%) | 7 (7.6%) | 0 (0%) | 5 (4.5%) | 15 (15.2%) |
| Grade 1 | 197 (21.4%) | 14 (20.0%) | 78 (29.1%) | 54 (31.2%) | 18 (19.6%) | 0 (0%) | 18 (16.4%) | 15 (15.2%) |
| Grade 2 | 299 (32.4%) | 27 (38.6%) | 138 (51.5%) | 48 (27.7%) | 7 (7.6%) | 0 (0%) | 47 (42.7%) | 32 (32.3%) |
| Grade 3 | 243 (26.4%) | 6 (8.6%) | 6 (2.2%) | 26 (15.0%) | 50 (54.3%) | 107 (98.2%) | 25 (22.7%) | 23 (23.2%) |
| Grade 4 | 64 (6.9%) | 7 (10.0%) | 2 (0.7%) | 14 (8.1%) | 10 (10.9%) | 2 (1.8%) | 15 (13.6%) | 14 (14.1%) |
| Body mass index, kg/m2 (SD) | 22.1 (4.2) | 24.0 (3.9) | 22.3 (3.2) | 23.0 (3.3) | 21.1 (3.7) | 20.8 (5.6) | 20.0 (4.0) | 23.8 (5.0) |
| Underweight | 179 (19.4%) | 7 (10.1%) | 37 (13.8%) | 17 (9.8%) | 23 (25.0%) | 35 (32.1%) | 52 (47.3%) | 8 (8.0%) |
| Overweight | 189 (20.5%) | 30 (42.9%) | 49 (18.3%) | 42 (24.3%) | 13 (14.1%) | 12 (11.0%) | 11 (9.1%) | 32 (32.0%) |
| Post-bronchodilator | 1.38 (0.61) | 1.25 (0.36) | 1.72 (0.67) | 1.64 (0.56) | 1.01 (0.41) | 1.09 (0.47) | 1.12 (0.35) | 1.03 (0.45) |
| FEV1, liters (SD) | ||||||||
| Post-bronchodilator | 54.7% (20.0) | 47.2% (14.1) | 63.4% (21.2) | 56.4% (17.2) | 47.3% (18.7) | 50.2% (19.5) | 52.3% (16.6) | 47.9% (21.0) |
| FEV1, % predicted (SD) | ||||||||
| GOLD | ||||||||
| Stage I† | 115 (12.5%) | 1 (1.4%) | 62 (23.1%) | 16 (9.2%) | 7 (7.6%) | 12 (11.0%) | 9 (8.2%) | 8 (8.1%) |
| Stage II† | 392 (42.5%) | 29 (41.4%) | 126 (47.0%) | 92 (53.2%) | 30 (32.6%) | 39 (35.8%) | 46 (40.0%) | 30 (30.0%) |
| Stage III† | 324 (35.1%) | 32 (45.7%) | 67 (25.0%) | 55 (31.8%) | 40 (43.5%) | 43 (39.4%) | 46 (43.6%) | 41 (41.4%) |
| Stage IV† | 86 (9.3%) | 8 (11.4%) | 13 (4.9%) | 10 (5.8%) | 15 (16.3%) | 15 (13.8%) | 9 (8.2%) | 16 (16.2%) |
| SGRQ | ||||||||
| Symptoms score | 50.5 | 41.3 | 53.5 | 45.4 | 55.1 | 51.0 | 62.0 | 40.2 |
| Activity score | 50.4 | 37.5 | 44.0 | 49.0 | 60.3 | 67.9 | 56 | 44.2 |
| Impact score | 26.1 | 22.1 | 22.8 | 23.3 | 26.9 | 35.2 | 35.8 | 20.9 |
| Total score (SD) | 37.5 (18.6) | 29.8 (16.0) | 34.5 (16.7) | 34.8 (18.0) | 41.5 (20.3) | 47.8 (15.0) | 46.1 (19.5) | 31.2 (19.2) |
Notes:
Number of subjects with percentages in parentheses, if not specified otherwise. Underweight = body mass index < 18.5 kg/m2; overweight ≥ 25 kg/m2. The Chisquare or Kruskal–Wallis test was performed for the evaluation of variation in the above characteristics among the seven Asian cities (Beijing, China; Colombo, Sri Lanka; Penang, Malaysia; Quezon City, Philippines; Sapporo, Japan; Seoul, Korea; and Taipei, Taiwan) (P < 0.001 for all comparisons among the cities).
Abbreviations: SD, standard deviation; MMRC, modified Medical Research Council; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SGRQ, St George’s Respiratory Questionnaire.
Table 1 shows the demographic and clinical characteristics of the 922 patients by city. Overall mean age was 68.2 years (standard deviation 8.5 years) and 864 of the subjects (93.7%) were male. We found that 296 patients (32.1%) had a history of exposure to biomass fuels and 412 (44.7%) had an occupational history of dusty jobs.
The prevalence of biomass exposure differed from city to city throughout Asia, ranging from 0% in Sapporo, Japan, to 97.2% in Quezon City, Philippines. Biomasses included wood (85.0%), charcoal (8.7%), and agricultural crop residues (4.2%). Of the 922 COPD patients, 201 (21.8%) had symptoms of chronic bronchitis and 693 (75.2%) had wheeze. Mean BMI was 22.1 kg/m2, mean predicted post-bronchodilator FEV1 was 54.7% and mean total SGRQ score was 37.5. The proportion of COPD patients with underweight and overweight were 19.4% and 20.5%, respectively.
Of the COPD patients, 43 (4.7%) had no history of cigarette smoking and 879 (95.3%) were cigarette smokers. Among them 222 (24.1%) were current cigarette smokers and 657 (71.3%) were past smokers (Table 2). We found that 63.9% of these patients had fathers who smoked and that 16.7% had mothers who smoked.
Table 2.
Cigarette smoking history
| Total | Beijing, China | Sapporo, Japan | Seoul, Korea | Penang, Malaysia | Quezon, Philippines | Colombo, Sri Lanka | Taipei, Taiwan | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Current smoker | 222* (24.1%) | 14 (20.0%) | 72 (26.9%) | 53 (30.6%) | 30 (32.6%) | 11 (10.1%) | 20 (18.2%) | 22 (22.0%) | <0.001 |
| Past smoker | 657 (71.3%) | 54 (77.1%) | 195 (72.8%) | 117 (67.6%) | 62 (67.3%) | 89 (81.7%) | 69 (62.7%) | 71 (71.0%) | <0.001 |
| Never smoker | 43 (4.7%) | 2 (2.9%) | 1 (0.4%) | 3 (1.7%) | 0 (0%) | 9 (8.3%) | 21 (19.1%) | 7 (7.0%) | <0.001 |
| Smoking amount† (pack-years) | 50.3 | 55.5 | 76.8 | 45.9 | 64.4 | 40.1 | 22.6 | 57.1 | <0.001 |
| Paternal smoking history | 418 (63.9%) | 48 (68.6%) | n/a | 116 (67.1%) | 56 (60.9%) | 74 (67.9%) | 53 (48.2%) | 71 (71.0%) | <0.001 |
| Maternal smoking history | 109 (16.7%) | 20 (28.6%) | n/a | 36 (20.8%) | 12 (13.0%) | 33 (30.3%) | 1 (0.9%) | 7 (7.0%) | <0.001 |
Notes:
Numbers of subjects with percentages in parentheses;
mean amount of cigarette smoking calculated only among current or past smokers. P-values were obtained by Chi-square test.
Abbreviation: n/a, not available.
Comorbidities and current respiratory medications
We found that 8.8% and 8.5% of the COPD patients had a history of diabetes mellitus and ulcer disease, respectively, and that 3.6% and 3.5% had a history of myocardial infarction and cerebrovascular disease, respectively.
Of the COPD patients, 81.8% reported that they were currently using medications to treat breathing problems. Of these patients, 38.7% were using theophylline, 37.6% an inhaled long-acting muscarinic antagonist, 35.1% an inhaler containing a combination of a corticosteroid and a long-acting beta-agonist, and 32.0% an inhaled short-acting beta-agonist.
Respiratory symptoms and exposure to biomass fuels or dusty jobs
Respiratory symptoms, including cough, phlegm, wheeze, and dyspnea, were more frequent in those COPD patients with a history of exposure to biomass fuels than without and in those with a history of exposure to dusty jobs than without (Table 3). For some of the symptoms, these relationships remained even after the adjustment for age, sex, GOLD stage, cigarette smoking, biomass fuel exposure, dusty job exposure, and city (Table 4). Multivariable analysis showed that, for biomass fuel exposure, the odds ratios (ORs) of cough, phlegm, wheeze, and dyspnea were 1.11 (95% confidence interval [CI]: 0.72–1.72), 1.39 (95% CI: 0.85–2.26), 1.37 (95% CI: 0.82–2.27), and 1.08 (95% CI: 0.70–1.66), respectively, and, for dusty job exposure, the ORs of these symptoms were 1.47 (95% CI: 1.01–2.14), 1.77 (95% CI: 1.16–2.69), 1.51 (95% CI: 0.98–2.35), and 1.19 (95% CI: 0.81–1.77), respectively.
Table 3.
Comparison of subjects who were exposed to biomass fuels or a dusty job with nonexposed subjects
| Biomass exposure
|
Dusty job
|
|||||
|---|---|---|---|---|---|---|
| 296 subjects answered “yes” | 626 subjects answered “no” | P-value | 412 subjects answered “yes” | 496 subjects answered “no” | P-value | |
| Sex | 0.032 | 0.003 | ||||
| Male | 270* (91.2%) | 594 (94.9%) | 395 (95.9%) | 457 (92.1%) | ||
| Female | 26 (8.8%) | 32 (5.1%) | 17 (4.1%) | 39 (7.9%) | ||
| Cigarette Smoking | ||||||
| Current smoker | 58 (19.6%) | 164 (26.2%) | <0.001 | 91 (22.1%) | 126 (25.4%) | 0.313 |
| Past smoker | 209 (70.6%) | 444 (70.9%) | 299 (72.6%) | 346 (69.8%) | ||
| Never smoker | 29 (9.8%) | 18 (2.9%) | 22 (5.3%) | 24 (4.8%) | ||
| Smoking amount† (pack-years) | 46.1 | 52.9 | 0.018 | 47.2 | 52.5 | 0.195 |
| Cough | 202 (68.2%) | 255 (40.7%) | <0.001 | 256 (62.1%) | 200 (40.3%) | <0.001 |
| Phlegm | 237 (80.1%) | 303 (48.4%) | <0.001 | 290 (70.4%) | 247 (49.8%) | <0.001 |
| Chronic bronchitis | 99 (33.4%) | 137 (21.9%) | <0.001 | 127 (30.8%) | 108 (21.8%) | 0.001 |
| Wheeze | 245 (82.8%) | 448 (71.6%) | <0.001 | 330 (80.1%) | 358 (72.2%) | 0.003 |
| Dyspnea, MMRC | <0.001 | |||||
| dyspnea grade | ||||||
| 0 | 25 (8.5%) | 93 (14.9%) | 36 (8.7%) | 80 (16.1%) | <0.001 | |
| 1 | 49 (16.6%) | 147 (23.5%) | 70 (17.0%) | 121 (24.4%) | ||
| 2 | 64 (21.6%) | 236 (37.7%) | 118 (28.6%) | 178 (35.9%) | ||
| 3 | 137 (46.3%) | 106 (16.9%) | 159 (38.6%) | 82 (16.5%) | ||
| 4 | 21 (7.1%) | 43 (6.9%) | 28 (6.8%) | 35 (7.1%) | ||
| Post-bronchodilator | 52.2% predicted | 55.9% predicted | 0.009 | 51.2% predicted | 57.3% predicted | <0.001 |
| FEV1, % predicted† | ||||||
| Total score of SGRQ† | 40.4 | 36.2 | 0.001 | 41.0 | 34.6 | 0.006 |
Notes:
Numbers of subjects with percentages in parentheses, if not specified;
mean values.
Abbreviations: MMRC, Modified Medical Research Council; FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire.
Table 4.
Odds ratios of risk factors for respiratory symptoms
| Cough | Phlegm | Chronic bronchitis | Wheeze | Dyspnea | |
|---|---|---|---|---|---|
| Age, years | 1.00 (0.98–1.02)* | 1.01 (0.98–1.03) | 1.01 (0.98–1.03) | 0.98 (0.95–1.01) | 1.02 (1.00–1.05) |
| Male | 1.78 (0.83–3.85) | 1.04 (0.42–2.59) | 0.77 (0.34–1.73) | 0.63 (0.22–1.75) | 0.41 (0.16–1.10) |
| GOLD | |||||
| Stage II† | 1.05 (0.54–2.05) | 1.31 (0.65–2.62) | 1.10 (0.53–2.27) | 0.80 (0.39–1.65) | 1.49 (0.74–2.99) |
| Stage III† | 1.43 (0.72–2.83) | 2.02 (0.98–4.14) | 1.51 (0.73–3.12) | 1.41 (0.67–2.98) | 3.83 (1.86-7.87)‡ |
| Stage IV† | 1.40 (0.61–3.21) | 3.49 (1.36-8.97)‡ | 2.36 (1.01-5.49)‡ | 6.61 (1.71-25.51)‡ | 10.99 (3.68-32.79)‡ |
| Biomass exposure | 1.11 (0.72–1.72) | 1.39 (0.85–2.26) | 1.12 (0.72–1.74) | 1.37 (0.82–2.27) | 1.08 (0.70–1.66) |
| Dusty job | 1.47 (1.01-2.14)‡ | 1.77 (1.16-2.69)‡ | 1.59 (1.07-2.34)‡ | 1.51 (0.98–2.35) | 1.19 (0.81–1.77) |
| Cigarette smoking | |||||
| Past§ | 1.31 (0.56–3.03) | 1.45 (0.65–3.23) | 1.27 (0.54–2.98) | 1.25 (0.46–3.37) | 2.14 (0.89–5.16) |
| Current§ | 2.36 (0.95–5.84) | 2.13 (0.87–5.20) | 2.43 (0.98–5.99) | 1.22 (0.42–3.50) | 1.67 (0.66–4.22) |
Notes:
Odds ratios with 95% confidence intervals in parentheses were presented by multiple logistic regression analysis after adjustment for age, sex, GOLD stage of COPD, smoking, and also city;
reference was GOLD stage I;
statistically significant relationship with P < 0.05;
reference was never smoker. The outcome (dependent) variables were binary variables of cough, phlegm, chronic bronchitis (combined symptoms of cough and phlegm), wheeze, and dyspnea. Dependent variables were age, sex, COPD severity of GOLD stage, history of exposure to biomass fuels, exposure to dusty jobs, cigarette smoking, and city.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; COPD, chronic obstructive pulmonary disease.
Airflow limitation and exposure to biomass fuels or dusty jobs
Airflow limitation appeared to be more severe in the COPD subjects with a history of exposure to biomass fuels or dusty jobs than without (Table 3). The mean of post-bronchodilator FEV1 was 52.2% of predicted value versus 55.9% for the COPD subjects with versus without biomass exposure; 51.2% of predicted value versus 57.3% for the subjects with versus without dusty job exposure (both comparisons, P < 0.01).
Health-related quality of life and exposure to biomass fuels or dusty jobs
Quality of life appeared to be poorer in the COPD subjects with a history of exposure to biomass fuels or dusty jobs than without (Table 3). The mean of the total SGRQ score was 40.4 versus 36.2 for the COPD subjects with versus without biomass exposure (P = 0.001); 41.0 versus 34.6 for the subjects with versus without dusty job exposure (P = 0.006). Multivariable analysis showed that being female or in a higher GOLD stage was related to poor quality of life. The OR of male to female was 0.48 (95% CI: 0.25–0.91) for poor quality of life. The ORs of GOLD stage II, III, and IV to GOLD I were 1.8 (95% CI: 1.1–2.8), 4.4 (95% CI: 2.8–7.0), and 12.0 (95% CI: 6.0–24.0), respectively.
Discussion
We have shown here that there were variations of COPD patients from seven Asian cities in the exposure history to biomass fuels and dusty jobs and also in respiratory symptoms. The symptoms were more frequent, airflow limitation was more severe, and quality of life was poorer in those COPD patients with a history of exposure to biomass fuels than without and those with a history of exposure to dusty jobs than without. Our findings suggest that both biomass fuel exposure and dusty job exposure might contribute to COPD morbidity in Asian cities.
In this study symptoms of COPD, cough, phlegm, wheeze, and dyspnea were more frequent in the COPD patients with a history of exposure to biomass fuels or dusty jobs than without. Among the symptoms, cough and phlegm were more frequent in COPD patients with exposure to dusty jobs even after the adjustment for age, sex, GOLD stage, cigarette smoking, exposure to biomass fuels, and city. These findings suggest that exposure to dusty jobs might impose bronchitis symptoms in the COPD patients, most of whom were past or current cigarette smokers in this study.
We found that the proportion of COPD subjects exposed to biomass fuels differed from city to city in Asia, with a range from 0% to 97.2%. Biomass exposure in women (44.8% = 100 × 26/58) was higher than that in men (31.3% = 100 × 270/864) (Table 3). This might be related to women’s cooking and heating with biomass fuels. Although men’s exposure to biomass fuels appeared to be less than women’s, men’s exposure percentage of 31% was also considerable, which suggests that biomass fuel exposure may be important for men as well as for women. Exposure to biomass fuels has been reported to be an important risk factor for the development of COPD in both Asian and non-Asian cities.22,23 Similar to previous findings, we found that 32.1% of our COPD subjects had been exposed to biomass combustion,24 and that this exposure may be related to respiratory symptoms. This finding is in agreement with a report showing the development of respiratory symptoms in Indians using domestic cooking fuels.23 In Sapporo, no patient had an exposure history of biomass fuels. They did not use biomass fuels at all as energy resources because oil and/or gas supply by the local government or by private companies were almost perfect in all areas throughout Japan including Sapporo.
Exposure to dusty jobs has been reported to be a risk factor for the development of COPD.24 In addition to having a role in the development of COPD, these jobs were associated with an increase in respiratory symptoms in patients with COPD in our study. Our findings therefore emphasize the importance of asking for history about exposure to dusty jobs, as well as cigarette smoking and biomass fuels, when examining COPD patients in Asia.
Of our COPD subjects, 8.8%, 8.5%, 3.6%, 4.3%, and 3.5% had histories of diabetes mellitus, ulcer disease, myocardial infarction, peripheral vascular disease, and cerebrovascular disease, respectively. The prevalence of diabetes mellitus in the general populations of Asia-Pacific regions has been reported to range from 3.7% to 13.3%.25 In addition, the prevalence of diabetes mellitus may not differ between individuals with and without COPD. For example, the prevalence of diabetes mellitus in individuals in Saskatchewan, Canada, with and without COPD has been reported to be 14.5% and 12.4%, respectively.26 The prevalence of ulcer disease in our study was comparable to the 8.5% previously reported,27 and the prevalence of myocardial infarction and cerebrovascular disease in our study was comparable to previous findings.26,28 We found that 38.7%, 35.1%, 32.0%, and 37.6% of our COPD patients were taking theophylline, an inhaler containing a combination of a corticosteroid and a long-acting beta-agonist, an inhaled short-acting beta-agonist, or an inhaled long-acting muscar-inic antagonist, respectively. The usage of each medication appeared to differ from city to city. The various combinations of these treatments may differ within Asian countries and also differ to those used in Western countries. Theophyl-line was the most prevalent and appears to be higher than in Western countries,29 perhaps due to its relatively low cost.
Limitations
The COPD patients were recruited not with random or systematic sampling but with convenient sampling, which inherently has a potential limitation of selection bias. Thus, the data in this manuscript may not accurately represent the cities of Asian countries. Despite the potential limitation of selection bias, this study provides a meaningful profle of COPD patients’ characteristics in seven Asian cities.
Secondly, the percentage of females was low in our study, perhaps due to the relatively low percentage of Asian females who smoke cigarettes.2 Besides the low percentage of cigarette smokers in females of these countries, there might be other possibilities why the female COPD subjects were very low in percentage. It would be less likely that Asian women may be referred to subspecialty clinics for COPD because biomass exposure is relatively unknown as a risk factor and also less likely that Asian women will consent to participate in clinical studies. Another possibility would be that health care might be less accessible for Asian women. Because this study is a clinic-based cross-sectional study, there seem to be several sorts of selection biases that might influence the characteristics of COPD patients. Future population-based studies could reveal the reasons why the percentage of women COPD patients was so low in Asian referral clinics.
Thirdly, the questions for biomass fuel exposure and dusty job exposure have not been validated yet. Further study with a validated questionnaire is needed to confirm our findings. On the contrary, the questions of cough, phlegm, and wheeze were adopted from the American Thoracic Society Division of Lung Diseases Respiratory Epidemiology Questionnaire that was validated as were the Charlson comorbidity conditions and the SGRQ.11–13
Finally, it is difficult to draw a definite conclusion with this study of a cross-sectional design. A follow-up study is needed to draw a confirmative conclusion from our findings.
Despite these possible limitations, our study showed that the characteristics of Asian COPD patients might differ from city to city in Asia, and might also differ from those of Western COPD patients.
Conclusion
The characteristics of COPD patients in Asian cities are various and a history of exposure to biomass fuels or dusty jobs was related to more frequent symptoms, severe airflow limitation, and poor quality of life.
Acknowledgments
We thank Jinkyeong Park, Hyejin Joo, Kyung-Wook Jo, and Jeong-Eun Lim for the data preparation, and the members of the KOLD Study Group for the data collection of Korean patients. This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Author contributions
Yeon-Mok Oh: study design, data collection, data analysis, choosing results, discussing the significance of results, writing a draft of the manuscript. Arvind Bhome: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Watchara Boonsawat: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Kirthi Dias Gunasekera: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Dushantha Madegedara: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Luisito Idolor: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Camilo Roa: study design, data collection, reviewing the manuscript. Woo Jin Kim: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Han-Pin Kuo: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Chun-Hua Wang: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Le Thi Tuyet Lan: study design, discussing the significance of analyzed data and results, reviewing the manuscript. Li-Cher Loh: study design, data collection, discussing the significance of analyzed data and results, reviewing and commenting on the manuscript. Choo-Khoon Ong: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Alan Ng: study design, discussing the significance of analyzed data and results, reviewing the manuscript. Masaharu Nishimura: study design, data collection, discussing the significance of analyzed data and results, reviewing and commenting on the manuscript. Hironi Makita: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Edwin Silverman: study design, discussing the significance of analyzed data and results, reviewing the manuscript. Jae Seung Lee: study design, discussing the significance of analyzed data and results, reviewing the manuscript. Ting Yang: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Yingxiang Lin: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Chen Wang: study design, data collection, discussing the significance of analyzed data and results, reviewing the manuscript. Sang-Do Lee: study design, data collection, discussing the significance of analyzed data and results, choosing results, reviewing the manuscript.
Disclosure
Yeon-Mok Oh has been an investigator in industry-sponsored studies (MSD Korea, AstraZeneca Korea, Boehringer Ingelheim Korea, Handok and GlaxoSmithKline) and in university-sponsored studies (Asan Institute for Life Science, University of Ulsan College of Medicine). YMO has participated as a speaker at scientific meetings organized and financed by pharmaceutical companies (Handok, Pfizer Korea, GlaxoSmithKline, AstraZeneca Korea, MSD Korea, and Boehringer Ingelheim Korea) and a magazine company (Korea Doctors’ Weekly). YMO developed an educational presentation for a pharmaceutical company (Diachi Sankyo Korea). Le Thi Tuyet Lan is a respiratory adviser for GSK, AstraZeneca, Nycomed, and Boehringer Ingelheim and received honorariums for lectures and grants for attending Respiratory Conferences. Edwin K Silverman received grant support and consulting fees from GlaxoSmithKline for studies of COPD genetics. EKS received honoraria and consulting fees from AstraZeneca. SSang-Do Lee serves as a consultant to GlaxoSmithKline and Nycomed, and has participated as a speaker at scientific meetings organized and financed by various pharmaceutical companies (GlaxoSmithKline, Astra-Zeneca Korea, Nycomed and Boehringer Ingelheim). Arvind Bhome has received grant support and logistic support from Glaxo SmithKline for Asthma ARIAP_II study. The authors have no other conflicts of interest to report.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regional COPD Working Group. COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology. 2003;8(2):192–198. doi: 10.1046/j.1440-1843.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan WC, Seale P, Ip M, et al. Trends in COPD mortality and hospitalizations in countries and regions of Asia-Pacific. Respirology. 2009;14(1):90–97. doi: 10.1111/j.1440-1843.2008.01415.x. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(Suppl 5):121S–126S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 5.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest. 1991;100(2):385–388. doi: 10.1378/chest.100.2.385. [DOI] [PubMed] [Google Scholar]
- 7.Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64(1):6–12. doi: 10.1136/thx.2008.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asian Network for Obstructive Lung Disease [homepage on the Internet] Seoul: ANOLD; 2011. [cited July 25, 2011]. Available from http://www.anold.org. Accessed October 10, 2012. [Google Scholar]
- 9.Makita H, Nasuhara Y, Nagai K, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62(11):932–937. doi: 10.1136/thx.2006.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Huh J W, Chae EJ, et al. Predictors of pulmonary function response to treatment with salmeterol/fluticasone in patients with chronic obstructive pulmonary disease. J Korean Med Sci. 2011;26(3):379–385. doi: 10.3346/jkms.2011.26.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33–37. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.[No authors listed]. Guideline of respiratory function tests – spirometry, flow-volume curve, diffusion capacity of the lung. Nihon Kokyuki Gakkai Zasshi. 2004:1–56. [PubMed] [Google Scholar]
- 18.Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis (Seoul) 2005;58(3):230–242. [Google Scholar]
- 19.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103(1):57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Offcial Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 21.WHO Global database on body mass index [webpage on the Internet] Geneva: World Health Organization; 2006. [updated November 14, 2012; July 29, 2011]. Available from http://apps.who.int/bmi/index.jsp. Accessed November 14, 2012. [Google Scholar]
- 22.Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62(10):889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 25.Lee CM, Huxley RR, Lam TH, et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr. 2007;16(1):187–192. [PubMed] [Google Scholar]
- 26.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Schneider C, Jick SS, Bothner U, Meier CR. Reflux disease, gastrointestinal ulcer or weight loss in patients with COPD. COPD. 2010;7(3):172–178. doi: 10.3109/15412555.2010.481698. [DOI] [PubMed] [Google Scholar]
- 28.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 29.Seaman J, Leonard AC, Panos RJ. Health care utilization history, GOLD guidelines, and respiratory medication prescriptions in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:89–97. doi: 10.2147/copd.s8822. [DOI] [PMC free article] [PubMed] [Google Scholar]