Abstract
Comprehensive information on the effects of cytochrome P450 2B6 (CYP2B6) polymorphisms, clinical factors, and drug-drug interactions on efavirenz concentrations in HIV/tuberculosis-coinfected (HIV/TB) patients is unavailable. A total of 139 HIV/TB adults, 101 of whom received a rifampin-containing anti-TB regimen, were prospectively enrolled to receive efavirenz (600 mg)/tenofovir/lamivudine. Nine single nucleotide polymorphisms (SNPs) within CYP2B6 were genotyped. Plasma efavirenz concentrations were measured at 12 weeks. The median (interquartile range [IQR]) efavirenz concentration was 2.3 (1.4 to 3.9) mg/liter. The SNPs (frequencies of heterozygous and homozygous mutants) were 64C>T (10% and 1%), 499C>G (0% and 0%), 516G>T (47% and 8%), 785A>G (54% and 10%), 1375A>G (0% and 0%), 1459C>T (3% and 0%), 3003C>T (44% and 27%), 18492T>C (39% and 6%), and 21563C>T (57% and 5%). The four most frequent CYP2B6 haplotypes identified were *1/*6 (41%), *1/*1 (35%), *1/*2 (7%), and *6/*6 (7%). The heterozygous/homozygous mutation associated with low efavirenz concentrations was 18492T>C (P < 0.001), and those associated with high efavirenz concentrations were 516G>T, 785A>G, and 21563C>T (all P < 0.05). Haplotype *1/*1 was associated with low efavirenz concentrations, and *6/*6, *1/*6, and *5/6 were associated with high efavirenz concentrations. As shown by multivariate analysis, low efavirenz concentrations were significantly associated with the *1/*1 haplotype (beta = −1.084, P = 0.027) and high body weight (beta = −0.076, P = 0.002). In conclusion, pharmacogenetic markers of CYP2B6 have the greatest impact with respect to inducing low plasma efavirenz concentrations in HIV/TB Thai patients.
INTRODUCTION
In the resource-limited countries, patients with human immunodeficiency virus (HIV) infection often present late with advanced acquired immune deficiency syndrome (AIDS) and major opportunistic infections, of which tuberculosis (TB) is one of the most common (1–4). A rifamycin-containing antituberculosis regimen is essential in treatment of tuberculosis. Rifampin is a potent hepatic cytochrome P450 enzyme inducer, leading to accelerated drug clearance and a significant reduction in plasma concentrations of particular antiretroviral drugs (5). Efavirenz is a nonnucleoside reverse transcriptase inhibitor (NNRTI) which is mainly metabolized by hepatic cytochrome P450 2B6 (CYP2B6). Coadministration of efavirenz and two nucleoside reverse transcriptase inhibitors (NRTIs) with rifampin is currently recommended as a preferred antiretroviral regimen in treating patients who are coinfected with HIV and Mycobacterium tuberculosis, particularly where rifabutin is not available (6).
The range of acceptable plasma concentrations of efavirenz at 12 h is currently proposed to be 1 to 4 mg/liter (6, 7). Subtherapeutic efavirenz concentrations occur when efavirenz is coadministered with rifampin, leading to subsequent failure of treatment with an efavirenz-based regimen (7, 8). In contrast, concentrations above the therapeutic range increase the risk of drug-related toxicity outcomes, such as neuropsychiatric side effects (7). The CYP2B6 gene is highly polymorphic (9, 10). Most previous studies have shown CYP2B6 single nucleotide polymorphisms (SNPs), particularly 516G>T, to be associated with high plasma concentrations of efavirenz and its drug-related toxicity (11–13). To date, data concerning these polymorphisms incorporated with clinical factors and drug-drug interactions that may be associated with low efavirenz concentrations are very limited. In addition, the differences in plasma efavirenz concentrations among previous published data may reflect different CYP2B6 SNPs and haplotypes. This study was therefore conducted to examine the impact of nine SNPs and haplotypes of the CYP2B6 enzyme, potential clinical factors, and drug-drug interactions on plasma efavirenz concentrations in Thai patients coinfected with HIV and tuberculosis, since these patients may have a subtherapeutic concentration of efavirenz and subsequent treatment failure.
(Part of this research was presented at the 52nd Interscience Conference of Antimicrobial Agents and Chemotherapy [ICAAC], San Francisco, CA, 2012 [poster round] [14].)
MATERIALS AND METHODS
Patients coinfected with HIV and tuberculosis were prospectively enrolled between October 2009 and May 2011 at the Bamrasnaradura Infectious Diseases Institute, Ministry of Public Health, Nonthaburi, Thailand. The institutional ethics committees of the Bamrasnaradura Infectious Diseases Institute and the Thai Ministry of Public Health approved the study. All patients provided written, informed consent prior to enrollment.
Patients were categorized according to antituberculosis regimen, rifampin-containing regimen, and other regimens without rifampin. They were followed through 12 weeks after initiation of antiretroviral therapy (ART). The objectives were to study the frequencies of nine CYP2B6 polymorphisms in HIV-infected Thai adults and to identify SNPs, haplotypes, and clinical factors that are associated with plasma efavirenz concentrations at 12 h after dosing. Inclusion criteria were as follows: (i) HIV-infected individuals 18 to 60 years of age, (ii) newly clinically diagnosed active tuberculosis, positive acid-fast staining, or a positive culture for Mycobacterium tuberculosis, (iii) treatment with an antituberculosis regimen 4 to 12 weeks prior to enrollment, (iv) naïveté to ART, (v) baseline CD4 cell count < 350 cells/mm3, and (vi) participation and informed consent. Exclusion criteria were as follows: (i) inability to tolerate efavirenz due to any reason, (ii) serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) levels > 5 times the upper limit of the normal range, (iii) a serum creatinine level > 2 times the upper limit of the normal range, (iv) treatment with a medication that has drug-drug interactions with efavirenz or rifampin, (v) treatment with immunosuppressive drugs, and (vi) pregnancy or lactation.
All patients were started on a once-daily antiretroviral regimen of tenofovir (300 mg), lamivudine (300 mg), and efavirenz (600 mg) at bedtime irrespective of baseline patient body weight. ART was initiated at between 4 weeks and 12 weeks of TB treatment initiation. The dosage of rifampin was 450 mg/day for body weight ≤ 50 kg and 600 mg/day for body weight > 50 kg. The antituberculosis regimen was isoniazid, rifampin, pyrazinamide, and ethambutol for the first 2 months followed by isoniazid and rifampin for the subsequent 4 to 7 months. Patients who received other antituberculosis regimens without rifampin were those who initially could not tolerate rifampin due to adverse effects or hypersensitivity. The patients had follow-up visits at week 2, week 6, and week 12 after initiation of ART, when they were assessed clinically and/or blood samples were taken. Adherence counseling was given to the patients, and adherence to treatment was assessed with a questionnaire. Any patient with adherence of less than 80% was excluded from the analysis. In addition, all patients were instructed to take their medication regularly at least 2 weeks prior to blood collection.
The fasting plasma efavirenz concentration at 12 h after dosing (for patients observed taking dosing) was measured using a validated high-performance liquid chromatography assay at 12 weeks of ART initiation. This assay was developed at the Department of Clinical Pharmacology at the University Medical Centre Nijmegen, Nijmegen, The Netherlands. The sample peak heights were processed by ChromQuest software version 4.1. CD4 cell counts were determined by flow cytometry using monoclonal antibodies with three-color reagent (TriTEST; Becton, Dickinson BioSciences) and analyzed using a FACScan flow cytometer (Becton, Dickinson BioSciences). Plasma HIV-1 RNA levels determined by real-time PCR using a Cobas AmpliPrep/Cobas TaqMan HIV-1 test (Roche Molecular Systems Inc., Branchburg, NJ) and alanine aminotransferase (ALT) enzyme levels were assessed at week 0 and at week 12 after ART.
At week 0 of ART, DNA was isolated from the stored EDTA cell pellets using a QlAamp DNA blood minikit (Qiagen, Hilden, Germany). Genomic DNA was quantified by a ND-1000 UV spectrophotometer (NanoDrop Technologies, Wilmington, DE) at 260 nm. A total of nine SNPs within CYP2B6 were genotyped. SNPs 516G>T and 785A>G have been previously reported to influence plasma efavirenz concentrations (15), and three CYP2B6 SNPs, 3003T>C, 18492C>T, and 21563C>T, were identified using the International Haplotype Mapping Project (HapMap) (http://www.hapmap.org) in Japanese and Han Chinese subjects. SNP499C>G was associated with high plasma efavirenz concentrations in Japanese subjects, and the remaining three SNPs, i.e., 64C>T, 1375A>G, and 1459C>T, were reported in Chinese subjects (16). The CYP2B6 haplotype determination was interpreted using The Human Cytochrome P450 (CYP) Allele Nomenclature Database (http://www.cypalleles.ki.se/cyp2b6.htm). All SNPs were included for CYP2B6 haplotype interpretation except 3003T>C and 18492C>T.
All analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL). Frequencies (percentages), means ± standard deviations (SD), and medians (IQR at the 25th and 75th percentiles) were used to describe patients' characteristics and laboratory parameters. The independent variables were evaluated with simple linear regression analysis to identify the factors that were associated with plasma efavirenz concentration. Any independent variable with a P value of less than 0.1 was included in the model of multiple regression analysis. Possible predictive factors for plasma efavirenz concentrations were evaluated with a linear regression model by adjusting for confounding factors, i.e., body weight at week 12, receiving rifampin, having positive hepatitis C virus antibody (anti-HCV) results, and haplotype. The factors of receiving rifampin, having anti-HCV, and haplotype were examined as dichotomous variables, and the remaining factors were examined as continuous variables. The beta values were estimated. The Pearson's correlations were used to study the relationships between the plasma efavirenz concentration and patient body weight. Interpatient variability of plasma efavirenz concentration was expressed as percent coefficient of variation (CV).
RESULTS
A total of 150 patients were initially enrolled and started ART. Eight patients discontinued efavirenz due to adverse events prior to measurement of the plasma efavirenz concentration, and three patients were excluded due to poor adherence. Demographic features and baseline laboratory parameters of the remaining 139 patients are shown in Table 1. The median (IQR) CD4 cell count was 42 (range, 17 to 105) cells/mm3, and the median (IQR) plasma HIV-1 RNA level was 5.8 (5.4 to 6.3) log copies/ml. Of 139 patients, 38 received antituberculosis regimens without rifampin. At week 12, the median (IQR) plasma efavirenz concentration for all 139 patients was 2.3 (1.4 to 3.9) mg/dl. Figure 1 shows the frequencies of each of the SNPs and the box plots of plasma efavirenz concentrations for nine CYP2B6 SNPs. The three most frequent CYP2B6 SNPs detected were 3003C>T, 785A>G, and 21563C>T. Nucleotide substitutions were not detected at positions 499 and 1375. Three CYP2B6 SNPs, including 516G>T, 785A>G, and 21563C>T, were found to be associated with high plasma efavirenz concentrations, but 18492T>C was associated with a low plasma efavirenz concentration. Figure 2 shows the frequencies of CYP2B6 haplotypes and plasma efavirenz concentrations by haplotype. Frequent CYP2B6 haplotypes were *1/*6 (41%), *1/*1 (35%), *1/*2 (7%), *6/*6 (7%), *4/*6 (4%), *1/*4 (3%), *2/*4 (1%), *2/*6 (1%), and *5/*6 (1%). Three of 9 haplotypes identified, including *6/*6, *1/*6, and *5/*6, were associated with high plasma efavirenz concentrations. There was no CYP2B6 genetic mutation in 35% of all patients for whom the haplotype was determined to be *1/*1.
Table 1.
Baseline characteristics of 139 patients coinfected with HIV and M. tuberculosis
| Characteristic | Values (n = 139) |
|---|---|
| Demographics | |
| No. (%) of males | 108 (78) |
| Mean age (yr) ± SD | 37 ± 8 |
| Mean body wt (kg) ± SD | 54 ± 10 |
| No. (%) of patients with indicated site of TB | |
| Lung | 64 (46) |
| Cervical lymph node | 14 (10) |
| Disseminated TB | 56 (40) |
| Meninges | 3 (3) |
| Colon | 2 (1) |
| Laboratory parameters | |
| Median (IQR) CD4 cell count/mm3 | 42 (17–105) |
| Median (IQR) % CD4 cells | 6 (3–11) |
| Median (IQR) log plasma HIV-1 RNA copies/ml | 5.8 (5.4–6.3) |
| Median (IQR) g hemoglobin/dl ± SD | 10.8 (9.5–11.9) |
| Median (IQR) mg serum alkaline phosphatase/dl ± SD | 105 (73–170) |
| Median (IQR) U alanine aminotransferase/liter ± SD | 30 (19–46) |
| Median (IQR) mg albumin/dl ± SD | 3.4 (3.0–3.8) |
| Median (IQR) mg total bilirubin/dl ± SD | 0.41 (0.31–0.71) |
| Median (IQR) mg serum creatinine/dl ± SD | 0.7 (0.6–0.8) |
| No. (%) of hepatitis B virus antigen-positive results | 6 (4) |
| No. (%) of hepatitis C antibody-positive results | 18 (13) |
Fig 1.
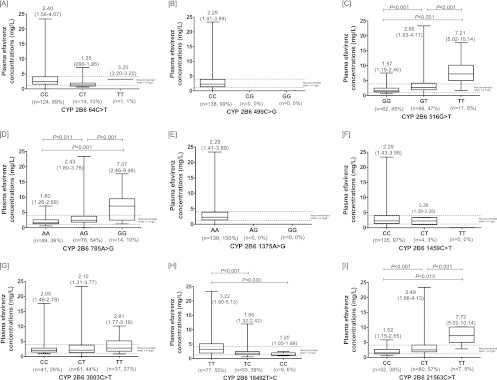
Nine single nucleotide polymorphisms of CYP2B6 and plasma efavirenz concentrations at 12 h after dosing. The middle bar indicates the median, and the upper and lower bars indicate the 25th and 75th interquartile ranges. The medians and 25th and 75th interquartile ranges are displayed on each box. P values are shown only for significant differences between genotypes.
Fig 2.
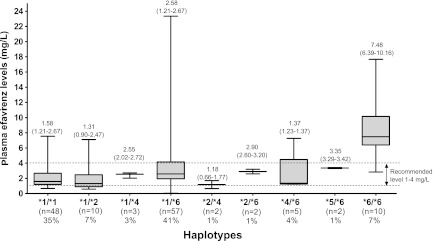
Distributions of CYP2B6 haplotypes and plasma efavirenz concentrations at 12 h after dosing by haplotypes. The middle bar indicates the median, and the upper and lower bars indicate the 25th and 75th interquartile ranges. The medians and 25th and 75th interquartile ranges are displayed on each box.
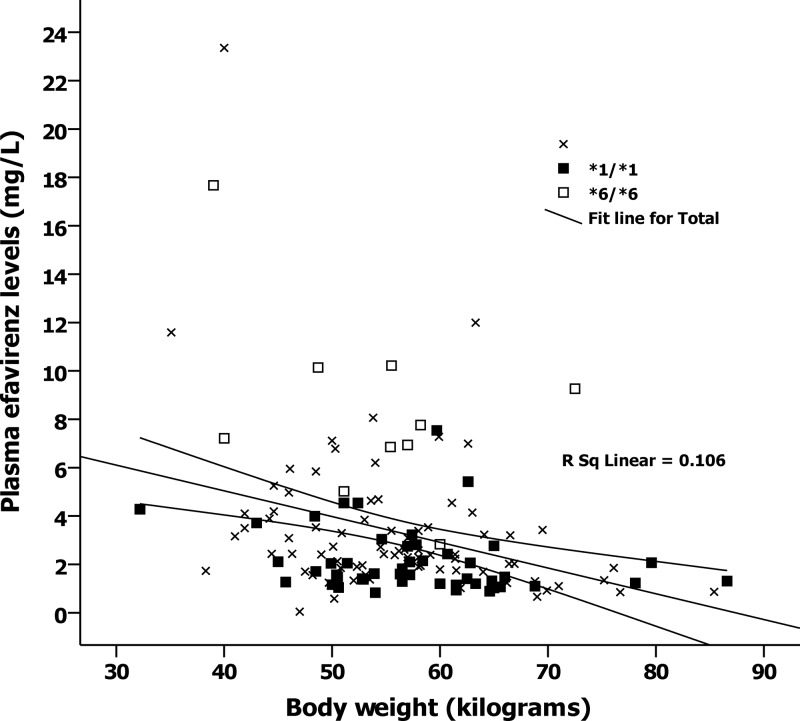
Univariate and multivariate analysis of possible factors associated with the plasma efavirenz concentration is shown in Table 2. By multivariate analysis, factors associated with a low plasma efavirenz concentration included specific haplotype and high body weight (P < 0.05), but the factor “receiving rifampin” did not reach a significant level. Figure 3 displays the relationship between body weight at week 12 and the plasma efavirenz concentration by haplotype. By correlation analysis, there appears to be a relationship between high body weight and a low plasma efavirenz concentration (P < 0.05). Median (IQR) plasma efavirenz concentrations for all patients, 101 patients who were concurrently receiving efavirenz and rifampin, and 38 patients who did not receive rifampin were 2.3 (1.4 to 3.9) mg/dl, 2.1 (1.3 to 3.5) mg/dl, and 2.7 (1.8 to 5.4) mg/dl, respectively. The interpatient variabilities of plasma efavirenz concentrations in the corresponding groups were 95%, 75%, and 107%, respectively. Seven of 101 (7%) of the patients who were concurrently receiving rifampin and 3 of 38 (8%) of those who were not concurrently receiving rifampin had a plasma efavirenz concentration below the recommended concentration.
Table 2.
Univariate and multivariate analysis of plasma efavirenz concentration as the dependent variable
| Parameter | Univariate analysis |
Multivariate analysisa |
||
|---|---|---|---|---|
| P | Beta | P | Beta | |
| Body wt at wk 12 | 0.001 | −0.086 | 0.002 | −0.076 |
| Male gender | 0.916 | −0.007 | ||
| Age | 0.221 | 0.040 | ||
| Baseline serum creatinine | 0.487 | −0.989 | ||
| Serum ALT | 0.498 | 0.007 | ||
| Hepatitis B virus antigen positive | 0.787 | −0.354 | ||
| Hepatitis C antibody positive | 0.033 | 1.678 | 0.332 | 0.674 |
| Patients receiving rifampin | 0.008 | −1.575 | 0.087 | −0.893 |
| CYP2B6 haplotypes | ||||
| *1/*1 | 0.001 | −1.777 | 0.027 | −1.084 |
| *1/*2 | 0.220 | −1.262 | ||
| *1/*4 | 0.638 | −0.864 | ||
| *1/*6 | 0.133 | 0.811 | ||
| *2/*4 | 0.342 | −2.126 | ||
| *2/*6 | 0.865 | −0.381 | ||
| *4/*6 | 0.748 | −0.512 | ||
| *5/*6 | 0.971 | 0.081 | ||
| *6/*6 | <0.001 | 4.893 | 0.001 | 3.076 |
| CYP2B6 nucleotide polymorphisms | ||||
| 64C>T | 0.105 | −1.387 | ||
| 499C>G | ||||
| 516G>T | <0.001 | 2.149 | ||
| 785A>G | <0.001 | 1.947 | ||
| 1375A>G | ||||
| 1459C>T | 0.500 | −1.077 | ||
| 3003C>T | 0.754 | 0.183 | ||
| 18492T>C | <0.001 | −1.635 | ||
| 21563C>T | <0.001 | 2.090 | ||
CYP2B6 nucleotide polymorphisms were not included in the multivariate analysis model because of a strong correlation between nucleotide polymorphisms and haplotypes.
Fig 3.
Relationship between body weight at the time of measurement and plasma efavirenz concentration by haplotype. Unfilled squares represent haplotype *6/*6, filled squares represent efavirenz haplotype *1/*1, and cross symbols represent other haplotypes. Broken lines represent regression predictions and 95% confidence intervals.
DISCUSSION
The emergence of HIV drug resistance is likely facilitated by prolonged exposure to a subtherapeutic concentration of antiretroviral drugs. Therefore, maintaining an adequate drug concentration is very important for achieving long-term virologic suppression in the treatment of HIV infection. To date, the utility of phamacogenetic markers to predict chance of antiretroviral failure has been limited. This is the first study aimed at examining the impact of pharmacogenetic markers of CYP2B6 and clinical factors on efavirenz concentrations as well as the effect of drug-drug interactions in patients coinfected with HIV and active tuberculosis who were receiving a antituberculosis regimen with or without rifampin.
Overall, the allelic frequencies of the CYP2B6 SNPs detected in this study are consistent with a recent study in Thais (17). CYP2B6 499C>G and 1375A>G were the absent SNPs in this study, and CYP2B6 1459C>T was rarely detected, which is consistent with previous reports from studies of Chinese patients (18, 19) and Japanese patients (16). No CYP2B6 genetic polymorphism was found in one-third of all patients whose haplotype was determined to be *1/*1, and this haplotype was one of the risk factors associated with a low plasma efavirenz concentration by multivariate analysis. CYP2B6 is genetically polymorphic; therefore, the determination of the haplotype would be another approach for predicting plasma efavirenz concentrations. Of note, the patients who carried CYP2B6 *1/*2 and *2/*4 had a median plasma efavirenz concentration that was less than that seen with those who carried CYP2B6 *1/*1, but those haplotypes were not found to be associated with low plasma efavirenz concentrations. Those data may be explained by the fact that only a small proportion of patients carried these genes. Taken together, these pharmacogenetic markers should raise concerns about maintaining an adequate drug concentration, especially in patients who have high body weight and concurrently receive rifampin. In addition, marked interpatient variability was observed during concurrent use of efavirenz and rifampin as well as during the use of efavirenz alone. This finding can be explained by the influences of these pharmacogenetic markers.
Another observation that arises from this study is that an increase in body weight after the time of initial measurement results in a decreasing efavirenz concentration. By multivariate analyses, efavirenz concentrations persistently decreased 0.7 mg/liter (coefficients = −0.07) for every 10-kg increase in patient body weight. A cross-sectional study from the Liverpool therapeutic drug monitoring registry and a study in Thais showed that body weight was an independent predictive factor for the plasma efavirenz concentration (20, 21), although some previous studies were not able to demonstrate this effect (22, 23). Moreover, patients' body weights increased over time while on treatment. Thus, a weight-based cutoff for efavirenz dosing is a reasonably practical therapeutic approach. To date, a body weight cutoff of 60 kg for the standard daily dosage of efavirenz has been proposed. According to the current U.S. Department of Health and Human Services (DHHS) guidelines, experts recommend a starting efavirenz dose of 600 mg/day and monitoring for virological response. The guidelines suggest considering an increase of the dose to 800 mg/day for patients who weigh more than 60 kg. On the other hand, rifampin is an essential drug for the treatment of tuberculosis and is also a potent inducer of expression of cytochrome P450 enzymes in the liver (8). Subtherapeutic efavirenz concentrations can occur when efavirenz is coadministered with rifampin, leading to subsequent treatment failure on an efavirenz-based antiretroviral regimen, as aforementioned (8). Approximately 7% of the patients who were and were not concurrently receiving rifampin had a plasma efavirenz concentration below the recommended concentration. Most patients in both groups achieved efavirenz concentrations above the minimum recommended concentration of 1 mg/liter. Thus, it is important that rifampin itself has a relatively small impact on the plasma efavirenz concentration compared to pharmacogenetic differences corresponding to CYP2B6 and patients' body weights.
On the other hand, three SNPs, including 516G>T, 785A>G, and 21563C>T, appear to be associated with high plasma efavirenz concentrations, with all means of plasma efavirenz concentrations being greater than 7 mg/liter in the patients who carried homozygous mutants even if concurrently receiving rifampin. Heterozygous mutants of these SNPs showed the same effect but at a lesser magnitude. Although the CYP2B6 516G>T SNP is well known to be useful for predicting plasma efavirenz concentrations, little is known about the plasma efavirenz concentration prediction potential of the other two SNPs. Previous studies in Thais (13, 17) and members of other ethnic groups (12, 15, 24) showed high plasma efavirenz concentrations in HIV-infected patients with the CYP2B6 516TT genotype while receiving rifampin. The frequency of this allele ranges from 15% in Asians up to 50% in Africans (11, 15, 25).
A number of limitations of this study should be addressed. First, other genetic variations associated with the plasma efavirenz concentration, for instance, CYP2B6 983T>C, were not investigated in this study. An association with high efavirenz concentrations in Africans has been previously indicated for CYP2B6 983T>C (26); nonetheless, it was absent in Asians (18, 27). Second, other pathways of efavirenz metabolism via CYP2A6 in patients with impaired CYP2BC function have been previously reported (28). CYP3A4 *1B was associated with lower efavirenz clearance. Neither CYP3A4 nor CYP2A6 SNPs were examined in this study; however, a relatively weak association between their variants and efavirenz concentrations was previously shown (13, 29). Third, data corresponding to the factor “receiving rifampin” did not reach a significant level, although this factor was found to be associated with low efavirenz concentrations in a previous study (30). This finding may have been due to a small sample size in the subgroup of patients who did not receive rifampin. Fourth, results from this study may not be completely applicable to members of other ethnic groups with inherited differences in their metabolisms. Ultimately, long-term treatment outcomes are needed to examine and confirm this finding.
This report provides interesting data regarding the factors potentially contributing to the pharmacokinetic variability of efavirenz in Thai patients coinfected with HIV and tuberculosis, including genetic factors, biological factors (i.e., body weight), and environmental factors (i.e., efavirenz-rifampin interactions). The patients with a particular haplotype and high body weight have the greatest probability of a low plasma efavirenz concentration. Pharmacokinetic variabilities reflect the combined influences and different magnitudes of such factors.
ACKNOWLEDGMENTS
We thank all patients who participated in this study.
This study was funded by grants from (i) The Thailand Research fund (RSA5380001), (ii) the Bamrasnaradura Infectious Diseases Institute, Department of Disease Control, Ministry of Public Health, Thailand, and (iii) The Mahidol University (MU)/The Thailand Research fund and Office of the Higher Education Commission (New Researcher grant MRG 5480136).
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009–1021 [DOI] [PubMed] [Google Scholar]
- 2. Raviglione MC, Snider DE, Jr, Kochi A. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220–226 [PubMed] [Google Scholar]
- 3. Ruxrungtham K, Phanuphak P. 2001. Update on HIV/AIDS in Thailand. J. Med. Assoc. Thai. 84(Suppl 1):S1–S17 [PubMed] [Google Scholar]
- 4. Sharma SK, Mohan A, Kadhiravan T. 2005. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J. Med. Res. 121:550–567 [PubMed] [Google Scholar]
- 5. Fox W, Ellard GA, Mitchison DA. 1999. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int. J. Tuberc. Lung Dis. 3:S231–S279 [PubMed] [Google Scholar]
- 6. Panel on Antiretroviral Guidelines for Adults and Adolescents 2012. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 15 July 2012 [Google Scholar]
- 7. Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75 [DOI] [PubMed] [Google Scholar]
- 8. Breen RA, Swaden L, Ballinger J, Lipman MC. 2006. Tuberculosis and HIV co-infection: a practical therapeutic approach. Drugs 66:2299–2308 [DOI] [PubMed] [Google Scholar]
- 9. Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. 2007. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 116:496–526 [DOI] [PubMed] [Google Scholar]
- 10. Phillips EJ, Mallal SA. 2008. Pharmacogenetics and the potential for the individualization of antiretroviral therapy. Curr. Opin. Infect. Dis. 21:16–24 [DOI] [PubMed] [Google Scholar]
- 11. Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, Masimirembwa C. 2008. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur. J. Clin. Pharmacol. 64:357–365 [DOI] [PubMed] [Google Scholar]
- 12. Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, Narendran G, Menon P, Gomathi C, Swaminathan S. 2009. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob. Agents Chemother. 53:863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uttayamakul S, Likanonsakul S, Manosuthi W, Wichukchinda N, Kalambaheti T, Nakayama EE, Shioda T, Khusmith S. 2010. Effects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adults. AIDS Res. Ther. 7:8 doi:10.1186/1742-6405-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manosuthi W, Sukasem C, Lueangniyomkul A, Mankatitham W, Thongyen S, Nilkamhang S, Manosuthi S, Sungkanuparph S. 2009. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), San Francisco, CA, 2012, poster H-894 [Google Scholar]
- 15. Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, Blievernicht J, Saussele T, Gunthard HF, Schwab M, Eichelbaum M, Telenti A, Zanger UM. 2007. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin. Pharmacol. Ther. 81:557–566 [DOI] [PubMed] [Google Scholar]
- 16. Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, Fujimoto K, Sato I, Ueda M, Horiba M, Hamaguchi M, Yamamoto M, Takata N, Kimura A, Koike T, Gejyo F, Matsushita S, Shirasaka T, Kimura S, Oka S. 2007. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin. Infect. Dis. 45:1230–1237 [DOI] [PubMed] [Google Scholar]
- 17. Sukasem C, Cressey TR, Prapaithong P, Tawon Y, Pasomsub E, Srichunrusami C, Jantararoungtong T, Lallement M, Chantratita W. 2012. Pharmacogenetic markers of CYP2B6 associated with efavirenz plasma concentrations in HIV-1 infected Thai adults. Br. J. Clin. Pharmacol. 74:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Sun J, Ma Q, Yao Y, Wang Z, Zhang L, Li L, Sun F, Lu H. 2010. CYP2B6 polymorphism and nonnucleoside reverse transcriptase inhibitor plasma concentrations in Chinese HIV-infected patients. Ther. Drug Monit. 32:573–578 [DOI] [PubMed] [Google Scholar]
- 19. Guan S, Huang M, Chan E, Chen X, Duan W, Zhou SF. 2006. Genetic polymorphisms of cytochrome P450 2B6 gene in Han Chinese. Eur. J. Pharm. Sci. 29:14–21 [DOI] [PubMed] [Google Scholar]
- 20. Manosuthi W, Sungkanuparph S, Tantanathip P, Mankatitham W, Lueangniyomkul A, Thongyen S, Eampokarap B, Uttayamakul S, Suwanvattana P, Kaewsaard S, Ruxrungtham K. 2009. Body weight cutoff for daily dosage of efavirenz and 60-week efficacy of efavirenz-based regimen in human immunodeficiency virus and tuberculosis coinfected patients receiving rifampin. Antimicrob. Agents Chemother. 53:4545–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stöhr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, Pillay D, Hill T, Ainsworth J, Pozniak A, Leen C, Bansi L, Fisher M, Orkin C, Anderson J, Johnson M, Easterbrook P, Gibbons S, Khoo S. 2008. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir. Ther. 13:675–685 [PubMed] [Google Scholar]
- 22. Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, Biollaz J, Buclin T. 2003. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 73:20–30 [DOI] [PubMed] [Google Scholar]
- 23. Friedland G, Khoo S, Jack C, Lalloo U. 2006. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J. Antimicrob. Chemother. 58:1299–1302 [DOI] [PubMed] [Google Scholar]
- 24. Kwara A, Lartey M, Sagoe KW, Xexemeku F, Kenu E, Oliver-Commey J, Boima V, Sagoe A, Boamah I, Greenblatt DJ, Court MH. 2008. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J. Clin. Pharmacol. 48:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, Wilkinson GR, Clifford DB, D'Aquila RT, De Gruttola V, Pollard RB, Merigan TC, Hirsch MS, George AL, Jr, Donahue JP, Kim RB. 2005. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an adult AIDS Clinical Trials Group study. J. Infect. Dis. 192:1931–1942 [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Sonnerborg A, Rane A, Josephson F, Lundgren S, Stahle L, Ingelman-Sundberg M. 2006. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet. Genomics 16:191–198 [DOI] [PubMed] [Google Scholar]
- 27. Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, Schwab M, Zanger UM. 2005. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet. Genomics 15:861–873 [DOI] [PubMed] [Google Scholar]
- 28. di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, Furrer H, Gunthard HF, Colombo S, Csajka C, Eap CB, Decosterd LA, Telenti A. 2009. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet. Genomics 19:300–309 [DOI] [PubMed] [Google Scholar]
- 29. Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. 2009. CYP2B6 (c. 516G→T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br. J. Clin. Pharmacol. 67:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manosuthi W, Sungkanuparph S, Tantanathip P, Lueangniyomkul A, Mankatitham W, Prasithsirskul W, Burapatarawong S, Thongyen S, Likanonsakul S, Thawornwa U, Prommool V, Ruxrungtham K. 2009. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R study. Clin. Infect. Dis. 48:1752–1759 [DOI] [PubMed] [Google Scholar]