Abstract
Emerging fungal diseases due to black yeasts and relatives in domestic or wild animals and in invertebrates or cold- and warm-blooded vertebrates are continually being reported, either as novel pathogens or as familiar pathogens affecting new species of hosts. Different epidemiological situations can be distinguished, i.e., occurrence as single infections or as zoonoses, and infection may occur sporadically in otherwise healthy hosts. Such infections are found mostly in mammals but also in cold-blooded animals, are frequently subcutaneous or cerebral, and bear much similarity to human primary disorders. Infections of the nervous system are mostly fatal, and the source and route of infection are currently unknown. A third epidemiological situation corresponds to pseudoepidemics, i.e., infection of a large host population due to a common source. It is often observed and generally hypothesized that the susceptible animals are under stress, e.g., due to poor housing conditions of mammals or to a change of basins in the case of fishes. The descriptions in this article represent an overview of the more commonly reported and recurring black fungi and the corresponding diseases in different types of animals.
INTRODUCTION
Phaeohyphomycosis is an umbrella term covering subcutaneous and systemic infections caused by pigmented fungi, where the agents are present in host tissue with brownish to olivaceous hyphal elements (1, 2). Fungal cells may also be unicellular and yeast-like, which makes the term somewhat confusing. The dark coloration of these fungi is due to the presence of melanin in the cell wall. This distinguishes them from other hyphomycetes that in tissue show similar morphology but lack pigmentation, the hyaline fungi (3), causing hyalohyphomycoses. Two more histopathological types are described, viz., chromoblastomycosis and mycetoma, characterized by spherical, cruciately septate “muriform cells” and multicellular grains, respectively. Clinically, phaeohyphomycoses range from superficial colonization to systemic abscess formation and dissemination (4).
Among the melanized fungi, the so-called “black yeasts” and their relatives are particularly significant as agents of disease in cold-blooded vertebrates (5). The term “black yeasts” indicates those melanized fungi that are able to reproduce in culture by unilateral budding (6). Not all members of the group causing infection have this ability, and therefore the fungi are united on the basis of phylogenetic relationships. They belong to a limited number but phylogenetically dispersed orders of ascomycetes (7) and show divergent adaptations to extreme conditions (8), among which are the bodies of animal hosts.
During the last decades, the potential pathogenicity of black yeast infections in crustaceans, captive and farmed fish, amphibians, aquarium animals, and other cold-blooded vertebrates has increasingly been recognized (9–12). Reports of black yeast-like infections in warm-blooded animals are relatively scant (13, 14), and these infections are mostly neurological. Similar to infections in humans, systemic phaeohyphomycoses frequently occur in otherwise healthy hosts and thus may be expected as emerging opportunists in immunocompromised or otherwise debilitated vertebrates and then may take (pseudo)epidemic proportions, as is frequently observed in farmed fish (5). Given the potential significance of black yeasts and their relatives to animal health and the scattered knowledge on these fungi in the veterinary literature, the present paper reviews the most important infections of animals due to these microorganisms.
CHARACTERISTICS OF BLACK YEASTS AND RELATIVES
A hierarchic taxonomy of black yeasts and relatives of veterinary importance is shown in Table 1, classified as described by Hibbett et al. and de Hoog et al. (15, 16). Mainly members of three orders are involved: the Pleosporales, the Ochroconiales, and particularly the Chaetothyriales. The orders belong to different subclasses and thus are totally unrelated. A fourth order, the Capnodiales, has been listed because of frequent reports of “Cladosporium” infections. However, Cladosporium is a very common genus of saprobic contaminants, and thus far none of the cases has been proven by sequencing. Morphologically similar species of Fonsecaea (Chaetothyriales) probably were involved.
Table 1.
Schematic classification of black fungi causing animal diseases
| Fungal pathogen (kingdom Fungi, phylum Ascomycota, subphylum Pezizomycotina) | Target animala | Reference(s) |
|---|---|---|
| Class Dothideomycetes | ||
| Order Capnodiales, family Davidiellaceae | ||
| Cladosporium cladosporioides | Ba (toad) | 31 |
| M (dog) | 107 | |
| M (sheep) | 14 | |
| Cladosporium sp. | M (cat) | 83, 84, 99, 100 |
| Order Pleosporales, family Pleosporaceae | ||
| Alternaria alternata | M (cat) | 79, 86, 87, 88, 89, 90, 91, 92, 93, 94 |
| M (horse) | 110, 111 | |
| M (zebra) | 112 | |
| Alternaria infectoria | M (dog) | 87 |
| Bipolaris spicifera | M (dog) | 103, 104 |
| Phoma herbarum | F (chinook salmon) | 46 |
| Class Eurotiomycetes | ||
| Order Chaetothyriales, family Herpotrichiellaceae | ||
| Capronia moravica | I (mussel) | 24 |
| Cladophialophora bantiana | M (cat) | 79, 87, 88, 89, 90, 91, 92, 94 |
| M (leopard) | 97, 98 | |
| M (dog) | 105, 108 | |
| M (alpaca) | 13 | |
| Exophiala angulospora | F (weedy seadragons) | 38 |
| Exophiala aquamarina | F (leafy seadragons) | 38 |
| F (sand lance) | 38 | |
| Exophiala attenuata | M (cat) | 95 |
| Exophiala cancerae | I (crab) | 11, 12 |
| Exophiala equina | R (Galapagos tortoise) | 66 |
| Exophiala jeanselmei | I (earthworm) | 25 |
| R (eastern box turtle) | 67 | |
| Exophiala oligosperma | R (Aldabra tortoise) | 68 |
| Exophiala pisciphila | F (Atlantic salmon) | 40 |
| F (Channel catfish) | 42 | |
| F (smooth dogfish) | 41 | |
| Exophiala salmonis | F (cutthroat trout, Atlantic salmon) | 36 |
| Exophiala spinifera | M (cat) | 90 |
| Exophiala sp. | F (seahorse) | 39 |
| F (Japanese flounder) | 43 | |
| F (King George whiting) | 45 | |
| Fonsecaea brasiliensis | I (crab) | 5, 21 |
| Fonsecaea multimorphosa | M (cat) | 101 |
| Fonsecaea pedrosoi | Ba (toad) | 34 |
| Fonsecaea sp. | Ba (frog) | 10,32 |
| Phialophora verrucosa | M (cat) | 84, 85, 88 |
| M (dog) | 85 | |
| Phialophora sp. | Ba (frog) | 10, 32 |
| Rhinocladiella sp. | Ba (frog) | 10, 32 |
| Order Ochroconiales, family Ochroconiaceae | ||
| Ochroconis gallopava | Bd (turkey, chicken) | 20, 71, 72, 73, 75, 76, 77 |
| Bd (gray-winged trumpeter) | 77 | |
| Bd (quail) | 78 | |
| Bd (owl) | 74 | |
| M (cat) | 102 | |
| Ochroconis humicola | Ba (toad) | 27 |
| F (coho salmon) | 47 | |
| F (Atlantic salmon) | 48 | |
| F (rainbow trout) | 49 | |
| F (scorpion fish) | 50 | |
| F (walking catfish) | 51 | |
| R (tortoise) | 70 | |
| Ochroconis tshawytschae | F (chinook salmon) | 52 |
I, invertebrates; Ba, batracians; R, reptiles; F, fishes; M, mammals; Bd, birds.
Factors which are of significance for pathogenicity of black fungi include the presence of melanin and carotene, formation of thick cell walls and meristematic growth (production of swollen, isodiametrically enlarging cell clumps with thick cell walls, in which melanin is usually deposited), presence of yeast-like phases, presence of additional forms of conidiogenesis, thermo- and osmotolerance, adhesion, hydrophobicity, assimilation of aromatic hydrocarbons, production of extracellular polysaccharides, siderophores, and acidic or alkaline secondary metabolites (6). The melanin pigment is believed to contribute to the organism's ability to elude host immune responses. This is thought to be achieved through blocking the effects of hydrolytic enzymes on the cell wall and scavenging free radicals released by phagocytic cells during the oxidative burst (3, 17).
Black yeasts of the Chaetothyriales present a complex ecological behavior. Numerous species are isolated from odd environments, such as those polluted with aromatic hydrocarbons. A role of an assimilative ability of these compounds has been surmised on the basis of their structural similarity with neurotransmitters, explaining their predilection for nervous system tissue (18, 19). Black yeasts species able to grow at temperatures of 37°C or above (in the bantiana, dermatitidis, and jeanselmei clades) (5) may cause systemic or disseminated infections in mammals, while those with a maximum growth temperature of around 35 to 37°C (carrionii and europaea clades) cause (sub)cutaneous and superficial infections in humans but are prevalent in cold-blooded animals. In addition, black yeast species with a maximum growth temperature of 50°C (Ochroconis gallopava) are able to infect birds, which have a body temperature well above that of humans, systemically (20). Nonthermotolerant species with maximum growth temperatures of 27 to 33°C may cause diseases in cold-blooded animals and, rarely, invasive infections in other animals (5).
DISEASES IN INVERTEBRATES
Recent reports underline that black yeasts are able to infect a large spectrum of invertebrates, including arthropods (especially crustaceans), mollusks, and annelids. To our knowledge, insects are not susceptible to particular black fungi. Fatal diseases may be observed in insects due to other pathogens, such as Beauveria spp., Cordyceps spp., Conidiobolus spp., Metarrhizium spp., and Hirsutella spp. These hyaline fungi are considered an environmentally friendly management option to control crop pest agents or vectors.
Crustaceans
Farmed and captured crustaceans (shrimps, crabs, lobsters, etc.) contribute to a significant proportion of global seafood production. Fungal disease therefore has been identified as a significant bottleneck for the successful harvesting of wild and cultured crustaceans. For example, lethargic crab disease (LCD), a systemic infection characterized by hematogenous dissemination, has been reported to cause extensive population depressions in the mangrove land crab (Ucides cordatus) in the northeastern region of the Brazilian coast (11, 12, 21). The impact of this infirmity is likely to be severe (22), as crabs represent an important fishery resource for local communities (23), Causative agents were described as Exophiala cancerae (5) and Fonsecaea brasiliensis (21). Clinical signs included increasingly weak motor control, particularly of pereiopods and chelae, causing lethargy and poor balance, followed by the death of the affected crab. Tetany of the claws was also observed in many crabs with other signs of the disease. The histopathology of crabs with variable signs of LCD indicated that the most affected tissues were the epidermis, connective tissue, heart, hepatopancreas, nervous system, and gills. Necrosis, tissue degeneration, and congestion of hemal sinuses (the two principal empty areas along the digestive tube) and vessels were present in heavily infected organs. Nerve fibers were compressed by accumulations of yeast-like cells. In heavy infections, the tissue of gill lamellae was destroyed with subsequent dilatation or compression. Cellular immune responses included hemocytic infiltration, agglutination and encapsulation, and phagocytosis. Phagocytosis of yeast-like cells was abundant in the connective tissue associated with the exoskeleton (12).
Mollusks
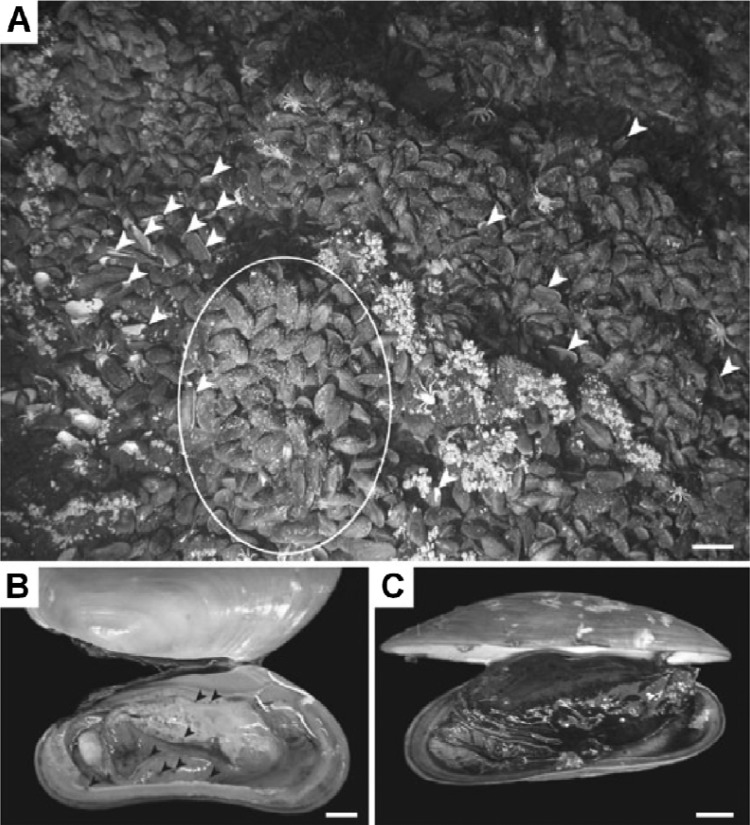
Dover et al. reported massive mortalities in populations of mussels (Bathymodiolus brevior) due to an epizootic event of Capronia moravica in the deep sea at the Mussel Hill hydrothermal vent in the Fiji Basin (24). The mussels presented a range of disease states, from apparently healthy individuals with normal creamy coloration, fleshy tissues, and well-developed gonads to mussels with scattered spots of brownish discoloration of mantle tissues to progressively more discolored bodies to, in the most dramatic cases, black-body stages. General deterioration of mussel condition was evident. Black-body mussels had a distinctive odor, which was neither that of hydrogen sulfide, as is typical for mussels from hydrothermal vents, nor that of putrescence (Fig. 1). Histological examination of brown spots on mantle tissues revealed the presence of dense populations of thick-walled yeast cells up to 15 μm in length. Connective tissues surrounding the gonads and digestive diverticula of brown-spotted mussels were also infected with black yeast and were infiltrated by hemocytes.
Fig 1.
Mussels. Massive mussel mortality due to Capronia (a member of Chaetothyriales)-like black yeast infection is shown. (Reproduced from reference 24 with permission of the publisher.) (A) The paired empty mussel shells (arrowheads) and area of broken shells on the left side of the photograph were observed in mussel beds. Scale bar, 10,000 mm. (B and C) Clinical signs of diseased mussels. (B) Brown-spot stage of infection (highlighted with black arrowheads). Scale bar, 1,000 mm. (C) Black-body stage of infection, involving the whole body. Scale bar, 1,000 mm.
Annelids
Exophiala jeanselmei was isolated by Vakili (25) from naturally infected late embryonic stages of the earthworm Octolasian tyrtaeum and from the cocoon albumen of Eisenia foetida. Spherical black granules, consisting of brown to black hyphae and cell aggregates, were observed in cocoon albumen and tissues of infected embryos of Octolasian tyrtaeum and albumen of E. foetida, which included both healthy-appearing and necrotic eggs. Excised black granules, when suspended in water, released both single and budding black yeast cells. The disease was reproduced by artificial inoculation and reisolation of the organism from cocoons (25).
DISEASES IN COLD-BLOODED VERTEBRATES
Outbreaks of infections by melanized fungi in cold-blooded animals appear to be relatively frequent in captive and farmed fishes, amphibians, and aquarium animals (5). This may cause severe losses in aquaculture and fishery industries.
Amphibians
Amphibians are distinctive among animals physiologically, morphologically, and medically. Their collectively unique life histories and the considerable gaps in our knowledge concerning diseases and veterinary care make it comparatively challenging to diagnose their disease, to treat them, and to maintain them successfully in live condition (10, 26). Fungal diseases of amphibians are closely related to diseases of other ectothermic vertebrates. Transmission usually occurs through contamination from the environment, interclass transmission (e.g., from fish to amphibian), and traumatic injury to the skin, which may contribute to the infection (27). The possibility that infection switches from a fish population to amphibians seems to be an important epidemiological consideration for amphibian disease (10).
Fungi and fungus-like organisms cause a number of diseases among amphibians, including chytridiomycosis (see below), phaeohyphomycosis, zygomycoses, saprolegniasis (a fungal disease caused by species of Saprolegnia mainly in freshwater salmonids), and ichthyophoniasis (a systemic granulomatous disease caused by Ichthyophonus hoferi) (10). Currently, the most significant and well-described pathogen of amphibians is a chytrid fungus, Batrachochytrium dendrobatidis, which is an ubiquitous, keratinophilic or chitinophilic, sporozooic fungus located in moist and aquatic environments.
Batrachochytrium dendrobatidis is responsible for fatal infections in amphibians and, despite being in a phylum not known for pathogenicity in vertebrates, is now recognized as a primary driver of amphibian declines (28, 29).
Toads appear to be more susceptible to the black yeast infection when they are debilitated and stressed (27, 30). Spontaneous disease occurs predominantly in stressed animals after removal from their natural habitat (10, 31). Two forms of infection, cutaneous and disseminated systemic, have been reported in both wild and captive animals. Disseminated systemic mycosis was reported in epizootics among a number of wild and captive frogs (Hyla caerule, Pternohylaf odiens, Phyllobatest rinitatis, Rhacophorus spp., and Hyla septentrionalis) in the United States, with granulomatous infection of internal organs (10, 32). The main etiologic agents were attributed to the genera Phialophora, Fonsecaea, Rhinocladiella, while a member of the order Ochroconiales, Ochroconis humicola, also was involved (27). Clinical signs of disease included anorexia, weight loss, ulcers or nodules in the skin, and swelling and lesions of internal organs, including the spleen, liver, and kidney (33). Pigmented hyphae invaded multiple organs with mild cell necrosis and minimal inflammatory cell response (27, 34).
In postmortem examination of laboratory-housed frogs, i.e., the marine toad (Bufo marinus), neurological disorders and multifocal dermatitis were observed. The lesions consisted of severe granulomatous encephalomyelitis, and multiple granulomas were observed in the nasal cavity, lungs, heart, bone marrow, ovaries, and skin (31, 34, 35). Skin lesions were raised papular to vesicular and were ulcerative. Lower limbs were typically affected.
Fishes
Systemic black yeast infections in fish have been described on many occasions (9). The infections are generally considered to be secondary to metabolic factors or stress of captivity or a consequence of water quality problems, trauma (rough handling or aggression), bacterial disease, or parasites (36, 37). Bacterial or parasitic diseases and toxic or environmental conditions may mimic fungal disease to various extents. Furthermore, mycoses may be masked by overwhelming secondary bacterial infection and therefore remain undiagnosed (9). Captivity may be a contributory factor to reduced immune function.
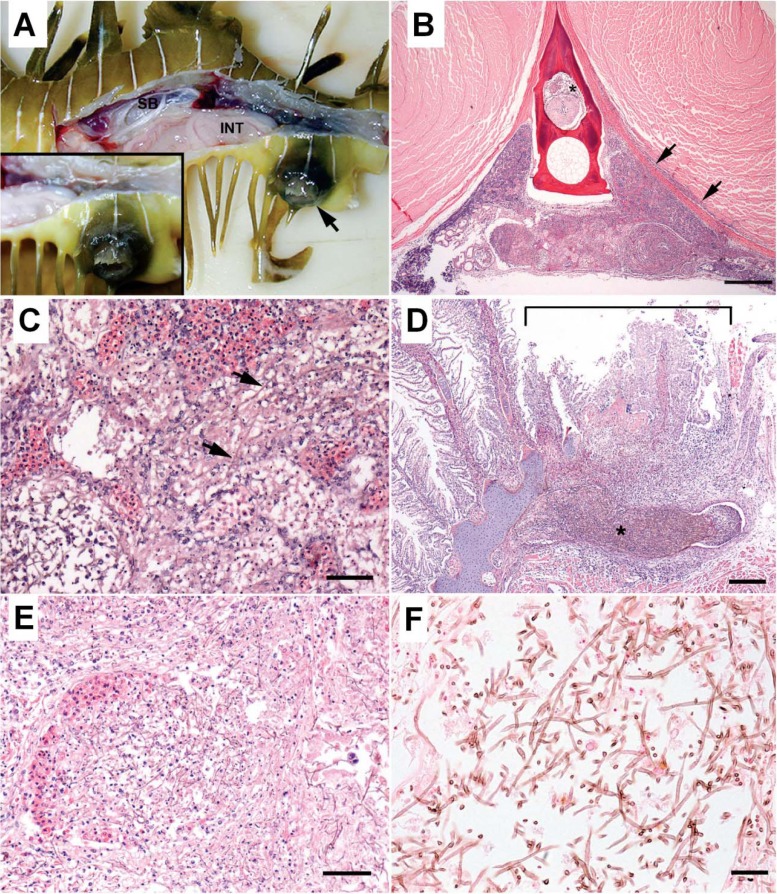
Disseminated infections by Exophiala angulospora were identified repeatedly in aquarium-maintained weedy seadragons (Phyllopteryx taeniolatus) (38). A novel species of Exophiala, now described as E. aquamarina, caused infections in leafy seadragons (Phycodurus eques). Systemic necrotizing lesions and invasion of blood vessels were consistent features (Fig. 2). The host's inflammatory infiltrates, consisting mainly of histiocytes, were mild compared to the extent of necrosis. Granulomas and abscesses were not consistently identified in seadragons. Infection in these seadragons could be the result of an inadequate or deficient host immunological response. In another report on Exophiala infection in seahorses (39), clinical signs included lethargy, disoriented swimming, and nonulcerative dermal masses. Internally raised white to yellow areas were seen in multiple organs, particularly in the liver. Chronic cases had granulomatous inflammation similar to that seen in cases of mycobacteriosis.
Fig 2.
Seadragon. (A) Skin ulcer. (B to F) Histopathological features in renal parenchyma (B), dorsal trunk (C), gill (D) blood vessel in kidney (E) and kidney tissue (F). Disseminated phaeohyphomycosis is caused by an Exophiala sp. (Reproduced from reference 38 with permission of the publisher.) (A) Leafy seadragon with lateral body wall removed to expose coelomic viscera. A skin ulcer (arrow) is located in the skin adjacent to the cloaca. Inset, closer view of the ulcer with raised black margins. SB, swim bladder; INT, intestine. (B) Extensive necrosis involving approximately two-thirds of the renal parenchyma, including the presence of fibrin and cells in the extradural sinus (asterisk) and an infiltrate along the fascia and margin of adjacent epaxial muscle (arrows). Hematoxylin and eosin staining was used. Scale bar, 0.5 mm. (C) Transverse section of dorsal trunk with high magnification of renal parenchyma reveals innumerable filamentous brown fungal hyphae (arrows) coursing through necrotic tubules, interstitium, and sinusoids. Hematoxylin and eosin staining was used. Scale bar, 0.05 mm. (D) Focally extensive necrosis of several consecutive filaments and their lamellae (bracket) overlying a region of the arch where a mat of densely intertwined brown fungal hyphae (asterisk) resides within the venous sinus. Hematoxylin and eosin staining was used. Scale bar, 0.2 mm. (E) Intertwined hyphae are present in the blood vessel lumen, and there is necrosis of a segment of the wall. Hematoxylin and eosin staining was used. Scale bar, = 0.2 mm. (F) Fungal hyphae are slender, filamentous, and septate with occasional right-angle branches. Walls of hyphae stain brown, indicative of melanin. Fontana-Masson staining was used. Scale bar, 0.025 mm.
Systemic Exophiala aquamarina infection was also recently described in a sand lance (a fish belonging to the Ammodytidae family), an Ammodytes sp. (A. Nyaoke, E. S. Weber, C. Innis, D. Stremme, C. Dowd, L. Hinckley, T. Gorton, B. Wickes, D. Sutton, S. de Hoog, and S. Frasca, Jr., unpublished data). Gross lesions included extensive cutaneous ulcers with well-demarcated black margins and multiple dark foci in gills and viscera. Fungal hyphae were identified in lesions consisting of necrosis of skeletal muscle, kidney, gill, heart, spleen, pancreas, and mesentery. Hyphae were also present in the extradural sinus and spinal cord of the fish.
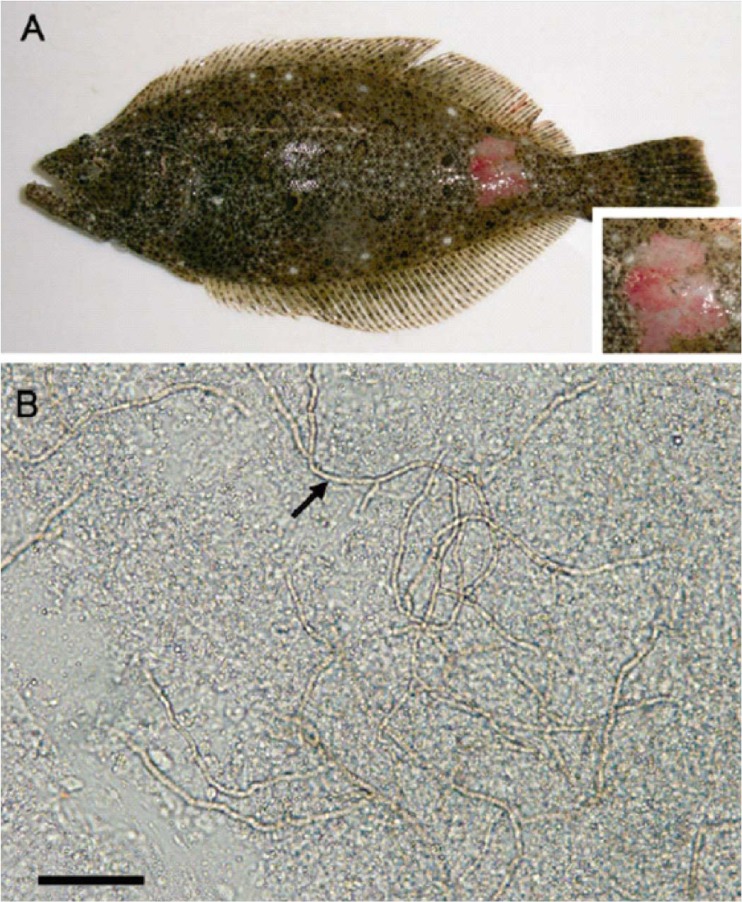
Localized and systemic Exophiala infections have been reported in several freshwater and marine teleosts (any member of a large and extremely diverse group of ray-finned fishes), resulting in a variety of inflammatory responses. Exophiala salmonis was reported to be a cause of cerebral infections in cutthroat trout fingerlings (Oncorhynchus clarkii). Lesions were characterized by granuloma formation with numerous giant cells in brain and cranial tissues. The infection extended peripherally to include surrounding cranial structures, such as eyes and gills (36). Exophiala salmonis was also described as a cause of up to 40% mortality in Atlantic salmon (Salmo salar) hatcheries (36). Lesions in these salmon were systemic and involved brain and kidney. The host's inflammatory response varied from granulomatous to granulocytic, with the formation of microabscesses (36). Similarly, Exophiala pisciphila infection was associated with high mortality in Atlantic salmon. Hyphae invaded cranial structures, including semicircular canals, and the lateral body line, accompanied by a granulomatous inflammatory reaction (40). Exophiala pisciphila was identified in cutaneous and visceral lesions with a predilection for the kidney in channel catfish (Ictalurus punctatus), and skin and brain lesions were observed in smooth dogfish (Mustelus canis) (41, 42). The ulcerative lesions in Japanese flounder (Paralichthys olivaceus) caused by a species of Exophiala (43), in captive American plaice (a right-eyed flatfish belonging to the Pleuronectidae family) and Hippoglossoides platessoides due to Exophiala pisciphila (44), and in captured King George whiting (Sillaginodes punctata) due to an Exophiala species (45) were limited to the skin (Fig. 3). Systemic phaeohyphomycosis has also been associated with Phoma herbarum from a variety of fish species, including the Chinook salmon (Oncorhynchus tshawytscha) (46). The outbreak was reported in hatchery-reared fingerlings. Affected fish exhibited abnormal swimming behavior, exophthalmia, multiple rounded areas of muscle softening, protruded hemorrhagic vents, and abdominal swelling. In all affected fishes, swim bladders were filled with a whitish creamy viscous fungal mass, surrounded by dark red areas in swim bladder walls, kidneys, and musculature (46).
Fig 3.
Skin of a fish (Japanese flounder) with cutaneous phaeohyphomycosis due to an Exophiala sp. (Reproduced from reference 43 with permission of the publisher.) (A) Ulcerative skin lesion in the caudal part of the ocular (eyed) side, a clinical sign of diseased fish. (B) Numerous fungal hyphae (arrow) in the skin lesion up to the edge of the slide. Scale bar, 0.05 mm.
In several cases, two relatives of Ochroconis gallopava mentioned above, viz., O. humicola and O. tshawytshae, were reported to occasionally cause infection in fish. Thus, the possible existence of virulence factors in Ochroconis species should be considered, as thermotolerance is excluded for pathogenicity in cold-blooded animals. Ochroconis humicola is a rare pathogen of cold-blooded vertebrates, particularly fish such as coho salmon (Oncorhynchus kisutch) (47), Atlantic salmon (Salmo salar) (48), rainbow trout (Oncorhynchus mykiss) (49), scorpion fish (Inimicus japonica) (50), and walking catfish (Clarias batrachus) (51). A case of infection in chinook salmon due to Ochroconis tshawytschae was also reported (52).
Reptiles
Fungal disease has been documented worldwide in all orders and suborders of the reptilians with the exception of the Rhynchocephala (Tuataras) (53, 54). As in other vertebrates, fungal pathogens in reptiles may be primary or secondary invaders. However, relatively few mycotic diseases have been described in free-ranging reptiles (55). Due to the terrestrial lifestyle of many reptiles, most of the infections are hyalohyphomycoses of skin caused particularly by Chrysosporium ancestors of dermatophytes (56–59). The fungi isolated from skin lesions in reptiles further include Paecilomyces, Penicillium, Fusarium, Geotrichium, Mucor, and Aspergillus. Systemic mycotic diseases such as histoplasmosis, coccidioidomycosis, and cryptococcosis are rarely seen in reptiles (55). Fungal diseases may be promoted by immune-compromising conditions, such as husbandry deficiencies or inappropriate temperatures, humidity, or enclosure hygiene (60, 61). Furthermore, reptiles are becoming more popular as companion animals and are therefore presented more frequently to veterinary care (62).
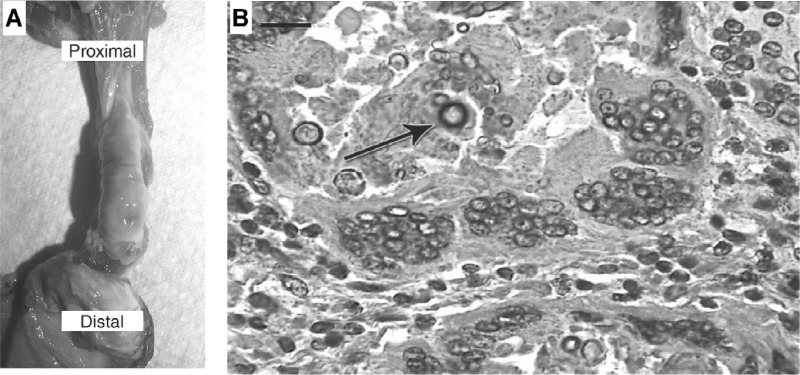
Black yeast diseases have been reported from a variety of reptiles. In a study by Jacobsen, muriform cell-like fungal elements were observed in different internal organs of a snake, mimicking human chromoblastomycosis (63). Similar histopathology was reported in a reticulated python (Python reticulatus) with a severe ulcerative dermatitis on the ventral scales (64) and in a boa constrictor (Constrictor constrictor) (65). Manharth et al. described phaeohyphomycosis in a captive Galapagos tortoise (Geochelone nigra) caused by an Exophiala species (now identified as E. equina), which had spread to the lungs and eyes (66). Joyner et al. explored a subcutaneous inflammatory mass in an eastern box turtle (Terrapene carolina) due to Exophiala jeanselmei (67). Gross necropsy revealed a firm, encapsulated subcutaneous mass filled with dark brown-black, friable necrotic material of the distal right hind limb. Microscopically, the mass was characterized by a granulomatous inflammatory process with numerous multinucleated histiocytic giant cells. Brown-pigmented fungal hyphae and conidia were present within necrotic centers and associated with multinucleated cells (Fig. 4).
Fig 4.
Skin of an Eastern box turtle with cutaneous phaeohyphomycosis due to an Exophiala sp. (Reproduced from reference 67 with permission of the publisher.) (A) Necroscopic observation of the right hind limb showed that skin is reflected distally to expose a subcutaneous mass. Small opening, edema, and pigmentation are demonstrated in the caudodistal portion of the capsule. (B) A histopathological section of lesion shows the granuloma with peripheral multinucleated histiocytic giant cells and a necrotic center with brown-pigmented fungal hyphae and conidia (arrow). Hematoxylin and eosin staining was used. Scale bar, 0.01 mm.
In a study by Stringer et al. (68), infection of bones in an Aldabra tortoise (Geochelone gigantea) presenting with a deep flaking area of the carapace (dorsal section of the shell) was reported. Histological examination of biopsy specimens from this area revealed phaeohyphomycosis of the superficial keratinized layers. After several weeks of regular debridement, deep bone involvement was evident and was confirmed through histological examination. PCR analysis of extracted DNA from the fixed tissue block identified the fungus as Exophiala oligosperma (68). In an older report, Cladosporium species were isolated from granulomatous lesions from the mandible of an adult anaconda (Eunectes mutinous) (69), but this identification is questionable.
In a study by Weitzman et al. (70), Ochroconis humicola was isolated from foot lesions in a tortoise, Terrapine carolina var. carolina. Dematiaceous hyphae were observed in KOH preparations of the biopsy specimen and in stained preparations (70).
DISEASES IN WARM-BLOODED VERTEBRATES
Poultry and Wild Birds
Infections by black fungi of the Chaetothyriales are absent in birds, but members of Ochroconiales have repeatedly been reported (71, 72). Most infections occur sporadically in wild birds maintained in captivity, but at times they may take the form of outbreaks in poultry. Transmission may then occur by inhalation of fungal elements from contaminated litter.
The pathogenicity of fungal species in birds seems to be of a basically different nature from that in amphibians and reptiles. For example, Ochroconis gallopava is a thermotolerant dematiaceous fungus with a maximum growth temperature of 50°C. This fungus is generally found in warm habitats such as hot springs and thermal soils and causes subcutaneous and systemic mycosis (encephalitis) in poultry and other birds (20, 71–74). Infections have been reported in birds, in which the body temperature is much higher (>40°C) than that in mammals, independent of their immunological status. However, some reports concerned 1-day-old chicks having an immature immunity.
A significant neurotropic potential of O. gallopava has been well described in epidemic encephalitis in turkeys (Meleagris gallopavo) less than 1 year old (71, 72, 75, 76). In these reports, epidemic encephalitis occurred in hundreds of turkeys and chickens from the same flocks. Investigators were able to reproduce the infection experimentally by inoculating Ochroconis gallopava into the bird's respiratory tract; the resultant central nervous system (CNS) disease mimicked the naturally acquired infection. Cerebral phaeohyphomycosis also has been recognized in other species, including gray-winged trumpeters (Psophia crepitans) and quail chicks (Coturnix spp.) (77, 78).
In a report by Salkin et al., Ochroconis gallopava was found to cause fatal encephalitis in a captivity-bred snowy owl chick (Nyctea scandiaca) (74). The healthy bird showed an acute onset of unusual neurological signs, i.e., the sudden development of ataxia, severe intermittent torticollis, and rigidity of the legs with repeated rocking backward to the point of falling over. Microscopic examination of sections of the brain stained with hematoxylin and eosin (H&E) revealed widespread invasion with septate, melanized hyphae.
Cats and Dogs
Numerous reports are available about phaeohyphomycoses in cats and dogs, where cats seem to be somewhat more susceptible than other carnivores (79). Reports of deep infections in cats include nasal (80), renal (81), and ocular (82) involvement, as well as cerebellar infection (83). Cutaneous, subcutaneous, and systemic phaeohyphomycoses due to Cladophialophora bantiana, Phialophora verrucosa, Fonsecaea pedrosoi, Exophiala spinifera, Alternaria alternata, and A. infectoria have been documented in cats (79, 84–92).
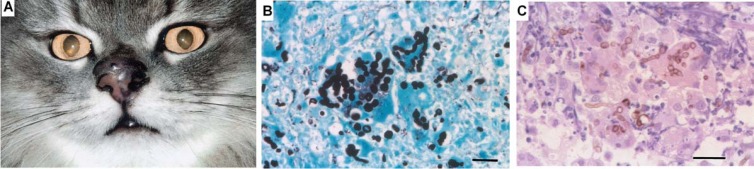
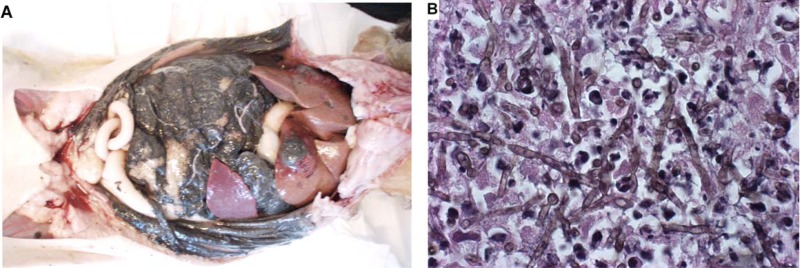
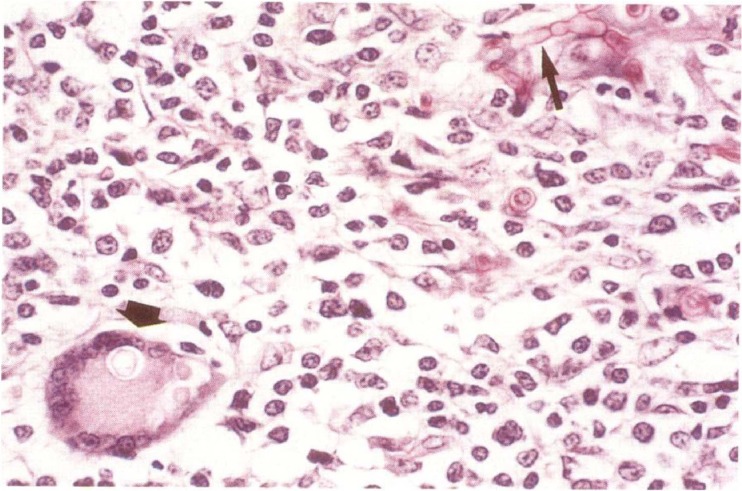
In a study by Abramo et al., a domestic shorthair cat presented with a history of breathing difficulty due to an edematous swelling on the dorsum of the nose and on the left side of the muzzle, which also affected the nostril (86). Histological examination of the nodule revealed a cystic granulomatous dermatitis characterized by neutrophils, macrophages, and giant cells. Pigmented, yeast-like fungus cells of Cladophialophora bantiana and hyphal elements were easily identified in hematoxylin-eosin-stained tissue sections (Fig. 5). Similarly, another case of fatal systemic phaeohyphomycosis in a cat was reported by Elies et al. and was due to Cladophialophora bantiana (79). The authors identified numerous pigmented and septate hyphae after dissecting scattered black granular necrotic areas throughout the liver (Fig. 6).
Fig 5.
Skin of a cat with cutaneous phaeohyphomycosis due to Cladophialophora bantiana. (A) Swollen, edematous, and dark-pigmented nodules on the dorsum of the nose and left nostril. (Reproduced from reference 86 with permission of the publisher.) (B) Several yeast-like fungus cells and hyphal fungal elements. Grocott staining was used. Scale bar, 0.02 mm. (C) Brown-pigmented, short unbranched hyphal elements and yeast-like fungus cells inside macrophages and giant cells. Hematoxylin and eosin staining was used. Scale bar, = 0.02 mm.
Fig 6.
Abdominal cavity of a cat with fatal systemic phaeohyphomycosis due to Cladophialophora bantiana. (Reproduced from reference 79 with permission of the publisher.) (A) At necropsy, the serosal surfaces were covered by a black granular substance, and necrotic foci are scattered throughout the liver. (B) Numerous pigmented and septate hyphae in a necrotic area. Hematoxylin and eosin staining was used.
Miller examined histological slides corresponding to 77 cases of nodular granulomatous skin disease associated with fungal infection in cats in the United Kingdom (93). Based on the aspect of the fungal elements, initial histological diagnoses were hyalohyphomycosis (64 cases), phaeohyphomycosis (5 cases), and dermatophytic pseudomycetoma (8 cases). All cases of hyalohyphomycosis were at first suspected to be alternariosis based on common features, including the anatomical distribution of lesions (48 of 64 cases involved the nostril and bridge of nose, face, and ears), pattern of histological changes, and, of course, results of fungal culture. Cases that were initially diagnosed as phaeohyphomycosis were histologically similar to those of alternariosis, except the causative organisms were deeply pigmented brown and had a variety of morphologies that were different from those of Alternaria. These findings indicate that alternariosis is by far the most common nodular fungal skin disease of cats in the United Kingdom. A recent investigation by Bernhardt et al. indicated that the same situation probably occurs in cats in Germany (94). In a study by Chermette et al., Exophiala attenuata was also reported as the agent of nasal granuloma in a cat (95).
Cladophialophora bantiana was isolated from a wild carnivore (snow leopard, Panthera uncia), underlying canine distemper with spastic paralysis of the hind legs and inability to defecate and urinate. Pigmented hyphae were numerous throughout the lesions and occasionally within lateral ventricles of brain (97, 98).
Cladosporium species have been reported from feline phaeohyphomycosis in cats (83, 84, 99, 100), but the identification of some of these may be questionable. In a study by Bostock et al., a rapidly growing subcutaneous nodule excised from the nose of a domestic shorthair cat was found to contain a granuloma caused by Exophiala jeanselmei (99). Recently, Fonsecaea multimorphosa from the cerebrum of an Australian spayed female cat that showed circling and incoordination was described (101).
Another neurotropic characteristic of melanized fungi has been found in a study by Padhye et al., who identified Ochroconis gallopava as the causal agent of fatal encephalitis in a young cat (102). The etiologic fungus was isolated from the brain and disseminated lesions in the lungs and the mesenteric lymph nodes. Histological examination of the brain, lung, and mediastinal lymph node lesions revealed large numbers of pigmented, septate, branched hyphal elements with swollen intercalary and terminal vesicles and short chains of moniliform hyphal cells. Cultures of the mediastinal lymph nodes yielded O. gallopava.
Canine systemic phaeohyphomycosis may occur due to Ochroconis gallopava or Bipolaris spicifera (103, 104). Black yeast infections in dogs cause mainly cerebral infection (encephalitis), osteomyelitis, or nephritis (105, 106). Clinical and histopathological findings for brain infections in dogs are similar to those for infections in humans. Lesions vary from multifocal encapsulated abscesses to pyogranulomatous inflammation.
In a recent study by Poutahidis et al., Cladosporium cladosporioides from a German shepherd dog with granulomatous encephalitis and nephritis was reported (107). In addition to systemic infections, Cladophialophora bantiana was also isolated as the main cause of eumycetoma in a dog (108). Macroscopic black granules were visible on the ribs, and direct microscopic examination revealed their fungal origin. Cultures yielded pure colonies of C. bantiana.
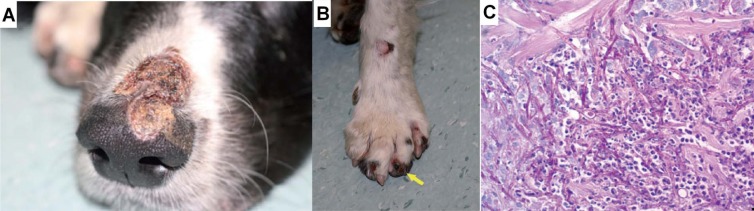
In another study by Dedola et al., Alternaria infectoria was isolated from multiple, purulent, crusting, and erosive to ulcerative lesions over different body areas in a dog under therapeutic immunosuppression (87). Onychorrhexis had occurred on digits, and the underlying corium had blackened (Fig. 7). There were also proliferative and black plaque-like lesions in the mouth. Thick-walled fungal hyphae were detected in impression smears, which were further identified as A. infectoria by sequencing the internal transcribed spacer 1 (ITS1) region of the rRNA gene.
Fig 7.
Skin of a dog with cutaneous infection due to Alternaria infectoria. (Reproduced from reference 87 with permission of the publisher.) (A) A large skin lesion localized over the dorsal muzzle, covered with crust and draining a small amount of purulent exudates. (B) The left forefoot, showing a number of erosive and crusty lesions and, on the fourth digit, onychorrhexis and blackening of the corium (arrow). (C) Histopathological section of ulcerated lesion on the lateral left thigh, which shows the presence of many fungal hyphae and sporulating body-like forms in the section. Periodic acid-Schiff staining was used. Scale bar, 0.02 mm. Magnification, ×400.
Ruminants and Equines
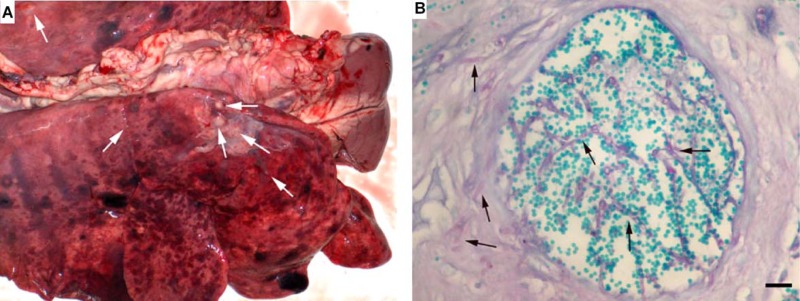
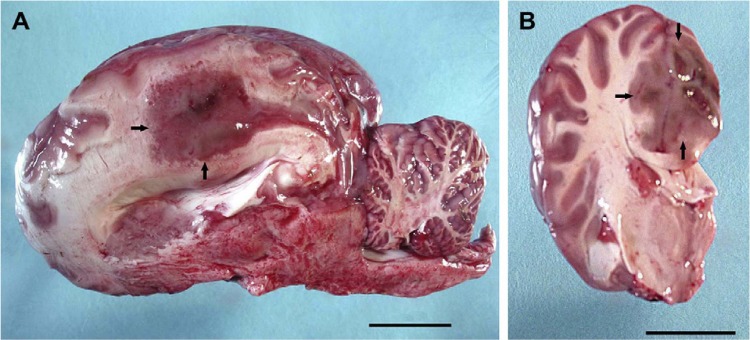
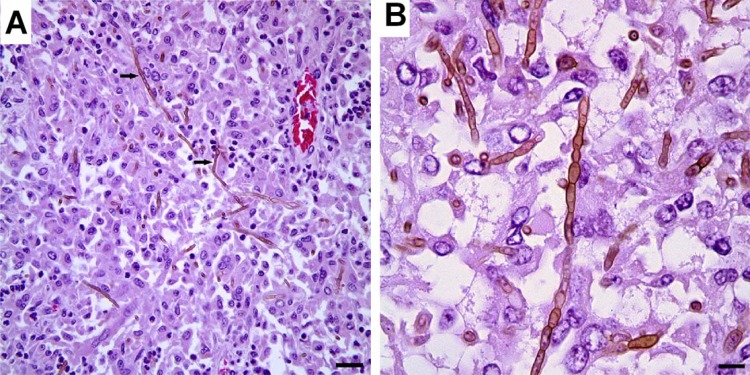
Only a few cases of ruminant and equine phaeohyphomycosis have been reported in the literature. Haligur et al. identified severe systemic phaeohyphomycoses with pulmonary signs of infection due to Cladosporium cladosporioides in merino sheep (14). The sheep were kept under insufficient management conditions in a pen that was humid and overcrowded. The animals had signs of respiratory distress, coughing, fever, and anorexia. All diseased animals died. At necropsy, abnormalities were confined to the lung, abomasum (the fourth and final stomach compartment), lymph nodes, kidneys, liver, and heart, but most of the lesions were localized in the lung and abomasum (Fig. 8). Granulomatous foci were flesh colored, regularly shaped, from 0.2 to 1 cm in size, and purulent. Severe hemorrhages were observed in the lungs, and vasculitis with thrombosis was apparent in various organs, which is suggestive of hematogenous dissemination. Cerebral phaeohyphomycosis was diagnosed in a camelid Huacaya alpaca (Vicugna pacos) (13). The fungal agent was identified as Cladophialophora bantiana by sequencing. Lesions were limited to the cerebrum and were well demarcated, firm, mottled, pale gray to brown red, and with a granular texture, centered on the left and right cingulate gyri (Fig. 9). Microscopically, granulomatous and intralesional necrotizing meningoencephalitis was observed (Fig. 10).
Fig 8.
Lung (A) and abomasum (B) of a merino sheep with fatal systemic phaeohyphomycosis due to Cladosporium cladosporioides. (Reproduced from reference 14 with kind permission from Springer Science+Business Media.) (A) Numerous flesh-colored, regularly shaped granulomatous foci in a lung, ranging from 200 to 1,000 mm (arrows). (B) Fungal agent in an abomasal vessel. Periodic acid-Schiff staining was used. Scale bar, 0.1 mm.
Fig 9.
Brain of a ruminant, huacaya alpaca (Vicugna pacos) with cerebral phaeohyphomycosis due to Cladophialophora bantiana. (Reproduced from reference 13 with permission of Elsevier.) (A) A parasagittal section of the brain demonstrates a 2.5- by 2-cm, fairly well-demarcated, slightly firm, red to gray mass dorsal to the corpus callosum and extending from the level of the post-cruciate gyrus caudally to the level of the occipital lobe (arrows). Scale bar, 2,000 mm. (B) A cross section of the left cerebral hemisphere at the level of the thalamus shows that the mass effaces the neuroparenchyma of the cingulate gyrus and extends into the corona radiata and adjacent gyri (arrows). Scale bar, 2,000 mm.
Fig 10.
Brain of a ruminant, huacaya alpaca (Vicugna pacos), with cerebral phaeohyphomycosis due to Cladophialophora bantiana. (Reproduced from reference 13 with permission of Elsevier.) (A) A photomicrograph of the cerebral granuloma shows that sheets of epithelioid macrophages, multinucleated giant cells, and rare neutrophils surround intracellular and extracellular pigmented fungal hyphae (arrows). Hematoxylin and eosin staining was used. Scale bar, 0.025 mm. (B) A histopathological section of brain shows brown septate hyphae with nonparallel walls, which are 4 by 6 mm. Hematoxylin and eosin staining was used. Scale bar, 0.01 mm.
Equine skin disease may take the form of a chromomycosis-like infection. Abid et al. (109) reported a sharply delimited, firm, brown-black dermal nodule at the right tuber ischium of a horse. Microscopically, the dermis of the excised specimen had multiple pyogranulomas, many of which contained thick-walled, dark brown fungal elements, some with internal septation compatible with muriform cells (109).
Genovese et al. described lymphoid follicle formation in facial nodules of an infected horse due to Alternaria alternata (110). The most striking feature common to all lesions was the infiltration of mature lymphocytes. Even the early lesions sampled did not show distinct pyogranuloma formation but did show a very distinct lymphoid component. The lesions were composed predominantly of small, mature lymphocytes admixed with plasma cells, histiocytes, and neutrophils in smaller numbers. A few plasma cells contained immunoglobulin-containing eosinophilic inclusions (Russell bodies). Lymphoid follicles with germinal centers were prominent in all but small, early lesions. Small aggregates of neutrophils were surrounded by macrophages and formed indistinct pyogranulomas. Periodic acid-Schiff (PAS)-stained sections revealed fungal hyphae (Fig. 11). In contrast to that study, in a case described by Coles et al., the inflammation associated with a fungal infection due to Alternaria alternata (reported under the obsolete name A. tenuis) was pyogranulomatous in multiple dermal nodules on the chest, head, and legs of a horse (111).
Fig 11.
Skin from a horse with cutaneous nodular phaeohyphomycosis due to Alternaria alternata. (Reproduced from reference 110 with permission of BMJ Publishing Group Ltd.) A histopathological section from a skin ulcer shows a Langhans-type giant cell containing fungal hyphae (arrow) and extracellular hyphae (arrowhead). Periodic acid-Schiff staining was used. Magnification, ×400.
Cabanes et al. reported multifocal areas of alopecia and scaling due to Alternaria alternata affecting the skin of the head and neck of a mare (112). Systemic phaeohyphomycosis occurred in a young adult zebra (Equus grevyi) with a history of a sudden onset of weight loss, lethargy, and hypothermia (113). Lesions consisted of focal pyogranulomatous pneumonia and myocarditis. Pigmented fungal hyphae were observed in the lungs, whereas the heart contained only yeast forms.
COMPARATIVE PATHOGENICITIES OF BLACK YEASTS IN ANIMALS
In humans, infections by most groups of melanized fungi occur in immunocompromised hosts, except in the case of black members of the order Chaetothyriales, where all type of infections frequently occur in healthy individuals. Although rare, invasive infections may take a fatal course (114).
Different animal groups harbor various fungal opportunist pathogens. In invertebrates and cold-blooded vertebrates with a moist or mucous tegument or having a water-associated life style, black yeast (Chaetothyriales) infections are prevalent. For these animals, the skin is a major organ, and microbial disintegration frequently leads to death. This also holds true for waterborne reptiles, such as turtles, whereas terrestrial reptiles have a thick, dry, water-repellent skin and are much more often subject to infection by phylogenetically ancestral dermatophyte species (genus Chrysosporium, order Onygenales). Birds and mammals have a protected, water-repellent coverage, and their black yeast infections are unusual. Cutaneous infections mostly involve dermatophytes, while Aspergillus (order Eurotiales) is frequently observed in the airways, especially in birds. Alternaria (Pleosporales) and Ochroconis infections may be encountered in healthy as well as debilitated hosts, such as wild animals kept in captivity; these infections are cutaneous or systemic, respectively.
The melanized members of Pleosporales, Capnodiales, and Ochroconis are found mostly in the environment as plant pathogens, saprobes on plant debris, or colonizing inert rocks, and vertebrate pathogenicity in each species is highly unusual. In contrast, the order Chaetothyriales includes a large number of species which are isolated from human and animal hosts. Several taxa are not even known outside the host. Therefore, the virulence of this order is much more pronounced. All black fungi are obligatorily melanized, and thus melanin alone does not explain this apparent intrinsic virulence. Thermotolerance is not a decisive factor either, since many hosts of Chaetothyriales are cold-blooded. Thermotolerance determines the choice of host: species with maximum growth temperatures of 27 to 33°C may cause diseases in cold-blooded animals, whereas those growing at up to 40°C may cause systemic infections in mammals.
Besides integumental barriers, the complexity of the immune system determines the type of tissue response. Crustaceans have poorly developed innate immunity and no adaptive immunity. An absence of advanced immune responses, such as granuloma formation or a significant inflammatory response, is observed in systemic infections in fish, seahorses, and crabs. In the case of invasive infection, hyphae grow densely and regularly. Primitive fish such as seahorses, with poorly developed innate as well as adaptive immunity, show similar histopathology. Granulomata with lymphocytes, granulocytes, and giant cells are observed only in higher vertebrates, which are equipped with fully developed innate and adaptive cellular immunity.
DIAGNOSIS AND IDENTIFICATION
Diagnosis of infections due to black yeasts at present still requires direct microscopy, culture, histopathology, and mostly also molecular analysis for reliable identification down to the species level (2, 115–117). Fungi with similar morphology may be phylogenetically remote from each other, which has a large impact on evaluation of the clinical course, appropriate therapy, and prophylaxis. For example, saprobic Cladosporium species in the past may have been confused with the superficially similar pathogens Fonsecaea and Cladophialophora. Verification of fungal identity by a reference laboratory is required.
Appropriate sampling, transport, and processing procedures are important considerations to demonstrate melanized fungi in tissue and further their identification in culture (3). Collecting specimens from the source of infection is the most useful approach; however, specimens obtained from areas peripheral to the site of infection, such as blood cultures in hematogenously disseminated disease, may also be useful if the lesion is not accessible (3). Such appropriate sampling procedures have been reported by different investigators (118). In addition, the guidelines regarding the handling of potentially infectious fungi in the laboratory setting should be followed (119, 120). Gross examination may occasionally reveal evidence of melanized fungi as well (Fig. 5). Gram stain, different concentrations of KOH, and the fluorescent calcofluor white stain are the most commonly used methods for the direct examination of specimens (121).
Various selective as well as nonselective media can be used for culture and isolation. A nonselective medium frequently employed is Sabouraud dextrose agar (SDA). Selective media include those containing cycloheximide, media enriched for fastidious organisms such as brain heart infusion (BHI) agar, and also media containing antimicrobial agents to suppress bacteria in specimens collected from nonsterile sites (3, 118, 122).
For histopathology, several types of staining can be used for the demonstration of black yeasts, such as hematoxylin and eosin (H&E), which demonstrates pigmentation in hyphae that are strongly melanized, the melanin Fontana-Masson stain only for light pigmentation, Periodic acid-Schiff (PAS) stain, which is frequently preferred over H&E due to the more vivid colors of hyphae (which stain a bright pink-purple against a green background), and Gomori methenamine silver (GMS) stain for the dramatic visualization of hyphae as dark elements against a green background (123).
Not only does histopathology allow differentiation from other types of disease, including bacterial or foreign body granuloma or squamous cell carcinoma and melanoma, but the presence of melanized hyphae, budding cells, muriform cells, or hyaline swollen cells aids in establishment of the type of disease and the etiologic agent. The rate of successful culturing of fungi from histopathologically positive tissues may be as low as 50% (124). A small number of melanized fungi, e.g., Ochroconis gallopava and Exophiala spinifera, possess sufficient features for phenotypic characterization. For most species, sequencing of the rDNA internal transcribed spacer (ITS) is necessary. Zeng and de Hoog stated that this fragment at present is sufficient for reliable identification of most black yeast species in Chaetothyriales (125). In the remaining orders (Pleosporales and Capnodiales), only occasional agents are noted, which are sufficiently remote from each other to allow reliable identification with the ITS. In a study by Lau et al., a panfungal PCR assay targeting the ITS1 region of fungi was described, which was able to detect several species of black fungi from fresh, formalin-fixed, or paraffin-embedded tissue specimens, including Exophiala spp (126).
TREATMENT AND CONTROL
In the last 2 decades, significant progress in the development of antifungal agents has been made, and the number of available antifungal drugs has increased by 30% since 2000 (127). Despite the availability of various systemic antifungals, griseofulvin, which is the only antifungal approved for systemic administration by the Food and Drug Administration (FDA) for veterinary use, particularly dermatophytosis, is not efficacious against black fungi. Nowadays polyenes, azoles, and echinocandins are the most popular groups of antifungals used for treatment of black yeast infection in animals. From these groups, a variety of systemic antifungals are available, such as amphotericin B, itraconazole, voriconazole, posaconazole, caspofungin, and anidulafungin.
Diseases caused by black fungi are difficult to treat because of their recalcitrant nature, possibly enhanced by the presence of melanin in the cell wall. Infections are chronic and in humans have been observed to reside in tissue for decades (128). Culture and susceptibility testing may provide useful information for selecting appropriate treatment protocols. Black fungi are susceptible to most currently used antifungals (129–131). For instance, on the basis of in vitro results and the findings of previous clinical studies, posaconazole and the new azole isavuconazole seem to be good alternatives to amphotericin B as the standard treatment for C. bantiana cerebral infections (132). In a study by Feng et al., susceptibility testing of Phialophora strains yielded low MIC values for itraconazole, voriconazole, posaconazole, and micafungin, while most strains had high MICs for fluconazole and amphotericin B (133).
The in vitro susceptibility of clinical isolates of Fonsecaea spp. to antifungal drugs was investigated (134). The resulting MICs, in increasing order, were as follows: posaconazole, 0.063 mg/liter; itraconazole, 0.125 mg/liter; isavuconazole, 0.25 mg/liter; voriconazole, 0.5 mg/liter; amphotericin B, 2 mg/liter; caspofungin, 2 mg/liter; anidulafungin, 2 mg/liter; and fluconazole, 32 mg/liter. This indicates that azole antifungals could be better therapeutic options. However, despite antifungal treatment and surgery, relapses are often noted. Importantly, for some infections, such as Ochroconis gallopava, the best antifungal agent and duration of therapy have not yet been defined and need further investigation.
Prophylaxis in animals probably mainly requires prevention of stress. Management practices such as handling, crowding, transporting, fluctuating temperatures, poor water and stable quality, and other husbandry deficiencies may predispose for fungal disease. For waterborne animals, this particularly involves transfer to new basins and large-scale fish farming in the open sea (135). In animal husbandry, poor stable and pen conditions have led to pseudoepidemics in sheep and poultry. Therefore, both the animals and the environment should be treated completely in order to control fungal infection and prevent the possibility of transmission to humans. In addition, similar to the management strategies in other areas of veterinary medicine, education and prevention medicine should be applied by veterinarians and other health professionals to prevent infection and also reduce the risk of zoonosis.
CONCLUDING REMARKS
To the best of our knowledge, black fungi are not on the list of clinically important zoonotic fungi. In animals, three different epidemiological situations can be distinguished. The first feature is single infection, which may occur sporadically in otherwise healthy hosts. Such infections are found mostly in mammals and are frequently subcutaneous or cerebral, and the systemic infections bear much similarity to human primary cerebral infections by melanized fungi (20). Cerebral infections are mostly fatal, and the source and route of infection are currently unknown. Unlike in humans, traumatic infections in animals are uncommon. A second epidemiological profile is represented by pseudoepidemics, i.e., infection of a large host population due to a common source. It is often observed and generally hypothesized that the susceptible animals are under stress, e.g., due to poor housing conditions of mammals or change of basins in the case of fish. Third, true epidemics may occur, which is probably the case with lethargic crab disease.
The relatively frequent infection of amphibians is remarkable. They produce aromatic toxins in their skin, and it has been hypothesized that the presence of alkyl benzenes in host tissue may enhance susceptibility to black yeast infection. In mammals the relation with this type of compound would be expressed by neurotropism, given the alkylbenzene structure of several neurotransmitters.
ACKNOWLEDGMENTS
We thank Osamu Kurata, Cindy Lee Van Dover, and Jonathan M. Sleeman for kind preparation of the original versions of figures, and we thank Cindy Lee Van Dover, Salvatore Frasca, Jr., Osamu Kurata, Jonathan M. Sleeman, Jim Mills, F. Abramo, L. Elies, Carla Dedola, Mehmet Haligur, Gerry M. Dorrestein, C. Frank, and L. M. Genovese for permission to reprint figures.
This publication was prepared by Seyedmojtaba Seyedmousavi, Jacques Guillot, and G. Sybren de Hoog as a collaborative study between the ISHAM Veterinary Mycology and Black Yeasts Working Groups.
Biographies

Seyedmojtaba (Amir) Seyedmousavi obtained a degree in veterinary medicine (D.V.M.) in 2002 from Tehran University and completed his Ph.D. and training in medical and veterinary mycology in 2006. He was previously appointed as a senior lecturer and assistant professor of medical mycology in Iran (2002 to 2009). From 2009 to 2010, he did postdoctoral research and a traineeship in molecular mycology at CBS-KNAW, Netherlands, in the lab of Prof. Dr. G. S. de Hoog. In 2010, he joined the Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Netherlands, as a postdoctoral scientific researcher under the supervision of Prof. Dr. Paul E. Verweij, Prof. Dr. Johan W. Mouton, and Dr. Willem Melchers. His current research focuses on fungal infection models and on the pharmacokinetics and pharmacodynamics (PK/PD) of antifungal agents in experimental models of azole-resistant invasive aspergillosis. He also investigates the comparative utilities of molecular diagnostics as well as biomarkers. He is one of the founding members (2010) and coordinator of the ISHAM veterinary mycology working group.

Jacques Guillot, a graduate of the Veterinary College of Alfort France, completed his training in medical mycology at Pasteur Institute, Paris, France. His Ph.D., obtained from the University Paris Est Créteil in 1995, was about the classification of Malassezia yeasts. His research activity is now focused on the genetic diversity and virulence of airborne fungal pathogens such as Pneumocystis and Aspergillus spp. Jacques Guillot contributed to the description of several original clinical cases of animal mycoses. His entire career has been as a teacher at the Veterinary College of Alfort France, holding the position of full professor of parasitology and mycology since 2002. He has been a corresponding member of the French Academy of Medicine since 2008. With physicians and other veterinarians, Jacques Guillot recently created a research group devoted to the study of the colonization and invasion of pulmonary epithelium by fungal pathogens in mammals and birds.

Sybren de Hoog is senior researcher in phylogenetic and ecological mycology at the Centraalbureau voor Schimmelcultures KNAW Fungal Biodiversity Centre at Baarn, since 2000 in Utrecht. He is also appointed as professor of mycology at the Universities of Amsterdam, Beijing, and Guangzhou and has written over 480 scientific papers, in addition to editing numerous special journal issues. He was program chairman of the TIFI/ECMM congress in Amsterdam (2003). He has prepared the standard work Atlas of Clinical Fungi (with J. Guarro, Reus, Spain), for which an electronic version is available. He has been president of the International Society for Human and Animal Mycology (ISHAM). In this function he assisted in the organization of ISHAM and satellite congresses in Tokyo and Beijing in 2009. His teaching activities comprise the CBS course in medical mycology for hospital personnel and courses in various places in Europe and Asia.
REFERENCES
- 1. McGinnis MR. 1983. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J. Am. Acad. Dermatol. 8:1–16 [DOI] [PubMed] [Google Scholar]
- 2. Ajello L. 1986. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur. J. Epidemiol. 2:243–251 [DOI] [PubMed] [Google Scholar]
- 3. Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin. Microbiol. Rev. 23:884–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salfelder K. 1990. Atlas of fungal pathology. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 5. de Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S. 2011. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27:46–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Hoog GS. 1993. Evolution of black yeasts: possible adaptation to the human host. Antonie Van Leeuwenhoek 63:105–109 [DOI] [PubMed] [Google Scholar]
- 7. de Hoog GS, McGinnis MR. 1987. Ascomycetous black yeasts, p 187–199 In de Hoog GS, Smith MT, Weijman ACM. (ed), The expanding realm of yeast-like fungi. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 8. Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS. 2009. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud. Mycol. 64:123-133S127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanong RP. 2003. Fungal diseases of fish. Vet. Clin. North Am. Exot. Anim. Pract. 6:377–400 [DOI] [PubMed] [Google Scholar]
- 10. Densmore CL, Green DE. 2007. Diseases of amphibians. Ilar J. 48:235–254 [DOI] [PubMed] [Google Scholar]
- 11. Boeger WA, Pie MR, Ostrensky A, Patella L. 2005. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Mem. Inst. Oswaldo Cruz 100:161–167 [DOI] [PubMed] [Google Scholar]
- 12. Boeger WA, Pie MR, Vicente V, Ostrensky A, Hungria D, Castilho GG. 2007. Histopathology of the mangrove land crab Ucides cordatus (Ocypodidae) affected by lethargic crab disease. Dis. Aquat. Organ. 78:73–81 [DOI] [PubMed] [Google Scholar]
- 13. Frank C, Vemulapalli R, Lin T. 2011. Cerebral phaeohyphomycosis due to Cladophialophora bantiana in a Huacaya alpaca (Vicugna pacos). J. Comp. Pathol. 145:410–413 [DOI] [PubMed] [Google Scholar]
- 14. Haligur M, Ozmen O, Dorrestein GM. 2010. Fatal systemic cladosporiosis in a merino sheep flock. Mycopathologia 170:411–415 [DOI] [PubMed] [Google Scholar]
- 15. de Hoog GS, Guarro J, Gene J, Figueras MJ. 2009. Atlas of clinical fungi:the ultimate benchtool for diagnostics. A pilot version of the 3rd ed, CD-ROM. Centraalbureau voor Schimmelcultures, KNAW Fungal Biodiversity Centre/Universitat Rovira i Virgili, Utrecht, Netherlands [Google Scholar]
- 16. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. 2007. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 111:509–547 [DOI] [PubMed] [Google Scholar]
- 17. Nosanchuk JD, Casadevall A. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203–223 [DOI] [PubMed] [Google Scholar]
- 18. Prenafeta-Boldu FX, Summerbell R, de Hoog GS. 2006. Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiol. Rev. 30:109–130 [DOI] [PubMed] [Google Scholar]
- 19. Zhao J, Zeng J, de Hoog GS, Attili-Angelis D, Prenafeta-Boldu FX. 2010. Isolation and identification of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microb. Ecol. 60:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horre R, de Hoog GS. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176–193 [Google Scholar]
- 21. Vicente VA, Orelis-Ribeiro R, Najafzadeh MJ, Sun J, Guerra RS, Miesch S, Ostrensky A, Meis JF, Klaassen CH, de Hoog GS, Boeger WA. 2012. Black yeast-like fungi associated with Lethargic Crab Disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae). Vet. Microbiol. 158:109–122 [DOI] [PubMed] [Google Scholar]
- 22. Glaser M. 2003. Interrelations between mangrove ecosystem, local economy and social sustainability in Caeté Estuary, North Brazil. Wetlands Ecol. Manag. 11:265–272 [Google Scholar]
- 23. Alves RRN, Nishida AK. 2003. Socio-economical aspects and environ-mental perception of Caranguejo-uça U. cordatus (L. 1763) (Deca-poda, Brachyura) gatherers in the Mamanguape river estuary, northeast Brazil. Interciencia 28:36–43 [Google Scholar]
- 24. Dover CLV, Ward ME, Scott JL, Underdown J, Anderson B, Gustaf-son, Whalen CME, Carnegie RB. 2007. A fungal epizootic in mussels at a deep-sea hydrothermal vent. Mar. Ecol. 28:54–62 [Google Scholar]
- 25. Vakili NG. 1993. Exophiala jeanselmei, a pathogen of earthworm species. Med. Mycol. 31:343–346 [Google Scholar]
- 26. Pare JA. 2003. Fungal diseases of amphibians: an overview. Vet. Clin. North Am. Exot. Anim. Pract. 6:315–326 [DOI] [PubMed] [Google Scholar]
- 27. Juopperi T, Karli K, De Voe R, Grindem CB. 2002. Granulomatous dermatitis in a spadefoot toad (Scaphiopus holbrooki). Vet. Clin. Pathol. 31:137–139 [DOI] [PubMed] [Google Scholar]
- 28. Fisher MC, Garner TW, Walker SF. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 63:291–310 [DOI] [PubMed] [Google Scholar]
- 29. Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. 1999. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5:735–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller EA, Montali RJ, Ramsay EC, Rideout BA. 1992. Disseminated chromoblastomycosis in a colony of ornate-horned frogs (Ceratophrys ornata). J. Zoo Wildl. Med. 23:433–438 [Google Scholar]
- 31. Bube A, Burkhardt E, Weiss R. 1992. Spontaneous chromomycosis in the marine toad (Bufo marinus). J. Comp. Pathol. 106:73–77 [DOI] [PubMed] [Google Scholar]
- 32. Elkan E, Philpot CM. 1973. Mycotic infections in frogs due to a Phialophora-like fungus. Sabouraudia 11:99–105 [DOI] [PubMed] [Google Scholar]
- 33. Taylor SK, Green DE, Wright KM, Whitaker BR. 2001. Bacterial diseases, p 159–179 In Wright KM, Whitaker BR. (ed), Amphibian medicine and captive husbandry. Krieger Publishing Company, Malabar FL [Google Scholar]
- 34. Cicmanec JL, Ringler DH, Beneke ES. 1973. Spontaneous occurrence and experimental transmission of the fungus, Fonsecaea pedrosoi, in the marine toad, Bufo marinus. Lab. Anim. Sci. 23:43–47 [PubMed] [Google Scholar]
- 35. Velasquez LF, Restrepo A. 1975. Chromomycosis in the toad (Bufo marinus) and a comparison of the etiologic agent with fungi causing human chromomycosis. Sabouraudia 13:1–9 [DOI] [PubMed] [Google Scholar]
- 36. Otis EJ, Wolke RE, Blazer VS. 1985. Infection of Exophiala salmonis in Atlantic salmon (Salmo salar L.). J. Wildl. Dis. 21:61–64 [DOI] [PubMed] [Google Scholar]
- 37. Silphaduang U, Hatai K, Wada S, Noga E. 2000. Cladosporiosis in a tomato clownfish (Amphiprion frenatus). J. Zoo Wildl. Med. 31:259–261 [DOI] [PubMed] [Google Scholar]
- 38. Nyaoke A, Weber ES, Innis C, Stremme D, Dowd C, Hinckley L, Gorton T, Wickes B, Sutton D, de Hoog S, Frasca S., Jr 2009. Disseminated phaeohyphomycosis in weedy seadragons (Phyllopteryx taeniolatus) and leafy seadragons (Phycodurus eques) caused by species of Exophiala, including a novel species. J. Vet. Diagn. Invest. 21:69–79 [DOI] [PubMed] [Google Scholar]
- 39. Stoskopf MK. 1993. Fish medicine. WB Saunders Co., Philadelphia, PA [Google Scholar]
- 40. Langdon JS, McDonald WL. 1987. Cranial Exophiala pisciphila in Salmo salar in Australia. Bull. Eur. Assoc. Fish Pathol. Biol. 35:117–119 [Google Scholar]
- 41. Gaskins JE, Cheung PJ. 1986. Exophiala pisciphila. A study of its development. Mycopathologia 93:173–184 [DOI] [PubMed] [Google Scholar]
- 42. McGinnis MR, Ajello L. 1974. A new species of Exophiala isolated from channel catfish. Mycologia 66:518–520 [PubMed] [Google Scholar]
- 43. Kurata O, Munchan C, Wada S, Hatai K, Miyoshi Y, Fukuda Y. 2008. Novel Exophiala infection involving ulcerative skin lesions in Japanese flounder Paralichthys olivaceus. Fish Pathol. 43:35–44 [Google Scholar]
- 44. Strongman DB, Morrison CM, McClelland G. 1997. Lesions in the musculature of captive American plaice Hippoglossoides plaessoides caused by the fungus Hormoconis resinae (Deuteromycetes). Dis. Aquat. Organ. 28:107–113 [Google Scholar]
- 45. Reuter RE, Ham WHJ, Davis S. 2003. Exophiala sp. infection in captured King George whiting, Sillaginodes punctata. Bull. Eur. Assoc. Fish Pathol. Biol. 23:128–134 [Google Scholar]
- 46. Faisal M, Elsayed E, Fitzgerald SD, Silva V, Mendoza L. 2007. Outbreaks of phaeohyphomycosis in the chinook salmon (Oncorhynchus tshawytscha) caused by Phoma herbarum. Mycopathologia 163:41–48 [DOI] [PubMed] [Google Scholar]
- 47. Ross AJ, Yasutake WT. 1973. Scolecobasidium humicola, a fungal pathogen of fish. J. Fish Res. Bd. Can. 32:1648–1652 [Google Scholar]
- 48. Schaumann K, Priebe K. 1994. Ochroconls humicola causing muscular black spot disease of Atlantic salmon (Salmo salar). Can. J. Bot. 72:1629–1634 [Google Scholar]
- 49. Ajello L, McGinnis MR, Camper J. 1977. An outbreak of phaeohyphomycosis in rainbow trout caused by Scolecobasidium humicola. Mycopathologia 62:15–22 [DOI] [PubMed] [Google Scholar]
- 50. Wada S, Nakamura K, Hatai K. 1995. First case of Ochroconis humicola infection in marine cultured fish in Japan. Fish Pathol. 30:125–126 [Google Scholar]
- 51. Bhattacharya U. 1988. Scolebasidium humicola, a new fungal fish infection from India. Environ. Ecol. 6:532–533 [Google Scholar]
- 52. Doty MS, Slater DW. 1946. A new species of Heterosporium pathogenic on young Chinook salmon. Am. Mid. Natural. 36:663–665 [Google Scholar]
- 53. Parè JA, Sigler L, Rosenthal KL, Mader DR. 2006. Microbiology: fungal and bacterial diseases of reptiles, p 217–239 In Mader DR. (ed), Reptile medicine and surgery, vol 2 Saunders Elsevier, St Louis, MO [Google Scholar]
- 54. Schumacher J. 2003. Fungal diseases of reptiles. Vet. Clin. North Am. Exot. Anim. Pract 6:327–335 [DOI] [PubMed] [Google Scholar]
- 55. Jacobson ER, Cheatwood JL, Maxwell LK. 2000. Mycotic diseases of reptiles. Semin. Avian Exot. Pet Med. 9:94–101 [Google Scholar]
- 56. Thomas AD, Sigler L, Peucker S, Norton JH, Nielan A. 2002. Chrysosporium anamorph of Nannizziopsis vriesii associated with fatal cutaneous mycoses in the salt-water crocodile (Crocodylus porosus). Med. Mycol. 40:143–151 [DOI] [PubMed] [Google Scholar]
- 57. Pare A, Coyle KA, Sigler L, Maas AK, III, Mitchell RL. 2006. Pathogenicity of the Chrysosporium anamorph of Nannizziopsis vriesii for veiled chameleons (Chamaeleo calyptratus). Med. Mycol. 44:25–31 [DOI] [PubMed] [Google Scholar]
- 58. Bowman MR, Pare JA, Sigler L, Naeser JP, Sladky KK, Hanley CS, Helmer P, Phillips LA, Brower A, Porter R. 2007. Deep fungal dermatitis in three inland bearded dragons (Pogona vitticeps) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. Med. Mycol. 45:371–376 [DOI] [PubMed] [Google Scholar]
- 59. Johnson RS, Sangster CR, Sigler L, Hambleton S, Pare JA. 2011. Deep fungal dermatitis caused by the Chrysosporium anamorph of Nannizziopsis vriesii in captive coastal bearded dragons (Pogona barbata). Aust. Vet. J. 89:515–519 [DOI] [PubMed] [Google Scholar]
- 60. Chinnadurai SK, Devoe RS. 2009. Selected infectious diseases of reptiles. Vet. Clin. North Am. Exot. Anim. Pract. 12:583–596 [DOI] [PubMed] [Google Scholar]
- 61. Cushing A, Pinborough M, Stanford M. 2011. Review of bacterial and fungal culture and sensitivity results from reptilian samples submitted to a UK laboratory. Vet. Rec. 169:390. [DOI] [PubMed] [Google Scholar]
- 62. Taddei S, Dodi PL, Di Ianni F, Cabassi CS, Cavirani S. 2010. Conjunctival flora of clinically normal captive green iguanas (Iguana iguana). Vet. Rec. 167:29–30 [DOI] [PubMed] [Google Scholar]
- 63. Jacobson ER. 1984. Chromomycosis and fibrosarcoma in a mangrove snake. J. Am. Vet. Med. Assoc. 185:1428–1430 [PubMed] [Google Scholar]
- 64. Frank W, Roester U. 1970. Amphibia as carriers of Hormiscium (Hormodendrum) dermatitis Kano, 1937, a causative agent of chromoblastomycosis (chromomycosis) in man. Z. Tropenmed. Parasitol. 21:93–108 [PubMed] [Google Scholar]
- 65. Frank W. 1976. Mycotic infections in amphibians and reptiles, p 73–88 In Page LA. (ed), Wildlife diseases. Plenum, New York, NY [Google Scholar]
- 66. Manharth A, Lemberger K, Mylniczenko N, Pinkerton M, Pessier AP. 2005. Disseminated phaeohyphomycosis due to Exophiala species in a Galapagos tortoise, Geochelone nigra. J. Herpetol. Med. Surg. 15:20–26 [Google Scholar]
- 67. Joyner PH, Shreve AA, Spahr J, Fountain AL, Sleeman JM. 2006. Phaeohyphomycosis in a free-living eastern box turtle (Terrapene carolina carolina). J. Wildl. Dis. 42:883–888 [DOI] [PubMed] [Google Scholar]
- 68. Stringer EM, Garner MM, Proudfoot JS, Ramer JC, Bowman MR, Heng HG, Bradway DS. 2009. Phaeohyphomycosis of the carapace in an Aldabra tortoise (Geochelone gigantea). J. Zoo Wildl. Med. 40:160–167 [DOI] [PubMed] [Google Scholar]
- 69. Marcus LC. 1971. Infectious diseases of reptiles. J. Am. Vet. Med. Assoc. 159:1626–1631 [PubMed] [Google Scholar]
- 70. Weitzman I, Rosenthal SA, Shupack JL. 1985. A comparison between Dactylaria gallopava and Scolecobasidium humicola: first report of an infection in a tortoise caused by S. humicola. Sabouraudia 23:287–293 [PubMed] [Google Scholar]
- 71. Ranck FM, Jr, Georg LK, Wallace DH. 1974. Dactylariosis—a newly recognized fungus disease of chickens. Avian Dis. 18:4–20 [PubMed] [Google Scholar]
- 72. Georg LK, Bierer BW, Cooke WB. 1964. Encephalitis in turkey poults due to a new fungus species. Sabouraudia 3:239–244 [DOI] [PubMed] [Google Scholar]
- 73. Kralovic SM, Rhodes JC. 1995. Phaeohyphomycosis caused by Dactylaria (human dactylariosis): report of a case with review of the literature. J. Infect. 31:107–113 [DOI] [PubMed] [Google Scholar]
- 74. Salkin IF, Dixon DM, Kemna ME, Danneman PJ, Griffith JW. 1990. Fatal encephalitis caused by Dactylaria constricta var. gallopava in a snowy owl chick (Nyctea scandiaca). J. Clin. Microbiol. 28:2845–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blalock HG, Georg LK, Derieux WT. 1973. Encephalitis in turkey poults due to Dactylaria (Diplorhinotrichum) gallopava—a case report and its experimental reproduction. Avian Dis. 17:197–204 [PubMed] [Google Scholar]
- 76. Randall CJ, Owen DM, Kirkpatrick KS. 1981. Encephalitis in broiler chickens caused by a hyphomycete resembling Dactylaria gallopava. Avian Pathol. 10:31–41 [DOI] [PubMed] [Google Scholar]
- 77. Karesh WB, Russell R, Gribble D. 1987. Dactylaria gallopava encephalitis in two grey-winged trumpeters (Psophia crepitans). Avian Dis. 31:685–688 [PubMed] [Google Scholar]
- 78. Shane SM, Markovits J, Snider TG, III, Harrington KS. 1985. Encephalitis attributed to dactylariosis in Japanese quail chicks (Coturnix coturnix japonica). Avian Dis. 29:822–828 [PubMed] [Google Scholar]
- 79. Elies L, Balandraud V, Boulouha L, Crespeau F, Guillot J. 2003. Fatal systemic phaeohyphomycosis in a cat due to Cladophialophora bantiana. J. Vet. Med. 50:50–53 [DOI] [PubMed] [Google Scholar]
- 80. Dhein CR, Leathers CW, Padhye AA, Ajello L. 1988. Phaeohyphomycosis caused by Alternaria alternata in a cat. J. Am. Vet. Med. Assoc. 193:1101–1103 [PubMed] [Google Scholar]
- 81. Reed C, Fox JG, Campbell LH. 1974. Leukaemia in a cat with concurrent Cladosporium infection. J. Small Anim. Pract. 15:55–62 [DOI] [PubMed] [Google Scholar]
- 82. Miller DM, Blue JL, Winston SM. 1983. Keratomycosis caused by Cladosporium sp in a cat. J. Am. Vet. Med. Assoc. 182:1121–1122 [PubMed] [Google Scholar]
- 83. Mariani CL, Platt SR, Scase TJ, Howerth EW, Chrisman CL, Clemmons RM. 2002. Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthair cats. J. Am. Anim. Hosp. Assoc. 38:225–230 [DOI] [PubMed] [Google Scholar]
- 84. Pukay BP, Dion WM. 1984. Feline phaeohyphomycosis: treatment with ketaconazole and 5-fluorocytosine. Can. Vet. J. 25:130–134 [PMC free article] [PubMed] [Google Scholar]
- 85. Beccati M, Vercelli A, Peano A, Gallo MG. 2005. Phaeohyphomycosis by Phialophora verrucosa: first European case in a cat. Vet. Rec. 157:93–94 [DOI] [PubMed] [Google Scholar]
- 86. Abramo F, Bastelli F, Nardoni S, Mancianti F. 2002. Feline cutaneous phaeohyphomycosis due to Cladophialophora bantiana. J. Feline Med. Surg. 4:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dedola C, Stuart AP, Ridyard AE, Else RW, van den Broek AH, Choi JS, de Hoog GS, Thoday KL. 2010. Cutaneous Alternaria infectoria infection in a dog in association with therapeutic immunosuppression for the management of immune-mediated haemolytic anaemia. Vet. Dermatol. 21:626–634 [DOI] [PubMed] [Google Scholar]
- 88. Dion WM, Pukay BP, Bundza A. 1982. Feline cutaneous phaeohyphomycosis caused by Phialophora verrucosa. Can. Vet. J. 23:48–49 [PMC free article] [PubMed] [Google Scholar]
- 89. Fondati A, Gallo MG, Romano E, Fondevila D. 2001. A case of feline phaeohyphomycosis due to Fonsecaea pedrosoi. Vet. Dermatol. 12:297–301 [DOI] [PubMed] [Google Scholar]
- 90. Kettlewell P, McGinnis MR, Wilkinson GT. 1989. Phaeohyphomycosis caused by Exophiala spinifera in two cats. J. Med. Vet. Mycol. 27:257–264 [PubMed] [Google Scholar]
- 91. McKay JS, Cox CL, Foster AP. 2001. Cutaneous alternariosis in a cat. J. Small Anim. Pract. 42:75–78 [DOI] [PubMed] [Google Scholar]
- 92. Roosje PJ, de Hoog GS, Koeman JP, Willemse T. 1993. Phaeohyphomycosis in a cat caused by Alternaria infectoria E. G. Simmons. Mycoses 36:451–454 [DOI] [PubMed] [Google Scholar]
- 93. Miller RI. 2010. Nodular granulomatous fungal skin diseases of cats in the United Kingdom: a retrospective review. Vet. Dermatol. 21:130–135 [DOI] [PubMed] [Google Scholar]
- 94. Bernhardt A, von Bomhard W, Antweiler E, Tintelnot K. 2012. Molecular identification of fungal pathogens in nodular skin lesiosn in cats. Mycoses 55:145. [DOI] [PubMed] [Google Scholar]
- 95. Chermette R, Ferreiro L, De Bievre C, Camadro P, Mialot M, Vauzelle P. 1997. Exophiala spinifera nasal infection in a cat and a literature review of feline phaeohyphomycosis. J. Mycol. Med. 7:149–158 [Google Scholar]
- 96. Reference deleted.
- 97. Janovsky M, Grone A, Ciardo D, Vollm J, Burnens A, Fatzer R, Bacciarini LN. 2006. Phaeohyphomycosis in a snow leopard (Uncia uncia) due to Cladophialophora bantiana. J. Comp. Pathol. 134:245–248 [DOI] [PubMed] [Google Scholar]
- 98. Dillehay DL, Ribas JL, Newton JC, Jr, Kwapien RP. 1987. Cerebral phaeohyphomycosis in two dogs and a cat. Vet. Pathol. 24:192–194 [DOI] [PubMed] [Google Scholar]
- 99. Bostock DE, Coloe PJ, Castellani A. 1982. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat. J. Comp. Pathol. 92:479. [DOI] [PubMed] [Google Scholar]
- 100. Nuttal W, Woodgyer A, Butler S. 1990. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat. N. Z. Vet. J. 38:123. [DOI] [PubMed] [Google Scholar]
- 101. Najafzadeh MJ, Vicente VA, Sun J, Meis JF, de Hoog GS. 2011. Fonsecaea multimorphosa sp. nov, a new species of Chaetothyriales isolated from a feline cerebral abscess. Fungal Biol. 115:1066–1076 [DOI] [PubMed] [Google Scholar]
- 102. Padhye AA, Amster RL, Browning M, Ewing EP. 1994. Fatal encephalitis caused by Ochroconis gallopavum in a domestic cat (Felis domesticus). J. Med. Vet. Mycol. 32:141–145 [PubMed] [Google Scholar]
- 103. Waurzyniak BJ, Hoover JP, Clinkenbeard KD, Welsh RD. 1992. Dual systemic mycosis caused by Bipolaris spicifera and Torulopsis glabrata in a dog. Vet. Pathol. 29:566–569 [DOI] [PubMed] [Google Scholar]
- 104. Singh K, Flood J, Welsh RD, Wyckoff JH, Snider TA, Sutton DA. 2006. Fatal systemic phaeohyphomycosis caused by Ochroconis gallopavum in a dog (Canis familaris). Vet. Pathol. 43:988–992 [DOI] [PubMed] [Google Scholar]
- 105. Lobetti RG. 1996. Leukogram and serum globulin values in two dogs with systemic Xylohypha bantiana infection. J. South Afr. Vet. Assoc. 67:91–92 [PubMed] [Google Scholar]
- 106. Lomax LG, Cole JR, Padhye AA, Ajello L, Chandler FW, Smith BR. 1986. Osteolytic phaeohyphomycosis in a German shepherd dog caused by Phialemonium obovatum. J. Clin. Microbiol. 23:987–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Poutahidis T, Angelopoulou K, Karamanavi E, Polizopoulou ZS, Doulberis M, Latsari M, Kaldrymidou E. 2009. Mycotic encephalitis and nephritis in a dog due to infection with Cladosporium cladosporioides. J. Comp. Pathol. 140:59–63 [DOI] [PubMed] [Google Scholar]
- 108. Guillot J, Garcia-Hermoso D, Degorce F, Deville M, Calvie C, Dickele G, Delisle F, Chermette R. 2004. Eumycetoma caused by Cladophialophora bantiana in a dog. J. Clin. Microbiol. 42:4901–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Abid HN, Walter PA, Litchfield H. 1987. Chromomycosis in a horse. J. Am. Vet. Med. Assoc. 191:711–712 [PubMed] [Google Scholar]
- 110. Genovese LM, Whitbread TJ, Campbell CK. 2001. Cutaneous nodular phaeohyphomycosis in five horses associated with Alternaria alternata infection. Vet. Rec. 148:55–56 [DOI] [PubMed] [Google Scholar]
- 111. Coles BM, Stevens DR, Hunter RL. 1978. Equine nodular dermatitis associated with Alternaria tenuis infection. Vet. Pathol. 15:779–780 [DOI] [PubMed] [Google Scholar]
- 112. Cabanes FJ, Abarca L, Bragulat MR, Bruguera T. 1988. Phaeohyphomycosis caused by Alternaria alternata in a mare. J. Med. Vet. Mycol. 26:359–365 [PubMed] [Google Scholar]
- 113. Dillehay DL, Silberman MS. 1991. Systemic Phaeohyphomycosis in a Zebra(Equus grevyi). J. Zoo Wildl. Med. 22:237–240 [Google Scholar]
- 114. Revankar SG, Sutton DA, Rinaldi MG. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206–216 [DOI] [PubMed] [Google Scholar]
- 115. Balajee SA, Sigler L, Brandt ME. 2007. DNA and the classical way: identification of medically important molds in the 21st century. Med. Mycol. 45:475–490 [DOI] [PubMed] [Google Scholar]
- 116. Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. 2007. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 45:3713–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ferrer C, Colom F, Frases S, Mulet E, Abad JL, Alio JL. 2001. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 39:2873–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sutton DA. 2007. Specimen collection, transport, and processing: mycology, p 1728–1735 In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. (ed), Manual of clinical microbiology, 9th ed ASM Press, Washington, DC [Google Scholar]
- 119. Centers for Disease Control and Prevention 2009. Biosafety in microbiological and biomedical laboratories, 5th ed Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 120. European Community 2000. Directive 2000/54/EC, p 19 Official Journal of the European Communities. Office for Official Publications of the European Communities, Luxembourg [Google Scholar]
- 121. Ruchel R, Schaffrinski M, Seshan KR, Cole GT. 2000. Vital staining of fungal elements in deep-seated mycotic lesions during experimental murine mycoses using the parenterally applied optical brightener Blankophor. Med. Mycol. 38:231–237 [DOI] [PubMed] [Google Scholar]
- 122. Sutton DA. 2008. Basic mycology, p 15–35 In Hospenthal DR, Rinaldi MG. (ed), Diagnosis and treatment of human mycoses. Humana Press, Towata, NJ [Google Scholar]
- 123. Kimura M, McGinnis MR. 1998. Fontana-Masson-stained tissue from culture-proven mycoses. Arch. Pathol. Lab Med. 122:1107–1111 [PubMed] [Google Scholar]
- 124. Tarrand JJ, Lichterfeld M, Warraich I, Luna M, Han XY, May GS, Kontoyiannis DP. 2003. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am. J. Clin. Pathol. 119:854–858 [DOI] [PubMed] [Google Scholar]
- 125. Zeng JS, De Hoog GS. 2008. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med. Mycol. 46:193–208 [DOI] [PubMed] [Google Scholar]
- 126. Lau A, Chen S, Sorrell T, Carter D, Malik R, Martin P, Halliday C. 2007. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J. Clin. Microbiol. 45:380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Foy DS, Trepanier LA. 2010. Antifungal treatment of small animal veterinary patients. Vet. Clin. North Am. Small Anim. Pract. 40:1171–1188 [DOI] [PubMed] [Google Scholar]
- 128. Najafzadeh MJ, Rezusta A, Cameo MI, Zubiri ML, Yus MC, Badali H, Revillo MJ, De Hoog GS. 2010. Successful treatment of chromoblastomycosis of 36 years duration caused by Fonsecaea monophora. Med. Mycol. 48:390–393 [DOI] [PubMed] [Google Scholar]
- 129. Badali H, de Hoog GS, Sudhadham M, Meis JF. 2011. Microdilution in vitro antifungal susceptibility of Exophiala dermatitidis, a systemic opportunist. Med. Mycol. 49:819–824 [DOI] [PubMed] [Google Scholar]
- 130. Badali H, De Hoog GS, Curfs-Breuker I, Andersen B, Meis JF. 2009. In vitro activities of eight antifungal drugs against 70 clinical and environmental isolates of Alternaria species. J. Antimicrob. Chemother. 63:1295–1297 [DOI] [PubMed] [Google Scholar]
- 131. Vitale RG, Perez-Blanco M, De Hoog GS. 2009. In vitro activity of antifungal drugs against Cladophialophora species associated with human chromoblastomycosis. Med. Mycol. 47:35–40 [DOI] [PubMed] [Google Scholar]
- 132. Badali H, de Hoog GS, Curfs-Breuker I, Klaassen CH, Meis JF. 2010. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J. Clin. Microbiol. 48:2350–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Feng P, Najafzadeh MJ, Sun J, Ahmed S, Xi L, de Hoog GS, Lai W, Lu C, Klaassen CH, Meis JF. 2012. In vitro activities of nine antifungal drugs against 81 Phialophora and Cyphellophora isolates. Antimicrob. Agents Chemother. 56:6044–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Najafzadeh MJ, Badali H, Illnait-Zaragozi MT, De Hoog GS, Meis JF. 2010. In vitro activities of eight antifungal drugs against 55 clinical isolates of Fonsecaea spp. Antimicrob. Agents Chemother. 54:1636–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rodgers CJ, Furones MD. 2009. Antimicrobial agents in aquaculture: practice, needs and issues. Options Méditerranéennes A, no 86. The use of veterinary drugs and vaccines in Mediterranean aquaculture. Mediterranean Agronomic Institute of Zaragoza, Zaragoza, Spain [Google Scholar]