Abstract
Human cytomegalovirus (CMV) is a leading cause of congenital infections worldwide. In the developed world, following the virtual elimination of circulating rubella, it is the commonest nongenetic cause of childhood hearing loss and an important cause of neurodevelopmental delay. The seroprevalence of CMV in adults and the incidence of congenital CMV infection are highest in developing countries (1 to 5% of births) and are most likely driven by nonprimary maternal infections. However, reliable estimates of prevalence and outcome from developing countries are not available. This is largely due to the dogma that maternal preexisting seroimmunity virtually eliminates the risk for sequelae. However, recent data demonstrating similar rates of sequelae, especially hearing loss, following primary and nonprimary maternal infection have underscored the importance of congenital CMV infection in resource-poor settings. Although a significant proportion of congenital CMV infections are attributable to maternal primary infection in well-resourced settings, the absence of specific interventions for seronegative mothers and uncertainty about fetal prognosis have discouraged routine maternal antibody screening. Despite these challenges, encouraging results from prototype vaccines have been reported, and the first randomized phase III trials of prenatal interventions and prolonged postnatal antiviral therapy are under way. Successful implementation of strategies to prevent or reduce the burden of congenital CMV infection will require heightened global awareness among clinicians and the general population. In this review, we highlight the global epidemiology of congenital CMV and the implications of growing knowledge in areas of prevention, diagnosis, prognosis, and management for both low (50 to 70%)- and high (>70%)-seroprevalence settings.
INTRODUCTION
Cytomegalovirus (CMV) is highly adapted to its human host. A full appreciation of CMV as a pathogen contributing to morbidity and mortality in a variety of immunocompromised hosts is well established. In contrast, the fact that CMV is also a leading cause of congenital infections worldwide is barely appreciated, as is the socioeconomic impact of CMV as the commonest nongenetic cause of childhood hearing loss in the postrubella era and a significant cause of neurodevelopmental delay (1–4). Indeed, CMV causes more cases of congenital disease than the combination of 29 currently screened conditions in most American states (5) and is more common than several disorders included in newborn screening in European Union countries (6).
The worldwide neglect of this problem is underscored by the continued lack of awareness of congenital CMV among health care workers and the public. The low profile of congenital CMV can be explained by the following factors. First, most maternal and newborn infections are asymptomatic and therefore are not recognized at birth. Second, sequelae from congenital CMV infection are frequently delayed in onset, at which point a retrospective diagnosis is challenging. Third, the dogma that congenitally infected children who are born to women with preexisting antibodies have normal outcomes has led to inattention to congenital CMV in developing countries. Emerging data from highly seropositive populations, which are usually in developing countries, however, suggest that not only is the rate of congenital CMV infection higher than in developed countries but it is an important cause of hearing loss in resource-limited settings (7, 8). In fact, the higher prevalence of congenital CMV infection in highly seropositive populations coupled with recent hearing outcome data from Brazil suggests that the resource-limited settings may bear the greatest burden of congenital CMV infection (7, 8). However, population-based natural history studies that accurately estimate disease, disability, and mortality burden in resource-limited settings are lacking. Moreover, there are insufficient data about the feasibility of newborn screening and antiviral therapy and the cost of long-term care for affected children in developing countries.
The quest for active and passive immunization strategies that can prevent in utero infection remains an ongoing challenge. High virus diversity and the propensity for infection with multiple different virus strains pose an important biological barrier to the development of effective vaccines (9–13). Moreover, at the population level, the fact that most congenitally infected newborns are born to mothers with preexisting immunity limits the benefit of these approaches (14, 15). Therefore, interventions that can reduce the global burden of disease are presently restricted to behavioral measures (16–18).
In this review, we highlight the global epidemiology of congenital CMV and the implications of growing knowledge in areas of prevention, diagnosis, prognosis, and management for both low (50 to 70%)- and high (>70%)-seroprevalence settings.
BIOLOGY
CMV is a host-restricted member of the Herpesviridae family of viruses (19). Primary infection is characterized by a period of active virus replication with virus shedding in saliva, urine, milk, and genital secretions, a viremic phase, and, in some, an infectious mononucleosis syndrome (19, 20). This is followed by the development of a broad immune response involving all arms of the adaptive immune system, and after several weeks, viral latency is established (19). Latent infection is characterized by either a low level or absence of detectable virus replication with the maintenance of viral genomes as episomes in CD14+ peripheral blood mononuclear cells and CD34+ and CD33+ cells in the bone marrow, which will allow subsequent production of endogenous virus (reactivation) (21, 22). Sequence variability across the large viral genome generates extensive viral strain diversity (genotypes), the biological and clinical significance of which remains unknown (11, 23). In immunocompetent mothers, reactivation of endogenous virus and/or reinfection with new strains occurs periodically, and DNAemia and viruria may be present in both (24).
EPIDEMIOLOGY AND CLINICAL OUTCOMES
CMV is a global infection, although significant differences in the seroepidemiology exist between and within countries. CMV acquisition in a population is characterized by an age-dependent rise in seroprevalence, and correlates most closely with socioeconomic level and race (25–29). As a result, up to 50% of women of child-bearing age are seronegative in industrialized countries (25, 30). In this population, CMV acquisition occurs at a rate of 1 to 7% per year (31) and usually follows frequent and prolonged contact with young children (less than 3 years of age) (31–34). By comparison, in resource-poor communities in industrialized countries and in developing countries, CMV is usually acquired very early in life owing to breast milk transmission and crowded living conditions, and far fewer adult women are seronegative (7, 35–41).
The incidence of in utero CMV infection is highly population dependent (Fig. 1) and parallels maternal seroprevalence (Fig. 2), probably due to the fact that seroprevalence rates serve as a marker for the size of the reservoir of viruses. Thus, higher seroprevalence rates lead to an increased chance of either reactivation within a host, reinfection of seropositive hosts (together these constitute nonprimary infection), or primary infection of seronegative hosts within the population. This in turn probably leads to various degrees of maternal viremia and influences the risk for subsequent placental and/or fetal infection (42). In addition, seroprevalence levels in a population may reflect variation in host and environmental factors that also influence the risk of maternal (14, 43) and vertical (27) infection. Therefore, in industrialized countries, where the maternal seroprevalence is relatively low overall, rates of congenital CMV infection average 0.6 to 0.7% of live births (1 in every 100 to 150 newborns) (27, 44). However, even within a geographic region, variable rates of CMV seropositivity in mothers from different racial, ethnic, and socioeconomic backgrounds may translate to distinct epidemiological patterns of congenital infection (26, 27, 29, 45, 46). Similarly, in developing nations with highly seropositive populations, higher rates (1 to 5%) have often been reported (7, 47–51).
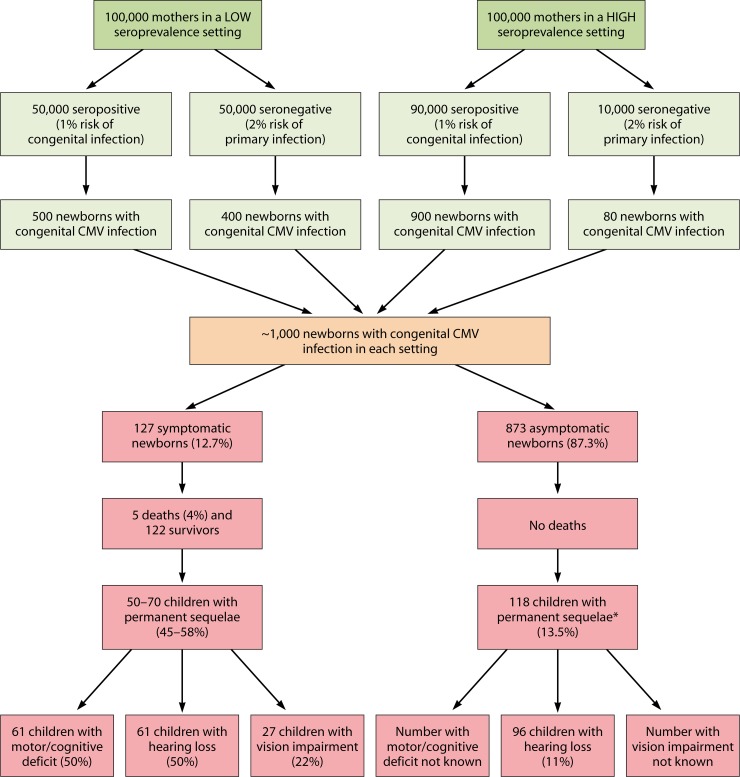
Fig 1.
Estimates of the prevalence of congenital CMV infection and sequelae in infected children in high (90%)- and low (50%)-seroprevalence settings. The following assumptions are made: the risk of primary infection is 2% in both settings, and the risk of intrauterine transmission is 40% during primary infection and 1% in CMV-seropositive mothers. The rates of sequelae are based on estimates from a systematic review of study populations from high-income countries with a range of maternal seroprevalence and congenital infection identified through universal screening (44). Proportions with each category of sequelae do not correspond to 100% because a child may have more than one complication. The figure does not take into account the effect of HIV infection in maternal populations, which would be expected to increase the risk of CMV vertical transmission and sequelae in infected infants. It also does not account for differences in congenital transmission rates observed in mothers of different racial or ethnic backgrounds. *, most of the children in the asymptomatic group will have hearing loss, and there are insufficient data to accurately estimate the number of children with cognitive/motor deficits and vision impairment.
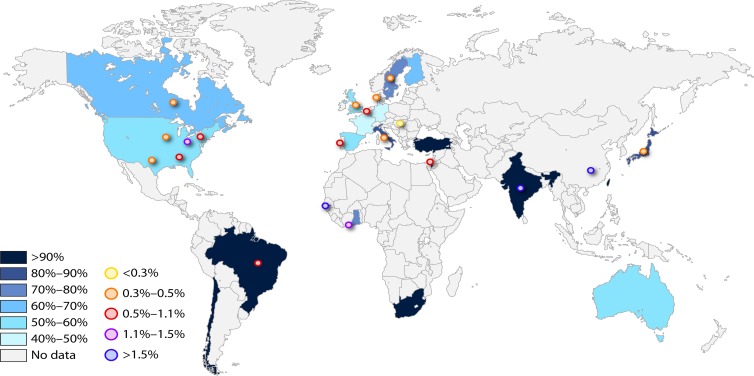
Fig 2.
Worldwide CMV seroprevalence rates among women of reproductive age and birth prevalence of congenital CMV infection. For CMV seroprevalences (shaded), percentages were obtained by adding the number of seropositive women from all studies within a given country and dividing that number by the total number of women tested. Reproductive age was generally defined as between 12 and 49 years of age. To reduce sampling variability, only countries that had at least 500 women tested were included. Studies were from Australia, Belgium, Brazil, Canada, Chile, England, Finland, France, Germany, Ghana, India, Israel, Italy, Japan, Scotland, South Africa, Spain, Sweden, Taiwan, Turkey, and the United States (26). For congenital CMV birth prevalences (circles), percentages were obtained from studies with a representative sample size (at least 1,000 newborns). To reduce detection bias, only studies using PCR or culture on saliva or urine were included, with the exception of Netherlands and Portugal, which tested DBS samples by PCR. When more than one representative study was available, percentages were obtained by adding the number of congenitally infected newborns from all studies within a given country and dividing that number by the total number of newborns tested. Countries for which maternal seroprevalence rates and birth prevalence of congenital CMV infection data were available are Brazil, Canada, England, India, Israel, Italy, Japan, Sweden, and the United States. (Adapted from reference 26.)
Most recent studies report lower transmission rates in early pregnancy (in comparison to later gestation) (52–58), with maternal primary infection leading to infection in 30 to 35% (Fig. 2) of fetuses and nonprimary infection having a transmission rate of 1.4% in study populations predominantly from industrialized countries (1.1 to 1.7%) (27). Data from screened populations indicate that while only one in 10 newborns infected in utero have obvious clinical signs of congenital infection (27, 44, 59), 10% to 15% of those without clinical findings (here referred to as having symptomatic and asymptomatic congenital CMV infection, respectively) develop long-term neurological sequelae (44). Specifically, sensorineural hearing loss (SNHL) occurs in about 35%, cognitive deficits in up to two-thirds, and death in around 4% of children with symptomatic infection. Visual impairment is thought to occur in 22 to 58% (60, 61) of symptomatic infants; however, there are insufficient data for this outcome from unbiased sampling. Far lower rates of sensory and cognitive sequelae have been reported in asymptomatic children. Hearing impairment has been reported in 7% to 10% (44, 62) of such infants, while the risk for cognitive deficits has not been studied systematically and the risk for visual impairment appears to be negligible (61). Overall (symptomatic and asymptomatic infections), permanent childhood hearing impairment is the commonest complication. In developed countries, congenital CMV accounts for 21% and 24% of cases of hearing loss at birth and 4 years of age, respectively (3, 59). Without early detection and prompt rehabilitation, this leads to speech, language, and social impairment in a significant number of children and deployment of continued medical care resources (63–66). Since newborn hearing screening may miss or underestimate hearing loss (the majority of children with CMV-associated SNHL have normal hearing at birth and develop subsequent late-onset hearing loss) and since the hearing loss is frequently progressive (50%), long-term monitoring is necessary (59, 67, 68). The public health impact of hearing loss may be even greater in high-seroprevalence settings where birth rates are substantially higher, although this has not been systematically studied to date.
The risk for long-term outcomes appears to be highest in infants born to mothers with primary infection in the first half of pregnancy (54, 55, 69–71). Following first-trimester maternal CMV infections, about a quarter of infants (20 to 25%) who are congenitally infected (Fig. 3 shows the risk of infection) will develop sensorineural hearing loss (SNHL), and 30 to 35% will suffer some form of central nervous system (CNS) sequelae (70). Since it is not possible to time nonprimary maternal infection, it is not known whether the timing of maternal infection is associated with the risk for sequelae in this group.
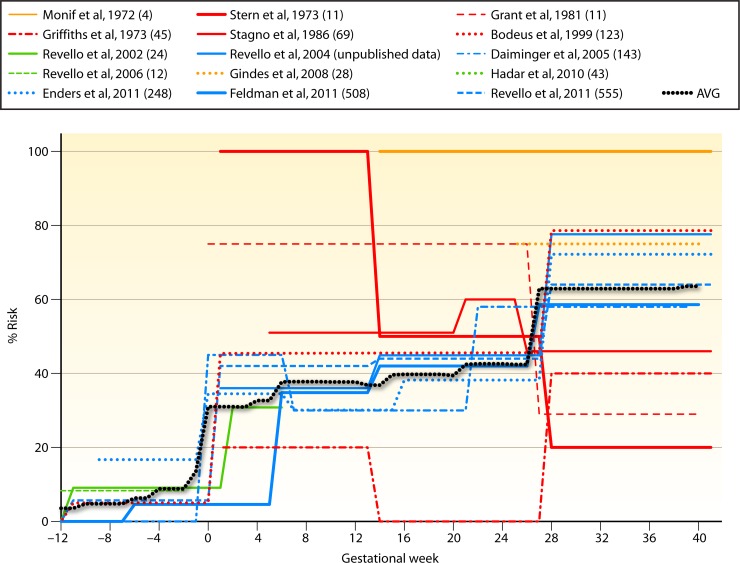
Fig 3.
Graph representing the risk of intrauterine CMV transmission following maternal primary infection from 15 studies. The transmission risk is the proportion of mothers undergoing a primary infection in a given trimester and/or the preconception period who transmitted CMV to the fetus. The risk is therefore uniform (represented by a flat line) for the time period defined as preconception (from 12 or more weeks prior to conception), first trimester (up to the 12th gestational week), second trimester (from 12 to 26 weeks), and third trimester (26 weeks to delivery) in each of the studies. Studies were grouped according to the number of weeks for which data were collected and are represented by lines of different colors: yellow, studies with late-gestation data; green, studies with preconception and/or first-trimester data; red, studies with first-, second-, and third-trimester data; blue, studies with preconception and first-, second-, and third-trimester data. The black dotted line represents pooling of the data (excluding unpublished data) for each gestational week. The denominator is the sum of mothers undergoing a primary infection from studies with data available for a particular gestational week. The numerator is the total number of transmitter mothers across these studies for that gestational week. Risks are shown as percentages. The number of women undergoing a primary infection in each study is shown in parentheses. (See references 43, 54, 55, 56, 71, 137, 143, 184, 209, 210, 211, 212, 213, and 214.)
It is estimated that more than two-thirds of infants with congenital CMV infection are born to mothers who are already CMV seropositive (14, 15, 72). Emerging observations demonstrate that the risk for symptomatic infection at birth and sequelae, especially hearing loss, in these children may be similar to that in infants born to mothers experiencing a primary infection (8, 73–77). In addition, in resource-poor settings, specific risk subgroups may exist, such as mothers with concomitant immunosuppressive chronic diseases (see below). Unfortunately, maternal and birth CMV prevalence and long-term follow-up data for congenitally infected children for many parts of the world are lacking, likely underestimating the global impact of congenital CMV infection.
Impact of the HIV Epidemic on Congenital CMV
HIV-infected women are often CMV seropositive and experience more frequent CMV recurrences with progressive immune impairment (78–80). Studies in Europe and the Americas support an increased risk for congenital CMV infection in neonates born to HIV-CMV-coinfected mothers (79, 81, 82). A French perinatal cohort that included 4,797 HIV-infected mothers between 1993 and 2004 demonstrated an increased risk for congenital CMV in HIV-infected newborns compared with HIV-negative infants (10.3% versus 2.2%). HIV-infected newborns also had a 3-fold-higher risk for symptomatic congenital CMV infection than uninfected newborns (23% versus 6.7%). Furthermore, CMV may act as a cofactor for HIV disease progression. The risk for infant mortality is increased in HIV-CMV-coinfected infants, and there is accelerated progression of CNS disease in survivors, especially developmental delay and worsening motor deficits (83, 84).
The French perinatal cohort study also showed that in the era of highly active antiretroviral therapy (HAART), the incidence of vertical CMV infection in HIV-positive mothers was falling, which was associated with improvements in CD4 count (81). However, in a more recent study, Frederick et al. have not observed a significant decrease in the prevalence of congenital CMV infection in children of HIV-infected mothers receiving prenatal antiretroviral therapy (85). The overall prevalence of congenital CMV infection in that study was 3.6%.
In resource-limited settings, the high rate of coinfections in pregnant women with HIV-1 and bacterial and parasitic pathogens likely influences the transplacental transmissibility of CMV (51, 79, 86–88). In sub-Saharan Africa, the burden of HIV-1 in women of reproductive age is alarming, reaching 40% in some regions (89). Despite improvements in, and access to, antiretroviral therapy, maternal HIV acquisition and mother-to-child transmission (MTCT) of HIV in developing countries continue, leading to a sizeable proportion of infants born HIV exposed or infected. In studies of HIV-1-infected and HIV-1-exposed Kenyan women and children, a strong correlation between CMV and HIV loads was observed in both mothers and infants (88), with CMV DNAemia in the mother being associated with an increased risk of maternal mortality and mortality in the HIV-infected infants by 24 months (88).
To our knowledge, there are no published data on the risk of transmission of CMV in HIV-positive mothers in resource-limited settings. Therefore, in order to illustrate the potential impact of HIV infection on congenital CMV infection, we extrapolate from findings in high-resource settings and use South Africa and Thailand as examples (Table 1). In South Africa, maternal HIV seroprevalence is about 30%, with the most recently reported overall HIV MTCT at around 3.5% (90). Assuming a 1%, 3%, and 10% (79, 81, 85) risk of CMV transmission in HIV-unexposed (n = 700,000), HIV-exposed uninfected (n = 265,000), and HIV-infected (n = 35,000) newborns, respectively, we estimate that around 18,450 newborns (an excess of 8,450 infected newborns due to maternal HIV) are born congenitally infected with CMV each year. The number of cases in South Africa equates to just over 40% of the total annual number of congenital CMV cases in the United States. In other words, South Africa is likely to have roughly 2.5 times the number of congenital CMV infections per capita as in the United States. In Thailand, which has an annual birth rate of 830,000 and a maternal HIV seroprevalence of 0.7% (91), assuming the above congenital CMV transmission risks and an HIV MTCT of 2.8% (T. Naiwatanakul, N. Punsuwan, N. Kullerk, W. Faikratok, R. Lolekha, and O. Sangwanloy, presented at the 5th International AIDS Society Conference on HIV Pathogenesis and Treatment, Cape Town, South Africa, 19 to 22 July 2009), we estimate that 8,427 newborns are born congenitally infected with CMV each year.
Table 1.
Estimates of the prevalence of congenital CMV infection in two resource-poor settings (South Africa and Thailand) according to maternal HIV-CMV coinfection
| Parameter | South Africa | Thailand |
|---|---|---|
| Annual birth rate | 1,000,000 | 830,000 |
| Antenatal HIV prevalence (%) | 30 | 0.7 |
| HIV perinatal transmission rate (%) | 3.5 | 2.8 |
| No. (no. of congenital CMV infections) | ||
| HIV unexposed (risk, 1%) | 700,000 (7,000) | 824,190 (8,242) |
| HIV exposed (risk, 3%) | 265,000 (7,950) | 5,647 (169) |
| HIV infected (risk, 10%) | 35,000 (3,500) | 163 (16) |
| Total no. of congenital CMV infections | 18,450 | 8,427 |
ADVANCES IN DIAGNOSIS AND MANAGEMENT OF THE NEWBORN
The majority of congenital CMV infections from both resource-poor and upper-income settings are asymptomatic at birth, and the diagnosis of intrauterine infection relies on virus detection by culture-based methods or PCR. Saliva or urine (see below) specimens should be obtained within the first 2 weeks of life (92), as virological testing cannot discriminate intrauterine from postnatal CMV infection beyond 2 weeks. When an early specimen is not available for testing, clinical features highly indicative of congenital CMV infection, such as CNS, retinal, or auditory findings, can suggest the diagnosis in symptomatic infants.
The presence of CNS disease in the symptomatic neonate with laboratory-confirmed congenital infection warrants the consideration of specific antiviral therapy. While there is no evidence for the effectiveness of treatment in children without CNS disease, it is reasonable to consider antiviral treatment in those with disseminated disease which is life threatening (93–95). Ganciclovir (GCV) or its prodrug valganciclovir (VGCV), an acyclic nucleoside analogue, is the preferred antiviral agent for the treatment of CMV disease (96). The efficacy of ganciclovir for the prevention of progressive hearing loss in infants with proven congenital CMV CNS disease as evidenced by microcephaly, other neurological findings, neuroimaging abnormalities, or hearing loss was evaluated in a randomized trial nearly a decade ago. At 12 months of follow-up, a considerably higher rate of preserved normal hearing, as well as improved hearing and prevention of worsening of hearing in those with a baseline hearing deficit, was demonstrated following a 6-week course of intravenous GCV, compared with no therapy (95). However, the frequency of drug toxicity, the absence of a placebo group, and high attrition rates in this study limit the significance of the findings. More recently, a secondary analysis on the same study population showed that infants who received GCV therapy appeared to have fewer developmental delays at 6 and 12 months than untreated infants (97). Based on these findings, a 6-week course of intravenous ganciclovir or oral valganciclovir (VGCV) is considered for children with CNS involvement (93, 98). Pharmaceutical liquid preparations of VGCV provide stable systemic exposure, and plasma levels equivalent to those for intravenous therapy can be achieved; however, a head-to-head comparison of efficacy has not been performed (98, 99). Rates of neutropenia during a 6-week course are, however, substantial (63% for ganciclovir and 38% for valganciclovir) (99), and biochemical/hematological parameters should be carefully monitored when either drug formulation is used (93). Such toxicity also precludes the treatment of neonates with asymptomatic infection because their risk of longer-term sequelae is only about 13% (44).
It has been suggested that ongoing viral replication in end organs may contribute to adverse long-term outcomes (progressive hearing impairment was reported for 21% of treated patients) (95), and prolonged antiviral therapy has been considered. A recent retrospective study of 6 weeks of intravenous GCV followed by VGCV up to a year showed that prolonged antiviral therapy may prevent hearing loss in children with normal baseline hearing and result in lower rates of deterioration in those with baseline deficits (100). However, these findings were limited by the absence of a control group. It is anticipated that the randomized multicenter placebo-controlled trial (CASG112) (NCT00466817) commenced in 2008 to compare the clinical benefit of 6 weeks versus 6 months of valganciclovir in symptomatic infants will define the role of prolonged antiviral therapy. A role for antiviral therapy in the prevention of SHNL and adverse psychomotor outcomes in asymptomatic infants has also been suggested (101). However, formally evaluating a toxic drug in a large cohort of asymptomatic children, most of whom will not go on to develop sequelae, remains problematic.
The prognostic value of clinical signs, imaging findings, and laboratory parameters in the newborn with confirmed congenital CMV has been extensively evaluated. Among infants with symptomatic congenital CMV infection, microcephaly (102, 103), chorioretinitis (102), abnormal neurological examination findings (102, 104), abnormal auditory brain stem evoked response (105), and petechiae and thrombocytopenia (104, 105) are each associated with an unfavorable clinical outcome. Furthermore, newborn neuroimaging (ultrasound [US], computed tomography [CT], and magnetic resonance imaging [MRI]) abnormalities (105, 106) carry a high risk for CNS sequelae. In asymptomatic infants, on the other hand, clinical or laboratory predictors of adverse outcomes have not been identified. However, low CMV blood viral loads (<103 copies/105 polymorphonuclear leukocytes) appear to predict normal development with reasonable certainty (>95%) (107–110). In the absence of well-defined predictors of outcome, monitoring of all congenitally infected newborns is advised. This includes regular neurological, developmental, auditory, and visual assessments at least until school age in symptomatic newborns, whereas recommendations for follow-up of asymptomatic newborns are usually restricted to audiology. Regular monitoring for progressive and late-onset deficits permits early rehabilitation (93, 94). At 1 year of follow-up, the absence of neurodevelopmental delay appears to predict a normal intellectual outcome (111).
Owing to the late presentation of most congenital CMV sequelae, diagnosing vertical infection in children beyond the newborn period is a key challenge for both the clinician and the epidemiologist. Dried blood spots (DBS), or Guthrie cards, which are collected routinely at birth in certain countries for newborn genetic and metabolic diseases screening, can be stored for extended periods of time. Since CMV DNA is stable on such DBS cards for up to 18 years, they offer an attractive tool for the retrospective molecular diagnosis of congenital infection in individual children who present with delayed-onset sequelae (112–115). DBS also have appeal for use in newborn congenital CMV screening programs. However, the variable and disappointing rates of detection of CMV DNA (34 to 100%) have made DBS unsuitable for these purposes (112, 114, 116–122), possibly because not all newborns are viremic at birth or due to technical factors (118, 122–124). However, a positive DBS PCR finding is diagnostic of congenital CMV infection and accordingly can be useful to retrospectively diagnose congenital CMV infection beyond the neonatal period.
The recent demonstration that real-time PCR detection of CMV in saliva swabs, either air dried or in viral transport medium, is equally sensitive as virus culture techniques has made wide-scale newborn screening realizable (125). Universal newborn CMV screening would identify infants at risk for hearing loss, who can then be targeted for prompt interventions that prevent significant speech and language deficits (44, 126). However, the cost of testing, the modest efficacy of available antiviral therapy, the high proportion of asymptomatic infections, and potentially adverse psychosocial effects are considered barriers to implementation, even in countries with newborn screening programs for the detection of genetic and metabolic disorders and hearing loss (126, 127). In spite of these issues, newborn virological screening can be justified on the grounds that congenital CMV infection is likely the most common nongenetic cause of sensorineural hearing loss and screening can now be undertaken noninvasively (125). In addition, the delayed onset of most cases of CMV-associated hearing loss makes newborn hearing screening an inadequate tool for the detection of CMV-associated hearing loss (67, 68). In resource-limited settings, reliable estimates of prevalence and disease burden from congenital CMV infection are needed before the cost-effectiveness and utility of newborn CMV screening can be determined.
ADVANCES IN PREVENTION OF ADVERSE OUTCOMES
Prenatal Screening and Diagnosis of Infection in the Mother and Fetus
Maternal (prenatal) screening may permit early identification of at-risk pregnancies or infected infants and thus the use of interventions to reduce morbidity has attracted increasing interest in recent years (128). The feasibility of prenatal screening has been argued on the basis that eight European countries have overcome commonly cited obstacles to this strategy (129, 130). However, universal antibody screening of pregnant women in most resource-rich countries has not been recommended because of the absence of proven specific interventions for maternal primary infection, and challenges in deciphering the prognosis of an individual mother and fetus have been discouraging. It is also becoming increasingly apparent that at a population level, the effectiveness of such prenatal screening programs will be limited, as around two-thirds of infants with congenital CMV infection in the United States and the vast majority in resource-limited settings are born to women who are seropositive preconceptionally (14, 15). In these settings, it has been assumed that reactivation of endogenous virus or reinfection with a different strain leads to intrauterine transmission (9, 13). However, since such events are clinically silent and simple virological or immunological markers for nonprimary infection do not exist, identifying women at risk of transmission is presently not possible.
Clinical suspicion of maternal primary infection, i.e., glandular fever or flu-like illness, and the detection during routine ultrasound screening of abnormalities suggestive of intrauterine CMV that lack an apparent cause are the common indications for specific diagnostic testing (131). Maternal primary infection can be confirmed reliably by the demonstration of seroconversion (CMV IgG negative to CMV IgG positive) when a baseline serum sample from either the earliest antenatal visit or prior to conception is available (Fig. 4). When such a comparison serum is not available, the detection of both CMV IgG and IgM antibodies may indicate a recent primary infection (132). However, as a reactive CMV IgM may be found in both primary and nonprimary infections and may persist for many months following primary infection, it does not reliably predict the risk for congenital infection (133). Therefore, a reactive CMV IgM should be further evaluated by determining the maturity of the CMV IgG antibodies using the avidity assay. Low-affinity CMV IgG antibodies (those that bind less tightly with their target protein) are produced in the first 18 to 20 weeks after infection (134). A subsequent maturation process generates IgG antibodies with higher avidities (affinity maturation). A high CMV IgG avidity index therefore excludes a recent primary infection and when detected before 12 to 16 weeks of gestation indicates a significantly lower risk of congenital infection (134, 135). Conversely, low-avidity IgG antibodies together with a reactive CMV IgM strongly supports the diagnosis of maternal primary infection in the preceding 3 or 4 months (136).
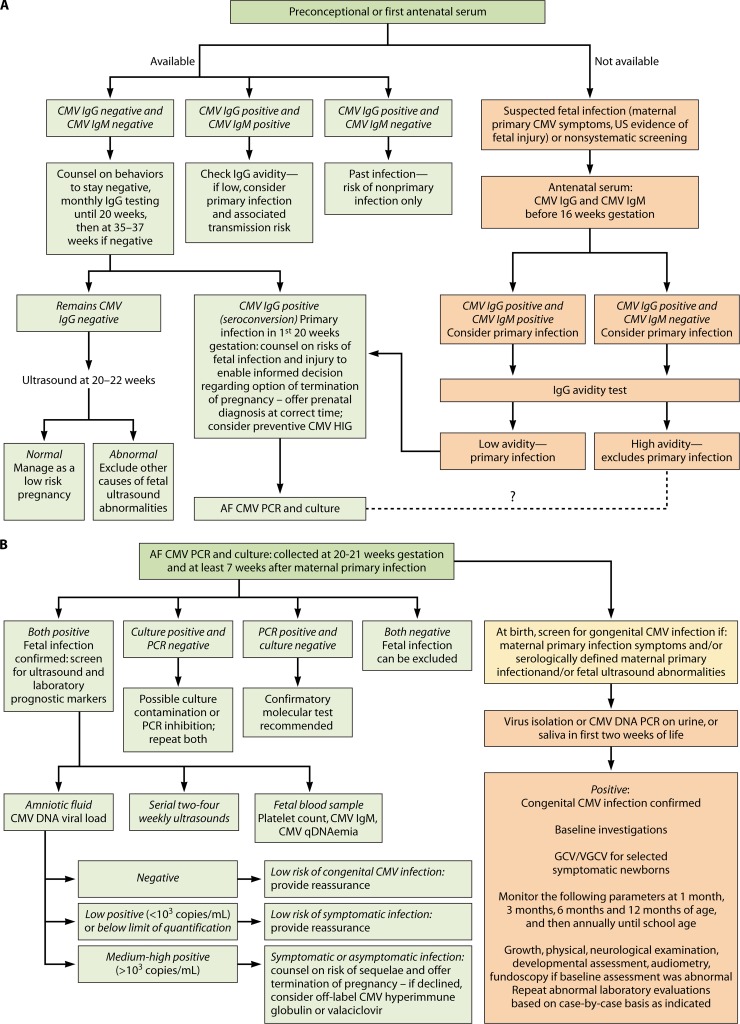
Fig 4.
Proposed diagnostic and management algorithm for maternal and congenital CMV infection. The presence of high-avidity CMV IgG antibodies before 16 weeks of gestation excludes primary infection; however, nonprimary infection is still a possibility. Indications for prenatal testing in nonprimary infections are less clear, and decisions should be made on case-by-case basis when sonographic findings are suggestive of congenital infection. Baseline investigations for newborns with symptomatic congenital CMV infection should include complete blood count, liver function tests, CMV real-time PCR (blood and urine), audiometry, ophthalmology screen, and cranial US/CT/MRI. A low CMV DNA blood viral load in the first month of life can predict a normal development in asymptomatic newborns. Since the cutoff values for amniotic fluid viral load measurements were derived from a few studies and have not been validated with international standards, they may not be generalizable.
The substantial risk of vertical transmission following primary maternal infection justifies invasive prenatal testing. Amniotic fluid (AF) CMV PCR is the test of choice for confirming fetal infection. As the interval between maternal and detectable fetal infection is at least 6 to 8 weeks, amniocentesis should be performed at 20 to 21 weeks of gestation and at least 7 weeks following maternal infection (69, 137–143). It is well established that the sensitivity of PCR (70 to 90%) for prenatal diagnosis is superior to that of virus culture techniques; when correctly timed, it approaches 100% (137, 143). However, as PCR may occasionally give false-positive results, it is generally recommended that screening be performed using a combination of PCR and virus culture or, where culture-based testing is not available, a second (confirmatory) molecular test (139, 144, 145) (Fig. 4). When both PCR and virus isolation tests are positive, congenital infection can be diagnosed with 100% certainty. On the other hand, when both tests are negative, fetal infection can be ruled out with a high degree of certainty (negative predictive value, >94%) (42). False-negative culture and PCR results have occasionally been reported and may be a result of delayed transmission of CMV to the fetus (69, 146, 147). Invasive prenatal testing may also be justified in nonprimary infections when sonographic findings suggest in utero CMV abnormalities (77). However, at present, firm guidelines in this area are lacking.
In the case of confirmed fetal infection, since a significant proportion of infected infants have a normal outcome, parents should be counseled on the established risks of symptomatic infection and long-term morbidity following intrauterine CMV infection, in order to guide decision-making regarding the options of termination of pregnancy (TOP) or expectant management (131), while intrauterine therapies (discussed below) remain experimental. In the absence of virological correlates or biomarkers that can definitively distinguish a symptomatic from an asymptomatic course of infection, defining the prognosis for an infected fetus may be aided by 2 to 4 weekly fetal ultrasound (US) examinations and appropriately timed (see above) amniotic fluid viral load testing (131). It has been shown that cerebral ultrasound abnormalities are strongly associated with a poor prognosis (148), and recent findings also show that combining ultrasound with magnetic resonance imaging improves the sensitivity of prenatal screening for cerebral lesions, in particular, after 30 to 34 weeks of gestation (149, 150). On the other hand, the predictive value of nonspecific findings, such as intrauterine growth restriction (IUGR), bowel hyperechogeneity, or isolated other noncerebral abnormalities, for symptomatic infection or adverse outcomes is relatively low (69, 148, 151). Amir et al. suggested that lenticulostriate vasculopathy (LSV) is a possible marker of hearing loss in congenital CMV infection (152). However, LSV is nonspecific, and other studies have not confirmed the prognostic value of this finding (148, 153). When no ultrasound findings are detected, the risk for symptomatic congenital infection and sequelae is significantly reduced, but these cannot be excluded (69, 139, 149, 151, 154).
Several studies suggest that low AF virus loads can provide reassurance for lower risks of both symptomatic infection and long-term sequelae (42, 140, 143, 155, 156). Although an association between high AF virus loads and symptomatic infection at birth has been documented in some studies (42, 140, 155), other studies have failed to show such an association (146, 157, 158). Virus load was also found to correlate with gestational age (146, 157). It is important to bear in mind that in the absence of international PCR quantification standard, the different assays deployed in these studies would have suffered from substantial inter- and intralaboratory variations, making the use of predictive cutoff values less generalizable. In addition, differing study designs make it difficult to compare the data among the various studies. The recently approved first WHO international standards for CMV PCR will reduce this variability and should be used to reevaluate the prognostic role of AF virus levels in future multicenter studies (159). Even if the predictive role for low AF virus load in symptomatic disease and sequelae is confirmed, these invasive diagnostics are beyond the reach of most public health systems in low-income countries, and therefore it is unlikely that the diagnosis and treatment of in utero CMV infection will become part of routine obstetric practice in these settings.
Antiviral Therapy and Passive Immunization
The results of ongoing controlled trials involving oral valaciclovir (NCT01037712), and CMV hyperimmune globulin (HIG) (NCT00881517) for prenatal intervention are awaited (160). Ganciclovir cannot be used for prenatal therapy due to its mutagenic potential in animals, but oral valaciclovir administered to mothers with evidence of fetal infection appears to be safe and decreases the circulating fetal viral load (161). However, evidence for improved outcomes with treatment has yet to be demonstrated.
The rationale for passive immunization of seronegative mothers comes from the observed lower risk of fetal infection in mothers with preexisting antibodies (162). This is further supported by evidence that CMV HIG can inhibit viral spread in vitro (163, 164), restore placental health in mothers with primary infection (165), and lead to regression of cerebral ultrasound abnormalities (166). A prospective study has demonstrated that monthly intravenous infusions of CMV HIG to mothers with confirmed primary infection (including those with virological evidence of fetal infection) are safe and can both prevent (adjusted odds ratio [OR], 0.32) and treat (adjusted OR, 0.02) fetal infection (167). Furthermore, recent retrospective studies have suggested that CMV HIG can protect against poor outcomes in infants (168, 169). In spite of these promising findings, though, a recent Cochrane Library Review underscored the lack of data from randomized controlled studies and accordingly the need for further research to assess the efficacy of antenatal interventions for the prevention of intrauterine transmission and adverse outcomes (170). Therefore, the results from two randomized controlled trials of CMV HIG that are under way (NCT00881517 and NCT01376778) should be awaited to confirm the effect on transmission or prevention of disease. Regardless of the outcome of these studies, since most seropositive individuals appear to have high levels of antiviral antibodies (as a result of boosting following frequent reactivation and/or reinfection), it can be inferred that CMV HIG will have little to no role in high-seroprevalence populations.
Maternal Antiviral Immune Responses and Intrauterine Transmission
CMV infection and risk of transmission to the fetus are intimately linked to immunity, although the temporal appearance and quality of the humoral and T-cell-mediated responses against CMV during primary and nonprimary maternal infection remain incompletely understood.
The importance of immune responses in protecting against intrauterine transmission of CMV is borne out by the significantly decreased risk of congenital infection in infants born to women who were seropositive prior to pregnancy (∼1%) in contrast to those with primary infection during pregnancy (∼30%) (14, 27) and the beneficial effects of administering hyperimmune globulin (HIG) in women with primary infection during pregnancy (167). The observation that differential levels of neutralizing anti-glycoprotein B titers exist at the time of delivery in transmitter and nontransmitter mothers undergoing a primary infection also supports the role of the humoral arm of the immune system in modulating intrauterine transmission of CMV (171). The gB protein is relatively well conserved among different virus strains and is considered the major target of the neutralizing antibody response to CMV (172). Recent data, however, suggest that the pentameric complex comprising gH, gL, UL128, UL130, and UL131 is the most important antigenic complex for neutralizing antibody responses (173). High titers of neutralizing antibody are thought to protect against transmission by blocking receptor-mediated transcytosis of CMV in the placenta (174) and by reducing viral replication (87). It will be interesting to investigate the temporal appearance of humoral responses against the pentameric glycoprotein complex in both primary and recurrent infections in pregnant women and to determine whether they are a potential marker for risk of transmission and/or congenital CMV disease.
There is increasing evidence in transplant recipients that high levels of virus replication and disease are associated with the suboptimal quality of the T-cell response against CMV (175, 176). In addition, in healthy adolescents, it has been shown that the plasma CMV DNAemia was still evident despite the detection of a strong neutralizing antibody response within 6 to 8 weeks following primary infection, although lymphoproliferative responses were weak (177). Therefore, cellular immunity is indispensable during the acute phase of infection, as well as for the control of chronic infection and the prevention of reinfection (10, 178–180). Although a range of CMV proteins are targeted by the CD4 and CD8 immune system (181), major targets include the pp65 tegument protein and the IE1 antigen (and to a lesser extent gB) (182). In pregnant women experiencing primary infection, the evolution of the lymphoproliferative response has been shown to be relatively slow until a memory T-cell response develops (183). The cytokine profile of these T cells is dominated by gamma interferon producers, with relatively little interleukin-2 (IL-2) production. In mothers who experienced primary infection and who transmitted the virus to their fetuses, CMV CD4+ T-cell responses appear to be delayed and of lower frequency, and there were lower levels of CMV CD45RA+ cells in mothers who transmitted CMV to their fetuses (183, 184). In seropositive pregnant women, it has been shown that naive CD8+ T cells were reduced by 50%, with the CD45RA effector population showing a more highly differentiated state (CD27 and CD28 low) while the CD45RA+ revertant memory cell population was expanded and was composed mainly of CMV-specific cells (185).
Notwithstanding these data, the precise components of protective immune responses against intrauterine transmission of CMV in women experiencing primary infection and in seropositive women remain to be defined and undoubtedly will contribute to the development of a successful vaccine.
Vaccines
The economic impact of congenital CMV was assessed by the Institute of Medicine nearly a decade ago. They estimated that the costs of medical and educational care for the thousands of children with asymptomatic and symptomatic congenital infection in the United States amounted to $1.9 billion per year, whereas the investment needed to develop a CMV vaccine would be approximately $360 million. The Institute of Medicine accordingly ranked the development of a CMV vaccine as the highest priority (4). Knowledge that CMV exhibits a high level of molecular diversity and carries an extensive array of virus immune evasion genes is increasing (10, 23, 186, 187). Consistent with this, it has been demonstrated that infection within a host can occur with multiple virus strains concomitantly, including at the time of initial infection, or sequentially (10, 23, 186, 187). Broad and cross-neutralizing cellular and humoral responses have therefore become a major goal of vaccine design (188). Whereas the traditional focus of CMV vaccines has been the prevention of primary maternal infection, this view has been challenged by recent data demonstrating that nonprimary infection drives most congenital infections, and that the rates of symptomatic infection at birth and hearing loss are similar in infants infected following primary and nonprimary maternal infections (7, 8, 74).
A recent phase II trial evaluated the efficacy of a recombinant genetically modified gB protein in a novel adjuvant, MF59 (189), in seronegative women and found a modest (∼50%) reduction in the rate of primary maternal infection in the vaccinated group compared to the placebo group (190). However, this protection was observed predominantly within the first year after immunization. Although boosting of both antibody and CD4 T-cell responses by the gB vaccine was also demonstrated in CMV-seropositive women, whether such boosting will provide protection against nonprimary infection in mothers with preexisting immunity is not known (191). The same vaccine deployed in patients awaiting solid organ transplantation (the cohort consisted of seropositive and seronegative patients) was immunogenic and reduced the duration of viremia in patients with CMV infection posttransplantation (192).
Several proof-of-concept studies of various candidate vaccines have also been conducted in recent years. A two-component alphavirus replicon vaccine containing gB and a pp65/IE1fusion protein has shown to be immunogenic in phase I clinical trials. In seronegative subjects, the vaccine elicited neutralizing antibodies and multifunctional T-cell responses (193), and it also boosted T-cell responses in CMV-seropositive renal transplant patients (194). A DNA vaccine comprising both gB and pp65 has also undergone phase I studies and a placebo-controlled phase II trial in stem cell transplant recipients. Although there was no difference in the number of vaccine and placebo recipients who received CMV-specific antiviral therapy, a significant reduction in the incidence and recurrence of DNAemia was seen (195). More recently, combining gB with a Toll-like receptor 9 (TLR9) agonist has produced durable polyfunctional cellular and cross-neutralizing humoral responses in transgenic mice (196). While there is some evidence for protection against nonprimary infection in these studies, evaluation of these candidate vaccines for the prevention of maternal and congenital infection seems to be far in the future. Even in low-seroprevalence settings, where vaccination of seronegative mothers could be cost-effective, it is unclear, in light of emerging findings on the epidemiology of congenital CMV, whether a CMV vaccine would provide substantial reductions in morbidity.
Behavioral Measures
In the absence of effective immunization strategies, the restriction of maternal infection relies predominantly on behavioral measures such as frequent hand washing after exposure to young children's body fluids and avoiding intimate contact with young children (197). Children, when infected vertically or in the first few years of life, can shed virus in urine and saliva for many years either continuously or intermittently (108, 198–200). CMV therefore spreads readily in settings where preschool children are concentrated, with fomites on wet absorbent surfaces most able to harbor viable viruses (33, 201). This places seronegative pregnant women who work in child care centers or who have a young child in the home or in day care at increased risk of seroconversion (31, 32, 34, 202). Accordingly, specific advice to seronegative women on measures that interrupt child-to-mother transmission has been shown to be effective (16, 18, 203). Besides contact with young children, sexual transmission from a seropositive male partner is an additional established route by which women may be infected with CMV (25, 160, 197, 204–207). It is quite likely that these modes of transmission are also responsible for reinfection of seropositive mothers with new or different virus strains. Indeed, sexual transmission probably frequently results in maternal reinfection in high-seroprevalence populations, where young women often report multiple sex partners and unsafe sex practices. However, the contribution of sexual and child-to-mother transmission to maternal reinfection in these settings remains to be virologically documented. Moreover, the relative role of reinfection compared with reactivation in delivering a child with CMV is also unknown. It is therefore difficult to speculate on the impact of behavioral changes in resource-limited settings. On the whole, promoting education and awareness of congenital CMV infection and ways to avoid exposure for all prospective mothers remains a key health educational objective (160, 208).
CONCLUSIONS
Congenital CMV is a major cause of disability in children, with little evidence for change in disease burden over time in high- and middle-income countries despite large scientific and clinical advances in the CMV field. This results from a general neglect of the problem, contributed to by the absence of clinical disease at birth in the majority of babies who develop complications and the lack of safe and effective antiviral therapy to prevent or reduce sequelae in most children with congenital CMV infection. Therefore, prevention of maternal infection and transmission is the main priority. Vaccines may offer protection against primary infection, and the efficacy of vaccines in mothers following nonprimary infection should be assessed.
The neglect of congenital CMV infection in the developing world reflects not only delayed onset of sequelae but also competing health priorities in such populations. Given that early detection of hearing loss can limit long-term disabilities, PCR-based newborn screening to identify those at risk of sequelae deserves consideration. However, it would be premature to consider newborn CMV screening in resource-poor settings because the disease burden from congenital CMV and the cost/benefit ratio of long term follow-up have not been defined. In addition, the cost and the competing health priorities for these settings make it difficult to envision such a screening program. While studies to define the disease burden should be undertaken as a matter of urgency, for the present, raising awareness of congenital CMV should be prioritized.
ACKNOWLEDGMENTS
We gratefully acknowledge Patrick Lane and Rajesh Narotam for assistance with the figures. Thanks also go to Dave le Roux, Michael Harrison, and Raveen Parboosing for reading an earlier version of this paper and to Noelle Nicholls for editorial assistance.
Biographies

Sheetal Manicklal is a Registrar in Medical Virology at the University of Cape Town, with a particular interest in vertically transmitted infections. She recently initiated a collaborative project to better understand the interrelationship between congenital CMV and HIV-1 and to define the disease burden in South Africa.

Vincent Emery is Pro-Vice-Chancellor (International Relations) and Professor of Translational Virology at the University of Surrey and holds an honorary Professorship of Virology at University College London (UCL). He started his scientific career as a biochemist but has been a virologist for the last 27 years. His current research aims to provide a holistic approach to understanding viral infections in immunocompromised hosts such as HIV-infected patients and transplant recipients. His particular interests have been focused on cytomegalovirus in solid organ and stem cell transplant recipients by combining viral replication measures with assessment of the immune response and mathematical biology to improve patient management. During his career, he has obtained in excess of £14.4 million of grant money from government agencies in the United Kingdom and the United States, charitable organizations, and the private sector. In addition, Professor Emery is also a named inventor on 5 patents in the area of biotechnology and molecular diagnostics, is a member of a UCL-Imperial College nanotechnology consortium funded by a £1.7-million grant from the EPSRC to develop novel nanodiagnostics for HIV, and is part of a team of researchers from UCL and OJ-Bio who have secured NIHR i4i funding of £1 million to develop novel point-of-care HIV diagnostics. Professor Emery has published in excess of 200 research articles, reviews, and books, including a “Pocket Guide to Cytomegalovirus” and “A Patient's Guide to Cytomegalovirus” published in 2009 and “A Spotlight on Cytomegalovirus Infection and Disease” as part of the Lectures in Transplantation series, published in 2010.

Tiziana Lazzarotto graduated with a degree in Biological Science from the University of Bologna and received scientific training for her specialty degree in Microbiology and Virology at the University of Bologna (Italy). She is Associate Professor of Microbiology and Clinical Microbiology, School of Medicine, Alma Mater Studiorum-University of Bologna (Italy). She works at the Operative Unit of Clinical Microbiology at St. Orsola Malpighi University General Hospital, Bologna, and she is the head of the Laboratory of Virology. For over 15 years, she has an expertise in the field of virology with specific reference to diagnosis and management of congenital human cytomegalovirus (CMV) infection. In particular she has made significant contributions to knowledge of the immune response to CMV, study on the pre- and postnatal diagnosis of congenital CMV infection, and identification of prognostic markers for in utero transmission.

Suresh Boppana is a Professor of Pediatrics and Microbiology at the University of Alabama at Birmingham and has been studying the natural history and pathogenesis of maternal and congenital cytomegalovirus (CMV) infection for the past 20 years. Dr. Boppana's work challenged the dogma that children with congenital CMV infection born to women with primary CMV infection during pregnancy experience most of the disease burden from this intrauterine infection. He has shown that CMV reinfections occur frequently in healthy seropositive women and that such reinfections could lead to intrauterine infection, symptomatic disease, and sequelae. His work with collaborators in Brazil and India is beginning to document the impact of congenital CMV infection in highly seropositive settings, including developing countries. He is currently the principal investigator of large multicenter study to define the contribution of congenital CMV infection to overall hearing loss and to develop diagnostic methodologies that can be used to screen large number of newborns. The findings from this study, demonstrating low sensitivity of the dried blood spot PCR assay and the development of a highly sensitive and specific saliva real-time PCR assay for congenital CMV infection, have been published in the Journal of the American Medical Association and the New England Journal of Medicine, respectively. His current research is focused on understanding the pathogenesis of CMV-associated hearing loss. He mentored several undergraduate, graduate, and postdoctoral trainees. He served as a consultant member of several NIH study sections.

Ravi Gupta is a consultant in infectious diseases at University College Hospital London and has a particular interest in viral infections. His research group studies host-virus interactions and HIV drug resistance. He is a member of the WHO HIV global surveillance resistance committee. His portfolio is broad, from teaching about HIV and viral hemorrhagic fever in the tropics to working on retroviral latency and HIV cure as part of the multicenter United Kingdom collaboration CHERUB.
REFERENCES
- 1. Demmler-Harrison GJ. 2009. Congenital cytomegalovirus: public health action towards awareness, prevention, and treatment. J. Clin. Virol. 46(Suppl. 4):S1–S5 [DOI] [PubMed] [Google Scholar]
- 2. Jeon J, Victor M, Adler SP, Arwady A, Demmler G, Fowler K, Goldfarb J, Keyserling H, Massoudi M, Richards K, Staras SA, Cannon MJ. 2006. Knowledge and awareness of congenital cytomegalovirus among women. Infect. Dis. Obstet. Gynecol. 2006:80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morton CC, Nance WE. 2006. Newborn hearing screening—a silent revolution. N. Engl. J. Med. 354:2151–2164 [DOI] [PubMed] [Google Scholar]
- 4. Stratton KR, Durch JS, Lawrence RS. (ed). 2000. Vaccines for the 21st century: a tool for decisionmaking. National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 5. Anonymous. 2008. Impact of expanded newborn screening—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 57:1012–1015 [PubMed] [Google Scholar]
- 6. de Vries JJ, Vossen AC, Kroes AC, van der Zeijst BA. 2011. Implementing neonatal screening for congenital cytomegalovirus: addressing the deafness of policy makers. Rev. Med. Virol. 21:54–61 [DOI] [PubMed] [Google Scholar]
- 7. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, Britt WJ. 2009. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin. Infect. Dis. 49:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto AY, Mussi-Pinhata MM, Isaac Mde L, Amaral FR, Carvalheiro CG, Aragon DC, Manfredi AK, Boppana SB, Britt WJ. 2011. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr. Infect. Dis. J. 30:1043–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366–1371 [DOI] [PubMed] [Google Scholar]
- 10. Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. 2010. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pignatelli S, Dal Monte P, Rossini G, Landini MP. 2004. Genetic polymorphisms among human cytomegalovirus (HCMV) wild-type strains. Rev. Med. Virol. 14:383–410 [DOI] [PubMed] [Google Scholar]
- 12. Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. 2010. Cytomegalovirus reinfections in healthy seroimmune women. J. Infect. Dis. 201:386–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliveira Pde F, Duarte G, Britt WJ. 2010. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am. J. Obstet. Gynecol. 202(3):297.e291-298 doi:10.1016/j.ajog.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stagno S, Pass RF, Dworsky ME, Henderson RE, Moore EG, Walton PD, Alford CA. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945–949 [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Zhang X, Bialek S, Cannon MJ. 2011. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin. Infect. Dis. 52:e11–13 [DOI] [PubMed] [Google Scholar]
- 16. Adler SP, Finney JW, Manganello AM, Best AM. 1996. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. Pediatr. Infect. Dis. J. 15:240–246 [DOI] [PubMed] [Google Scholar]
- 17. Picone O, Vauloup-Fellous C, Cordier AG, Parent Du Chatelet I, Senat MV, Frydman R, Grangeot-Keros L. 2009. A 2-year study on cytomegalovirus infection during pregnancy in a French Hospital. BJOG 116:818–823 [DOI] [PubMed] [Google Scholar]
- 18. Vauloup-Fellous C, Picone O, Cordier AG, Parent-du-Chatelet I, Senat MV, Frydman R, Grangeot-Keros L. 2009. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 46(Suppl. 4):S49–S53 [DOI] [PubMed] [Google Scholar]
- 19. Mocarski JE, Shenk T, Pass R. 2007. Cytomegaloviruses, p 2702–2772 In Knipe D, Howley P. (ed), Fields virology, 5th ed Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 20. Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. 1998. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J. Infect. Dis. 177:1170–1175 [DOI] [PubMed] [Google Scholar]
- 21. Bego MG, St Jeor S. 2006. Human cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp. Hematol. 34:555–570 [DOI] [PubMed] [Google Scholar]
- 22. Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 75:7543–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renzette N, Bhattacharjee B, Jensen JD, Gibson L, Kowalik TF. 2011. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 7:e1001344 doi:10.1371/journal.ppat.1001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. 2010. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J. Infect. Dis. 202:1800–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon MJ. 2009. Congenital cytomegalovirus (CMV) epidemiology and awareness. J. Clin. Virol. 46(Suppl. 4):S6–S10 [DOI] [PubMed] [Google Scholar]
- 26. Cannon MJ, Schmid DS, Hyde TB. 2010. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20:202–213 [DOI] [PubMed] [Google Scholar]
- 27. Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253–276 [DOI] [PubMed] [Google Scholar]
- 28. Marshall GS, Stout GG. 2005. Cytomegalovirus seroprevalence among women of childbearing age during a 10-year period. Am. J. Perinatol. 22:371–376 [DOI] [PubMed] [Google Scholar]
- 29. Stagno S, Dworsky ME, Torres J, Mesa T, Hirsh T. 1982. Prevalence and importance of congenital cytomegalovirus infection in three different populations. J. Pediatr. 101:897–900 [DOI] [PubMed] [Google Scholar]
- 30. Nishimura N, Kimura H, Yabuta Y, Tanaka N, Ito Y, Ishikawa K, Suzuki C, Morishima T. 1999. Prevalence of maternal cytomegalovirus (CMV) antibody and detection of CMV DNA in amniotic fluid. Microbiol. Immunol. 43:781–784 [DOI] [PubMed] [Google Scholar]
- 31. Hyde TB, Schmid DS, Cannon MJ. 2010. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev. Med. Virol. 20:311–326 [DOI] [PubMed] [Google Scholar]
- 32. Adler SP. 1989. Cytomegalovirus and child day care. Evidence for an increased infection rate among day-care workers. N. Engl. J. Med. 321:1290–1296 [DOI] [PubMed] [Google Scholar]
- 33. Hutto C, Ricks R, Garvie M, Pass RF. 1985. Epidemiology of cytomegalovirus infections in young children: day care vs. home care. Pediatr. Infect. Dis. 4:149–152 [DOI] [PubMed] [Google Scholar]
- 34. Pass RF, Hutto C, Ricks R, Cloud GA. 1986. Increased rate of cytomegalovirus infection among parents of children attending day-care centers. N. Engl. J. Med. 314:1414–1418 [DOI] [PubMed] [Google Scholar]
- 35. Akinbami AA, Rabiu KA, Adewunmi AA, Wright KO, Dosunmu AO, Adeyemo TA, Adediran A, Osunkalu VO. 2011. Seroprevalence of cytomegalovirus antibodies amongst normal pregnant women in Nigeria. Int. J. Womens Health 3:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamdan HZ, Abdelbagi IE, Nasser NM, Adam I. 2011. Seroprevalence of cytomegalovirus and rubella among pregnant women in western Sudan. Virol. J. 8:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kouri V, Correa CB, Verdasquera D, Martinez PA, Alvarez A, Aleman Y, Perez L, Golpe MA, Someilan T, Chong Y, Fresno C, Navarro MA, Perez E, Moro I, Sanchez R, Llanusa C, Melin P. 2010. Diagnosis and screening for cytomegalovirus infection in pregnant women in Cuba as prognostic markers of congenital infection in newborns: 2007-2008. Pediatr. Infect. Dis. J. 29:1105–1110 [DOI] [PubMed] [Google Scholar]
- 38. Krech U, Tobin J. 1981. A collaborative study of cytomegalovirus antibodies in mothers and young children in 19 countries. Bull. World Health Organ. 59:605–610 [PMC free article] [PubMed] [Google Scholar]
- 39. Saraswathy TS, Az-Ulhusna A, Asshikin RN, Suriani S, Zainah S. 2011. Seroprevalence of cytomegalovirus infection in pregnant women and associated role in obstetric complications: a preliminary study. Southeast Asian J. Trop. Med. Public Health 42:320–322 [PubMed] [Google Scholar]
- 40. Seo S, Cho Y, Park J. 2009. Serologic screening of pregnant Korean women for primary human cytomegalovirus infection using IgG avidity test. Korean J. Lab Med. 29:557–562 [DOI] [PubMed] [Google Scholar]
- 41. Wong A, Tan KH, Tee CS, Yeo GS. 2000. Seroprevalence of cytomegalovirus, toxoplasma and parvovirus in pregnancy. Singapore Med. J. 41:151–155 [PubMed] [Google Scholar]
- 42. Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 137:90–95 [DOI] [PubMed] [Google Scholar]
- 43. Stern H, Tucker SM. 1973. Prospective study of cytomegalovirus infection in pregnancy. Br. Med. J. 2:268–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dollard SC, Grosse SD, Ross DS. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17:355–363 [DOI] [PubMed] [Google Scholar]
- 45. Fowler KB, Stagno S, Pass RF. 1993. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980–1990. J. Infect. Dis. 168:552–556 [DOI] [PubMed] [Google Scholar]
- 46. Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 43:1143–1151 [DOI] [PubMed] [Google Scholar]
- 47. Dar L, Pati SK, Patro AR, Deorari AK, Rai S, Kant S, Broor S, Fowler KB, Britt WJ, Boppana SB. 2008. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr. Infect. Dis. J. 27:841–843 [DOI] [PubMed] [Google Scholar]
- 48. Noyola DE, Jimenez-Capdeville ME, Demmler-Harrison GJ. 2010. Central nervous system disorders in infants with congenital cytomegalovirus infection. Neurol. Res. 32:278–284 [DOI] [PubMed] [Google Scholar]
- 49. Rahav G, Gabbay R, Ornoy A, Shechtman S, Arnon J, Diav-Citrin O. 2007. Primary versus nonprimary cytomegalovirus infection during pregnancy, Israel. Emerg. Infect. Dis. 13:1791–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stagno S, Pass RF, Dworsky ME, Alford CA., Jr 1982. Maternal cytomegalovirus infection and perinatal transmission. Clin. Obstet. Gynecol. 25:563–576 [DOI] [PubMed] [Google Scholar]
- 51. van der Sande MA, Kaye S, Miles DJ, Waight P, Jeffries DJ, Ojuola OO, Palmero M, Pinder M, Ismaili J, Flanagan KL, Aveika AA, Zaman A, Rowland-Jones S, McConkey SJ, Whittle HC, Marchant A. 2007. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One 2:e492 doi:10.1371/journal.pone.0000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bodeus M, Hubinont C, Goubau P. 1999. Increased risk of cytomegalovirus transmission in utero during late gestation. Obstet. Gynecol. 93:658–660 [PubMed] [Google Scholar]
- 53. Bodeus M, Kabamba-Mukadi B, Zech F, Hubinont C, Bernard P, Goubau P. 2010. Human cytomegalovirus in utero transmission: follow-up of 524 maternal seroconversions. J. Clin. Virol. 47:201–202 [DOI] [PubMed] [Google Scholar]
- 54. Daiminger A, Bader U, Enders G. 2005. Pre- and periconceptional primary cytomegalovirus infection: risk of vertical transmission and congenital disease. BJOG 112:166–172 [DOI] [PubMed] [Google Scholar]
- 55. Enders G, Daiminger A, Bader U, Exler S, Enders M. 2011. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol. 52:244–246 [DOI] [PubMed] [Google Scholar]
- 56. Gindes L, Teperberg-Oikawa M, Sherman D, Pardo J, Rahav G. 2008. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG 115:830–835 [DOI] [PubMed] [Google Scholar]
- 57. Griffiths PD, Baboonian C. 1984. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br. J. Obstet. Gynaecol. 91:307–315 [DOI] [PubMed] [Google Scholar]
- 58. Revello MG, Gerna G. 2004. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J. Clin. Virol. 29:71–83 [DOI] [PubMed] [Google Scholar]
- 59. Grosse SD, Ross DS, Dollard SC. 2008. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J. Clin. Virol. 41:57–62 [DOI] [PubMed] [Google Scholar]
- 60. Anderson KS, Amos CS, Boppana S, Pass R. 1996. Ocular abnormalities in congenital cytomegalovirus infection. J. Am. Optom. Assoc. 67:273–278 [PubMed] [Google Scholar]
- 61. Coats DK, Demmler GJ, Paysse EA, Du LT, Libby C. 2000. Ophthalmologic findings in children with congenital cytomegalovirus infection. J. AAPOS 4:110–116 [DOI] [PubMed] [Google Scholar]
- 62. Saigal S, Lunyk O, Larke RP, Chernesky MA. 1982. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am. J. Dis. Child. 136:896–901 [DOI] [PubMed] [Google Scholar]
- 63. Ciorba A, Bovo R, Trevisi P, Bianchini C, Arboretti R, Martini A. 2009. Rehabilitation and outcome of severe profound deafness in a group of 16 infants affected by congenital cytomegalovirus infection. Eur. Arch. Otorhinolaryngol. 266:1539–1546 [DOI] [PubMed] [Google Scholar]
- 64. Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, Petrou S, Watkin P, Worsfold S, Yuen HM, Stevenson J. 2006. Language ability after early detection of permanent childhood hearing impairment. N. Engl. J. Med. 354:2131–2141 [DOI] [PubMed] [Google Scholar]
- 65. Robinshaw HM. 1995. Early intervention for hearing impairment: differences in the timing of communicative and linguistic development. Br. J. Audiol. 29:315–334 [DOI] [PubMed] [Google Scholar]
- 66. Yoshinaga-Itano C. 1999. Benefits of early intervention for children with hearing loss. Otolaryngol Clin. North Am. 32:1089–1102 [DOI] [PubMed] [Google Scholar]
- 67. Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. 2000. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol. 11:283–290 [PubMed] [Google Scholar]
- 68. Fowler KB, Dahle AJ, Boppana SB, Pass RF. 1999. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J. Pediatr. 135:60–64 [DOI] [PubMed] [Google Scholar]
- 69. Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, Rodesch F. 2000. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet. Gynecol. 95:881–888 [DOI] [PubMed] [Google Scholar]
- 70. Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. 2006. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J. Clin. Virol. 35:216–220 [DOI] [PubMed] [Google Scholar]
- 71. Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904–1908 [PubMed] [Google Scholar]
- 72. Ornoy A, Diav-Citrin O. 2006. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod. Toxicol. 21:399–409 [DOI] [PubMed] [Google Scholar]
- 73. Ahlfors K, Ivarsson SA, Harris S. 1999. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand. J. Infect. Dis. 31:443–457 [DOI] [PubMed] [Google Scholar]
- 74. Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55–60 [DOI] [PubMed] [Google Scholar]
- 75. Gaytant MA, Rours GI, Steegers EA, Galama JM, Semmekrot BA. 2003. Congenital cytomegalovirus infection after recurrent infection: case reports and review of the literature. Eur. J. Pediatr. 162:248–253 [DOI] [PubMed] [Google Scholar]
- 76. Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, Boppana SB. 2006. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J. Pediatr. 148:332–336 [DOI] [PubMed] [Google Scholar]
- 77. Zalel Y, Gilboa Y, Berkenshtat M, Yoeli R, Auslander R, Achiron R, Goldberg Y. 2008. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet. Gynecol. 31:417–420 [DOI] [PubMed] [Google Scholar]
- 78. Clarke LM, Duerr A, Feldman J, Sierra MF, Daidone BJ, Landesman SH. 1996. Factors associated with cytomegalovirus infection among human immunodeficiency virus type 1-seronegative and -seropositive women from an urban minority community. J. Infect. Dis. 173:77–82 [DOI] [PubMed] [Google Scholar]
- 79. Duryea EL, Sanchez PJ, Sheffield JS, Jackson GL, Wendel GD, McElwee BS, Boney LF, Mallory MM, Owen KE, Stehel EK. 2010. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr. Infect. Dis. J. 29:915–918 [DOI] [PubMed] [Google Scholar]
- 80. Schoenfisch AL, Dollard SC, Amin M, Gardner LI, Klein RS, Mayer K, Rompalo A, Sobel JD, Cannon MJ. 2011. Cytomegalovirus (CMV) shedding is highly correlated with markers of immunosuppression in CMV-seropositive women. J. Med. Microbiol. 60:768–774 [DOI] [PubMed] [Google Scholar]
- 81. Guibert G, Warszawski J, Le Chenadec J, Blanche S, Benmebarek Y, Mandelbrot L, Tubiana R, Rouzioux C, Leruez-Ville M. 2009. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 48:1516–1525 [DOI] [PubMed] [Google Scholar]
- 82. Mania A, Kemnitz P, Mazur-Melewska K, Figlerowicz M, Cudnoch K, Sluzewski W, Kowala-Piaskowska A, Mozer-Lisewska I. 2012. Human cytomegalovirus infection and clinical status of infants born to human immunodeficiency virus type 1 infected mothers. J. Matern. Fetal Neonatal Med. 25:180–186 [DOI] [PubMed] [Google Scholar]
- 83. Doyle M, Atkins JT, Rivera-Matos IR. 1996. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr. Infect. Dis. J. 15:1102–1106 [DOI] [PubMed] [Google Scholar]
- 84. Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, Pitt J, Cooper E, Goldfarb J, Hodes D, Kattan M, McIntosh K. 1999. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N. Engl. J. Med. 341:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frederick T, Homans J, Spencer L, Kramer F, Stek A, Operskalski E. 2012. The effect of prenatal highly active antiretroviral therapy on the transmission of congenital and perinatal/early postnatal cytomegalovirus among HIV-infected and HIV-exposed infants. Clin. Infect. Dis. 55:877–884 [DOI] [PubMed] [Google Scholar]
- 86. Khamduang W, Jourdain G, Sirirungsi W, Layangool P, Kanjanavanit S, Krittigamas P, Pagdi K, Somsamai R, Sirinontakan S, Hinjiranandana T, Ardonk W, Hongsiriwon S, Nanta S, Borkird T, Lallemant M, McIntosh K, Ngo-Giang-Huong N. 2011. The interrelated transmission of HIV-1 and cytomegalovirus during gestation and delivery in the offspring of HIV-infected mothers. J. Acquir. Immune Defic. Syndr. 58:188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77:13301–13314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Slyker JA, Lohman-Payne BL, John-Stewart GC, Maleche-Obimbo E, Emery S, Richardson B, Dong T, Iversen AK, Mbori-Ngacha D, Overbaugh J, Emery VC, Rowland-Jones SL. 2009. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 23:2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. South Africa Department of Health 2008. National antenatal sentinel HIV and syphilis prevalence survey, South Africa. Department of Health, Pretoria, South Africa [Google Scholar]
- 90. South African Medical Research Council 9 June 2011, posting date SA PMTCT evaluation shows that virtual elimination of paediatric HIV is possible with intensified effort. http://www.mrc.ac.za/pressreleases/2011/10press2011.htm
- 91. Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, Sheffield VC. 2006. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11). Proc. Natl. Acad. Sci. U. S. A. 103:6287–6292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. de Vries JJ, van der Eijk AA, Wolthers KC, Rusman LG, Pas SD, Molenkamp R, Claas EC, Kroes AC, Vossen AC. 2012. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 53:167–170 [DOI] [PubMed] [Google Scholar]
- 93. Gandhi RS, Fernandez-Alvarez JR, Rabe H. 2010. Management of congenital cytomegalovirus infection: an evidence-based approach. Acta Paediatr. 99:509–515 [DOI] [PubMed] [Google Scholar]
- 94. Kadambari S, Williams EJ, Luck S, Griffiths PD, Sharland M. 2011. Evidence based management guidelines for the detection and treatment of congenital CMV. Early Hum. Dev. 87:723–728 [DOI] [PubMed] [Google Scholar]
- 95. Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Kiell JM, Soong SJ, Whitley RJ. 2003. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 143:16–25 [DOI] [PubMed] [Google Scholar]
- 96. Faulds D, Heel RC. 1990. Ganciclovir: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39:597–638 [DOI] [PubMed] [Google Scholar]
- 97. Oliver SE, Cloud GA, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Soong SJ, Whitley RJ, Kimberlin DW. 2009. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J. Clin. Virol. 46(Suppl. 4):S22–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lombardi G, Garofoli F, Villani P, Tizzoni M, Angelini M, Cusato M, Bollani L, De Silvestri A, Regazzi M, Stronati M. 2009. Oral valganciclovir treatment in newborns with symptomatic congenital cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 28:1465–1470 [DOI] [PubMed] [Google Scholar]
- 99. Kimberlin DW, Acosta EP, Sanchez PJ, Sood S, Agrawal V, Homans J, Jacobs RF, Lang D, Romero JR, Griffin J, Cloud GA, Lakeman FD, Whitley RJ. 2008. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J. Infect. Dis. 197:836–845 [DOI] [PubMed] [Google Scholar]
- 100. Amir J, Wolf DG, Levy I. 2010. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur. J. Pediatr. 169:1061–1067 [DOI] [PubMed] [Google Scholar]
- 101. Lackner A, Acham A, Alborno T, Moser M, Engele H, Raggam RB, Halwachs-Baumann G, Kapitan M, Walch C. 2009. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: four to 10 year follow up. J. Laryngol Otol. 123:391–396 [DOI] [PubMed] [Google Scholar]
- 102. Conboy TJ, Pass RF, Stagno S, Alford CA, Myers GJ, Britt WJ, McCollister FP, Summers MN, McFarland CE, Boll TJ. 1987. Early clinical manifestations and intellectual outcome in children with symptomatic congenital cytomegalovirus infection. J. Pediatr. 111:343–348 [DOI] [PubMed] [Google Scholar]
- 103. Noyola DE, Demmler GJ, Nelson CT, Griesser C, Williamson WD, Atkins JT, Rozelle J, Turcich M, Llorente AM, Sellers-Vinson S, Reynolds A, Bale JF, Jr, Gerson P, Yow MD. 2001. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr. 138:325–331 [DOI] [PubMed] [Google Scholar]
- 104. Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 2002. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 110:762–767 [DOI] [PubMed] [Google Scholar]
- 105. Kylat RI, Kelly EN, Ford-Jones EL. 2006. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur. J. Pediatr. 165:773–778 [DOI] [PubMed] [Google Scholar]
- 106. Boppana SB, Fowler KB, Vaid Y, Hedlund G, Stagno S, Britt WJ, Pass RF. 1997. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 99:409–414 [DOI] [PubMed] [Google Scholar]
- 107. Boppana SB, Fowler KB, Pass RF, Rivera LB, Bradford RD, Lakeman FD, Britt WJ. 2005. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J. Pediatr. 146:817–823 [DOI] [PubMed] [Google Scholar]
- 108. Cannon MJ, Hyde TB, Schmid DS. 2011. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 21:240–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lanari M, Lazzarotto T, Venturi V, Papa I, Gabrielli L, Guerra B, Landini MP, Faldella G. 2006. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics 117:e76–e83 [DOI] [PubMed] [Google Scholar]
- 110. Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. 2009. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. Pediatr. Infect. Dis. J. 28:588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ivarsson SA, Lernmark B, Svanberg L. 1997. Ten-year clinical, developmental, and intellectual follow-up of children with congenital cytomegalovirus infection without neurologic symptoms at one year of age. Pediatrics 99:800–803 [DOI] [PubMed] [Google Scholar]
- 112. Barbi M, Binda S, Caroppo S. 2006. Diagnosis of congenital CMV infection via dried blood spots. Rev. Med. Virol. 16:385–392 [DOI] [PubMed] [Google Scholar]
- 113. Choi KY, Schimmenti LA, Jurek AM, Sharon B, Daly K, Khan C, McCann M, Schleiss MR. 2009. Detection of cytomegalovirus DNA in dried blood spots of Minnesota infants who do not pass newborn hearing screening. Pediatr. Infect. Dis. J. 28:1095–1098 [DOI] [PubMed] [Google Scholar]
- 114. Vauloup-Fellous C, Ducroux A, Couloigner V, Marlin S, Picone O, Galimand J, Loundon N, Denoyelle F, Grangeot-Keros L, Leruez-Ville M. 2007. Evaluation of cytomegalovirus (CMV) DNA quantification in dried blood spots: retrospective study of CMV congenital infection. J. Clin. Microbiol. 45:3804–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, Griffiths PD. 2008. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch. Dis. Child Fetal Neonatal ed 93:F280–F285 [DOI] [PubMed] [Google Scholar]
- 116. Barbi M, MacKay WG, Binda S, van Loon AM. 2008. External quality assessment of cytomegalovirus DNA detection on dried blood spots. BMC Microbiol. 8:2 doi:10.1186/1471-2180-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Britt WJ, Fowler KB. 2010. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 303:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Vries JJ, Claas EC, Kroes AC, Vossen AC. 2009. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 46(Suppl. 4):S37–S42 [DOI] [PubMed] [Google Scholar]
- 119. Johansson PJ, Jonsson M, Ahlfors K, Ivarsson SA, Svanberg L, Guthenberg C. 1997. Retrospective diagnostics of congenital cytomegalovirus infection performed by polymerase chain reaction in blood stored on filter paper. Scand. J. Infect. Dis. 29:465–468 [DOI] [PubMed] [Google Scholar]
- 120. Kharrazi M, Hyde T, Young S, Amin MM, Cannon MJ, Dollard SC. 2010. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. J. Pediatr. 157:191–197 [DOI] [PubMed] [Google Scholar]
- 121. Scanga L, Chaing S, Powell C, Aylsworth AS, Harrell LJ, Henshaw NG, Civalier CJ, Thorne LB, Weck K, Booker J, Gulley ML. 2006. Diagnosis of human congenital cytomegalovirus infection by amplification of viral DNA from dried blood spots on perinatal cards. J. Mol. Diagn. 8:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Soetens O, Vauloup-Fellous C, Foulon I, Dubreuil P, De Saeger B, Grangeot-Keros L, Naessens A. 2008. Evaluation of different cytomegalovirus (CMV) DNA PCR protocols for analysis of dried blood spots from consecutive cases of neonates with congenital CMV infections. J. Clin. Microbiol. 46:943–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bradford RD, Cloud G, Lakeman AD, Boppana S, Kimberlin DW, Jacobs R, Demmler G, Sanchez P, Britt W, Soong SJ, Whitley RJ. 2005. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J. Infect. Dis. 191:227–233 [DOI] [PubMed] [Google Scholar]
- 124. Gohring K, Dietz K, Hartleif S, Jahn G, Hamprecht K. 2010. Influence of different extraction methods and PCR techniques on the sensitivity of HCMV-DNA detection in dried blood spot (DBS) filter cards. J. Clin. Virol. 48:278–281 [DOI] [PubMed] [Google Scholar]
- 125. Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Tolan RW, Jr, Novak Z, Chowdhury N, Britt WJ, Fowler KB. 2011. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N. Engl. J. Med. 364:2111–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Grosse SD, Dollard S, Ross DS, Cannon M. 2009. Newborn screening for congenital cytomegalovirus: options for hospital-based and public health programs. J. Clin. Virol. 46(Suppl. 4):S32–S36 [DOI] [PubMed] [Google Scholar]
- 127. Dollard SC, Schleiss MR, Grosse SD. 2010. Public health and laboratory considerations regarding newborn screening for congenital cytomegalovirus. J. Inherit. Metab. Dis. 33:S249–254 [DOI] [PubMed] [Google Scholar]
- 128. Nigro G, Adler SP. 2011. Cytomegalovirus infections during pregnancy. Curr. Opin. Obstet. Gynecol. 23:123–128 [DOI] [PubMed] [Google Scholar]
- 129. Forsgren M. 2009. Prevention of congenital and perinatal infections. Euro Surveill. 14:2–4 [PubMed] [Google Scholar]
- 130. Rahav G. 2007. Congenital cytomegalovirus infection—a question of screening. Isr. Med. Assoc. J. 9:392–394 [PubMed] [Google Scholar]
- 131. Yinon Y, Farine D, Yudin MH, Gagnon R, Hudon L, Basso M, Bos H, Delisle MF, Menticoglou S, Mundle W, Ouellet A, Pressey T, Roggensack A, Boucher M, Castillo E, Gruslin A, Money DM, Murphy K, Ogilvie G, Paquet C, Van Eyk N, van Schalkwyk J. 2010. Cytomegalovirus infection in pregnancy. J. Obstet. Gynaecol. Can. 32:348–354 [DOI] [PubMed] [Google Scholar]
- 132. Lazzarotto T, Guerra B, Spezzacatena P, Varani S, Gabrielli L, Pradelli P, Rumpianesi F, Banzi C, Bovicelli L, Landini MP. 1998. Prenatal diagnosis of congenital cytomegalovirus infection. J. Clin. Microbiol. 36:3540–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Stagno S, Tinker MK, Elrod CFD, Cloud G, O'Beirne AJ. 1985. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infection in pregnant womne and newborn infants. J. Clin. Microbio 21:930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Grangeot-Keros L, Mayaux MJ, Lebon P, Freymoth F, Eugene G, Stricker R, Dussaix E. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944–946 [DOI] [PubMed] [Google Scholar]
- 135. Lazzarotto T, Spezzacatena P, Varani S, Gabrielli L, Pradelli P, Guerra B, Landini MP. 1999. Anticytomegalovirus (anti-CMV) immunoglobulin G avidity in identification of pregnant women at risk of transmitting congenital CMV infection. Clin. Diagn. Lab. Immunol. 6:127–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, Landini MP. 2000. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 13:137–141 [DOI] [PubMed] [Google Scholar]
- 137. Bodeus M, Hubinont C, Bernard P, Bouckaert A, Thomas K, Goubau P. 1999. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenat Diagn. 19:314–317 [DOI] [PubMed] [Google Scholar]
- 138. Donner C, Liesnard C, Brancart F, Rodesch F. 1994. Accuracy of amniotic fluid testing before 21 weeks' gestation in prenatal diagnosis of congenital cytomegalovirus infection. Prenat. Diagn. 14:1055–1059 [DOI] [PubMed] [Google Scholar]
- 139. Enders G, Bader U, Lindemann L, Schalasta G, Daiminger A. 2001. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat. Diagn. 21:362–377 [DOI] [PubMed] [Google Scholar]
- 140. Gouarin S, Gault E, Vabret A, Cointe D, Rozenberg F, Grangeot-Keros L, Barjot P, Garbarg-Chenon A, Lebon P, Freymuth F. 2002. Real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples from mothers with primary infection. J. Clin. Microbiol. 40:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lipitz S, Yagel S, Shalev E, Achiron R, Mashiach S, Schiff E. 1997. Prenatal diagnosis of fetal primary cytomegalovirus infection. Obstet. Gynecol. 89:763–767 [DOI] [PubMed] [Google Scholar]
- 142. Mulongo KN, Lamy ME, Van Lierde M. 1995. Requirements for diagnosis of prenatal cytomegalovirus infection by amniotic fluid culture. Clin. Diagn. Virol. 4:231–238 [DOI] [PubMed] [Google Scholar]
- 143. Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15:680–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gouarin S, Palmer P, Cointe D, Rogez S, Vabret A, Rozenberg F, Denis F, Freymuth F, Lebon P, Grangeot-Keros L. 2001. Congenital HCMV infection: a collaborative and comparative study of virus detection in amniotic fluid by culture and by PCR. J. Clin. Virol. 21:47–55 [DOI] [PubMed] [Google Scholar]
- 145. Revello MG, Lilleri D, Zavattoni M, Furione M, Middeldorp J, Gerna G. 2003. Prenatal diagnosis of congenital human cytomegalovirus infection in amniotic fluid by nucleic acid sequence-based amplification assay. J. Clin. Microbiol. 41:1772–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Goegebuer T, Van Meensel B, Beuselinck K, Cossey V, Van Ranst M, Hanssens M, Lagrou K. 2009. Clinical predictive value of real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples. J. Clin. Microbio. 47:660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Revello MG, Sarasini A, Zavattoni M, Baldanti F, Gerna G. 1998. Improved prenatal diagnosis of congenital human cytomegalovirus infection by a modified nested polymerase chain reaction. J. Med. Virol. 56:99–103 [DOI] [PubMed] [Google Scholar]
- 148. Benoist G, Salomon LJ, Jacquemard F, Daffos F, Ville Y. 2008. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 115:823–829 [DOI] [PubMed] [Google Scholar]
- 149. Benoist G, Salomon LJ, Mohlo M, Suarez B, Jacquemard F, Ville Y. 2008. Cytomegalovirus-related fetal brain lesions: comparison between targeted ultrasound examination and magnetic resonance imaging. Ultrasound Obstet. Gynecol. 32:900–905 [DOI] [PubMed] [Google Scholar]
- 150. Cheeran MC, Lokensgard JR, Schleiss MR. 2009. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 22:99–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Guerra B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T, Rizzo N. 2008. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 198:380.e1-7 doi:10.1016/j.ajog.2007.09.052 [DOI] [PubMed] [Google Scholar]
- 152. Amir J, Schwarz M, Levy I, Haimi-Cohen Y, Pardo J. 2011. Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch. Dis. Child. 96:846–850 [DOI] [PubMed] [Google Scholar]
- 153. Ancora G, Lanari M, Lazzarotto T, Venturi V, Tridapalli E, Sandri F, Menarini M. 2007. Cranial ultrasound scanning and prediction of outcome in newborns with congenital cytomegalovirus infection. J. Pediatr. 150:157–161 [DOI] [PubMed] [Google Scholar]
- 154. Azam AZ, Vial Y, Fawer CL, Zufferey J, Hohlfeld P. 2001. Prenatal diagnosis of congenital cytomegalovirus infection. Obstet. Gynecol. 97:443–448 [DOI] [PubMed] [Google Scholar]
- 155. Guerra B, Lazzarotto T, Quarta S, Lanari M, Bovicelli L, Nicolosi A, Landini MP. 2000. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 183:476–482 [DOI] [PubMed] [Google Scholar]
- 156. Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41:192–197 [DOI] [PubMed] [Google Scholar]
- 157. Picone O, Costa JM, Leruez-Ville M, Ernault P, Olivi M, Ville Y. 2004. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat. Diagn. 24:1001–1006 [DOI] [PubMed] [Google Scholar]
- 158. Revello MG, Zavattoni M, Furione M, Baldanti F, Gerna G. 1999. Quantification of human cytomegalovirus DNA in amniotic fluid of mother of congenitally infected fetuses. J. Clin. Microbio. 37:3350–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fryer JF, Heath AB, Anderson R, Minor PD. 2010. Collaborative study to evaluate the proposed 1st WHO international standard for human cytomegalovirus (HCMV) for nucleic acid amplification (NAT)-based assays WHO/BS/10.2138. World Health Organiztion, Geneva, Switzerland [Google Scholar]
- 160. Nyholm JL, Schleiss MR. 2010. Prevention of maternal cytomegalovirus infection: current status and future prospects. Int. J. Womens Health 2:23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jacquemard F, Yamamoto M, Costa JM, Romand S, Jaqz-Aigrain E, Dejean A, Daffos F, Ville Y. 2007. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 114:1113–1121 [DOI] [PubMed] [Google Scholar]
- 162. Fowler KB, Stagno S, Pass RF. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011 [DOI] [PubMed] [Google Scholar]
- 163. Maidji E, Nigro G, Tabata T, McDonagh S, Nozawa N, Shiboski S, Muci S, Anceschi MM, Aziz N, Adler SP, Pereira L. 2010. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am. J. Pathol. 177:1298–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Weisblum Y, Panet A, Zakay-Rones Z, Haimov-Kochman R, Goldman-Wohl D, Ariel I, Falk H, Natanson-Yaron S, Goldberg MD, Gilad R, Lurain NS, Greenfield C, Yagel S, Wolf DG. 2011. Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture. J. Virol. 85:13204–13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schleiss MR. 2006. The role of the placenta in the pathogenesis of congenital cytomegalovirus infection: is the benefit of cytomegalovirus immune globulin for the newborn mediated through improved placental health and function? Clin. Infect. Dis. 43:1001–1003 [DOI] [PubMed] [Google Scholar]
- 166. Nigro G, Torre RL, Pentimalli H, Taverna P, Lituania M, de Tejada BM, Adler SP. 2008. Regression of fetal cerebral abnormalities by primary cytomegalovirus infection following hyperimmunoglobulin therapy. Prenat. Diagn. 28:512–517 [DOI] [PubMed] [Google Scholar]
- 167. Nigro G, Adler SP, La Torre R, Best AM. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353:1350–1362 [DOI] [PubMed] [Google Scholar]
- 168. Nigro G, Adler SP, Parrutti G, Anceschi MM, Coclite E, Pezone I, Di Renzo GC. 2012. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy—a case-control study of the outcome in children. J. Infect. Dis. 205:215–227 [DOI] [PubMed] [Google Scholar]
- 169. Visentin S, Manara R, Milanese L, Da Roit A, Forner G, Salviato E, Citton V, Magno FM, Orzan E, Morando C, Cusinato R, Mengoli C, Palu G, Ermani M, Cosmi E, Gusetti G. 2012. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmuneglobulin therapy improves outcomes among infants at 1 year of age. Clin. Infect. Dis. 55:497–503 [DOI] [PubMed] [Google Scholar]
- 170. McCarthy FP, Giles ML, Rowlands S, Purcell KJ, Jones CA. 2011. Antenatal interventions for preventing the transmission of cytomegalovirus (CMV) from the mother to fetus during pregnancy and adverse outcomes in the congenitally infected infant. Cochrane Database Syst. Rev. 16(3):CD008371 doi:10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Boppana SB, Britt WJ. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115–1121 [DOI] [PubMed] [Google Scholar]
- 172. Britt WJ, Vugler L, Butfiloski EJ, Stephens EB. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 64:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J. Virol. 86:7444–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168:1210–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mattes FM, Vargas A, Kopyncinski J, Hainsworth EG, Sweny P, Nebbia G, Bazeos A, Lowdell M, Klenerman P, Phillips RE, Griffiths PD, Emery VC. 2008. Functional impairment of cytomegalovirus specific CD8 T cells predicts high-level replication after renal transplantation. Am. J. Transplant. 8:990–999 [DOI] [PubMed] [Google Scholar]
- 176. Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, Bell S, Gailbraith A, McNeil K, Jones S, Khanna R. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl. Infect. Dis. 9:165–170 [DOI] [PubMed] [Google Scholar]
- 177. Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. 1999. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J. Infect. Dis. 180:702–707 [DOI] [PubMed] [Google Scholar]
- 178. Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 101:2686–2692 [DOI] [PubMed] [Google Scholar]
- 179. Moss P, Khan N. 2004. CD8(+) T-cell immunity to cytomegalovirus. Hum. Immunol. 65:456–464 [DOI] [PubMed] [Google Scholar]
- 180. Sester M, Sester U, Gartner BC, Girndt M, Meyerhans A, Kohler H. 2002. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J. Am. Soc. Nephrol. 13:2577–2584 [DOI] [PubMed] [Google Scholar]
- 181. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Gyulai Z, Endresz V, Burian K, Pincus S, Toldy J, Cox WI, Meric C, Plotkin S, Gonczol E, Berencsi K. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537–1546 [DOI] [PubMed] [Google Scholar]
- 183. Lilleri D, Fornara C, Furione M, Zavattoni M, Revello MG, Gerna G. 2007. Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J. Infect. Dis. 195:1062–1070 [DOI] [PubMed] [Google Scholar]
- 184. Revello MG, Zavattoni M, Furione M, Fabbri E, Gerna G. 2006. Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J. Infect. Dis. 193:783–787 [DOI] [PubMed] [Google Scholar]
- 185. Lissauer D, Choudhary M, Pachnio A, Goodyear O, Moss PA, Kilby MD. 2011. Cytomegalovirus seropositivity dramatically alters the maternal CD8+ T cell repertoire and leads to the accumulation of highly differentiated memory cells during human pregnancy. Hum. Reprod. 26:3355–3365 [DOI] [PubMed] [Google Scholar]
- 186. Mocarski ES., Jr 2004. Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell. Microbiol. 6:707–717 [DOI] [PubMed] [Google Scholar]
- 187. Reddehase MJ. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831–844 [DOI] [PubMed] [Google Scholar]
- 188. Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233–239 [DOI] [PubMed] [Google Scholar]
- 189. Pass RF, Duliege AM, Boppana S, Sekulovich R, Percell S, Britt W, Burke RL. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970–975 [DOI] [PubMed] [Google Scholar]
- 190. Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sabbaj S, Pass RF, Goepfert PA, Pichon S. 2011. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J. Infect. Dis. 203:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P, Olmsted RA, Hoeper A, Morris J, Negri S, Maughan MF, Chulay JD. 2009. Randomized, double-blind, phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 28:484–493 [DOI] [PubMed] [Google Scholar]
- 194. Schleiss MR. 2009. VCL-CB01, an injectable bivalent plasmid DNA vaccine for potential protection against CMV disease and infection. Curr. Opin. Mol. Ther. 11:572–578 [PMC free article] [PubMed] [Google Scholar]
- 195. Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, Guterwill DF, Smith LRRAP, Kenney RT. 2012. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoetic stem-cell transplantation: a randomised, double-blin placebo-controlled, phase 2 trial. Lancet Infect. Dis. 12:209–299 [DOI] [PubMed] [Google Scholar]
- 196. Dasari V, Smith C, Zhong J, Scott G, Rawlinson W, Khanna R. 2011. Recombinant glycoprotein B vaccine formulation with Toll-like receptor 9 agonist and immune-stimulating complex induces specific immunity against multiple strains of cytomegalovirus. J. Gen. Virol. 92:1021–1031 [DOI] [PubMed] [Google Scholar]
- 197. Fowler KB, Pass RF. 2006. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 118:e286–292 [DOI] [PubMed] [Google Scholar]
- 198. Adler SP. 1991. Molecular epidemiology of cytomegalovirus: a study of factors affecting transmission among children at three day-care centers. Pediatr. Infect. Dis. J. 10:584–590 [PubMed] [Google Scholar]
- 199. Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid SD, Stagno S, Cannon MJ. 2009. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr. Infect. Dis. J. 28:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, Adler S, Arvin A, Lewis DB. 2004. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J. Immunol. 172:3260–3267 [DOI] [PubMed] [Google Scholar]
- 201. Stowell JD, Forlin-Passoni D, Din E, Radford K, Brown D, White A, Bate SL, Dollard SC, Bialek SR, Cannon MJ, Schmid DS. 2012. Cytomegalovirus survival on common environmental surfaces: opportunities for viral transmission. J. Infect. Dis. 205:211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Murph, Baron JC, Brown CK, Ebelhack CL, Bale JF., Jr 1991. The occupational risk of cytomegalovirus infection among day-care providers. JAMA 265:603–608 [PubMed] [Google Scholar]
- 203. Adler SP, Finney JW, Manganello AM, Best AM. 2004. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J. Pediatr. 145:485–491 [DOI] [PubMed] [Google Scholar]
- 204. Chandler SH, Handsfield HH, McDougall JK. 1987. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J. Infect. Dis. 155:655–660 [DOI] [PubMed] [Google Scholar]
- 205. Coonrod D, Collier AC, Ashley R, DeRouen T, Corey L. 1998. Association between cytomegalovirus seroconversion and upper genital tract infection among women attending a sexually transmitted disease clinic: a prospective study. J. Infect. Dis. 177:1188–1193 [DOI] [PubMed] [Google Scholar]
- 206. Handsfield HH, Chandler SH, Caine VA, Meyers JD, Corey L, Medeiros E, McDougall JK. 1985. Cytomegalovirus infection in sex partners: evidence for sexual transmission. J. Infect. Dis. 151:344–348 [DOI] [PubMed] [Google Scholar]
- 207. Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE, Jr, Cannon MJ. 2008. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988–1994. Sex. Transm. Dis. 35:472–479 [DOI] [PubMed] [Google Scholar]
- 208. Anonymous 2008. Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy—United States, 2007. MMWR Morb. Mortal. Wkly. Rep. 57:65–68 [PubMed] [Google Scholar]
- 209. Monif GR, Egan EA, II, Held B, Eitzman DV. 1972. The correlation of maternal cytomegalovirus infection during varying stages in gestation with neonatal involvement. J. Pediatr. 80:17–20 [DOI] [PubMed] [Google Scholar]
- 210. Grant S, Edmond E, Syme J. 1981. A prospective study of cytomegalovirus infection in pregnancy. I. Laboratory evidence of congenital infection following maternal primary and reactivated infection. J. Infect. 3:24–31 [DOI] [PubMed] [Google Scholar]
- 211. Hadar E, Yogev Y, Melamed N, Chen R, Amir J, Pardo J. 2010. Periconceptional cytomegalovirus infection: pregnancy outcome and rate of vertical transmission. Prenat. Diagn. 30:1213–1216 [DOI] [PubMed] [Google Scholar]
- 212. Feldman B, Yinon Y, Tepperberg Oikawa M, Yoeli R, Schiff E, Lipitz S. 2011. Pregestational, periconceptional, and gestational primary maternal cytomegalovirus infection: prenatal diagnosis in 508 pregnancies. Am. J. Obstet. Gynecol. 205:342.e1–342.e6 [DOI] [PubMed] [Google Scholar]
- 213. Revello MG, Fabbri E, Furione M, Zavattoni M, Lilleri D, Tassis B, Quarenghi A, Cena C, Arossa A, Montanari L, Rognoni V, Spinillo A, Gerna G. 2011. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: a 20-year experience. J. Clin. Virol. 50:303–307 [DOI] [PubMed] [Google Scholar]
- 214. Revello MG, Gerna G. 2004. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J. Clin. Virol. 29:71–83 [DOI] [PubMed] [Google Scholar]