Abstract
Severe sepsis and septic shock can alter the pharmacokinetics of broad-spectrum β-lactams (meropenem, ceftazidime/cefepime, and piperacillin-tazobactam), resulting in inappropriate serum concentrations. Obesity may further modify the pharmacokinetics of these agents. We reviewed our data on critically ill obese patients (body mass index of ≥30 kg/m2) treated with a broad-spectrum β-lactam in whom therapeutic drug monitoring was performed and compared the data to those obtained in critically nonobese patients (body mass index of <25 kg/m2) to assess whether there were differences in reaching optimal drug concentrations for the treatment of nosocomial infections. Sixty-eight serum levels were obtained from 49 obese patients. There was considerable variability in β-lactam serum concentrations (coefficient of variation of 50% to 92% for the three drugs). Standard drug regimens of β-lactams resulted in insufficient serum concentrations in 32% of the patients and overdosed concentrations in 25%. Continuous renal replacement therapy was identified by multivariable analysis as a risk factor for overdosage and a protective factor for insufficient β-lactam serum concentrations. The serum drug levels from the obese cohort were well matched for age, gender, renal function, and sequential organ failure assessment (SOFA) score to 68 serum levels measured in 59 nonobese patients. The only difference observed between the two cohorts was in the subgroup of patients treated with meropenem and who were not receiving continuous renal replacement therapy: serum concentrations were lower in the obese cohort. No differences were observed in pharmacokinetic variables between the two groups. Routine therapeutic drug monitoring of β-lactams should be continued in obese critically ill patients.
INTRODUCTION
The first antibiotic choice in the treatment of severe hospital-related infections is a broad-spectrum β-lactam. β-Lactam dosage regimens are based on pharmacokinetic (PK) data obtained in healthy, nonobese volunteers or patients who are not severely ill. However, PK variables can be altered by sepsis. Volume of distribution (Vd) may increase due to capillary leak syndrome, increased cardiac output, fluid resuscitation, and/or use of vasopressors. Antibiotic clearance (CL) may also either increase due to increased glomerular filtration or decrease due to organ failure (1–3). Obesity could further alter these PK variables: Vd could be further increased due to increased lean body mass and increased adipose tissue, and CL could be increased due to increased kidney mass and global filtration or decreased due to chronic hypertensive or diabetic nephropathy. Administration of standard drug regimens (SDRs) to obese critically ill patients may thus potentially result in more frequent inadequate serum drug concentrations than in nonobese individuals, which may be responsible for increased treatment failure, toxicity, and/or emergence of bacterial resistance.
Despite increasing numbers of obese patients worldwide, there is little information on when and how doses of β-lactams should be adjusted in these patients. A correction formula for weight has been proposed but never validated (4). In our intensive care unit (ICU), therapeutic drug monitoring (TDM) of broad-spectrum β-lactams (ceftazidime or cefepime [CEF], piperacillin-tazobactam [TZP], or meropenem [MEM]) is now routinely performed. We thus reviewed the serum drug concentrations obtained in critically ill obese patients and compared them to those obtained in critically nonobese patients to determine whether there were differences in reaching optimal drug concentrations for the treatment of nosocomial infections. We also evaluated whether β-lactam dose adjustment, using a correction formula for weight, would have optimized serum drug levels in this cohort of obese patients.
MATERIALS AND METHODS
Study design and data sources.
We reviewed data on obese, critically ill patients who had received broad-spectrum β-lactams (CEF, TZP, or MEM) in the 35-bed ICU at Erasme Hospital, an 858-bed university hospital, between 1 October 2009 and 31 December 2011. Routine TDM of broad-spectrum β-lactams was initiated in our ICU in October 2009. Patients were included if they had sepsis diagnosed according to standard criteria (5), had a body mass index (BMI) greater than or equal to 30 kg/m2, and had been treated with a broad-spectrum β-lactam (CEF, TZP, or MEM) and if TDM had been performed during antimicrobial therapy. Patients could be included more than once if a TDM was performed for a different antibiotic. When multiple TDMs were performed for the same antibiotic, they were all noted for evaluation.
Antibiotic treatment.
The clinician's choice of antibiotic therapy was based on local guidelines. Most patients received an SDR, consisting of a first dose of 2 g for CEF, 4 g for TZP, or 1 g for MEM, followed by doses adapted to creatinine clearance (CrCl), calculated using the Cockroft-Gault formula (6). Doses were also adjusted for continuous renal replacement therapy (CRRT) (see Table S1 in the supplemental material). Occasionally, dosage regimens were increased in obese patients after discussion between the attending physician and the infectious disease specialist. The higher dosage regimens either were based on the correction formula for weight or were greater than this correction formula. The correction formula for weight is as follows: dosing weight = 0.30(actual body weight − ideal body weight) + ideal body weight (4). Devine's formula was used to calculate ideal body weight (7). The new dosage regimens were always rounded to the closest unitary dose of the antibiotic: 2 g for CEF, 4 g for TZP, and 1 g for MEM.
CRRT.
Standard criteria for initiating CRRT were problematic metabolic acidosis, uncontrolled hyperkalemia (>5.5 mmol/liter), fluid overload, drug intoxication, and uremia (blood urea, greater than or equal to 200 mg/dl) (8). When CRRT was used, blood flow rate was fixed between 150 and 200 ml/min, and the minimum ultrafiltrate rate was set at 20 ml/kg/h, associated with a dialysate rate of 10 to 15 ml/kg/h for the first 1 or 2 days of therapy.
Data collection.
We recorded demographic data, comorbidities, primary reason for ICU admission, source of infection, pathogens responsible for the infection, antibiotic dosage regimen administered, use of CRRT, intensity of CRRT (calculated ultrafiltrate delivered), number of days of antibiotic therapy, the day of TDM, and biological data. The acute physiology and chronic health evaluation II (APACHE II) score (9) was determined on the day of admission of the patient to the ICU, and the sequential organ failure assessment (SOFA) score (10) was determined on the day of TDM.
Serum samples for TDM.
Two blood samples (3 ml each) were taken to assess drug concentrations: one just before the administration of the next dose of the β-lactam (T0), and the other 2 h (T2) after the onset of the 30-min infusion of the drug. The exact sampling times were recorded. Samples were kept on ice and sent directly to the clinical chemistry laboratory, where they were centrifuged at 3,000 rpm at 4°C for 10 min before the supernatant was removed and analyzed. High-performance liquid chromatography connected to UV spectrophotometry (HPLC-UV) was used to measure the serum concentrations of β-lactams (11, 25). For measurements of TZP concentrations, only piperacillin levels were measured because the PKs of piperacillin and tazobactam are highly correlated (12, 25). The lower and upper limits of quantification for each β-lactam analyzed were 2 and 200 μg/ml, respectively. The coefficient of variation for repeatability or intermediate precision estimated at six levels of concentration for each β-lactam never exceeded 11%.
Pharmacokinetic analyses.
A one-compartment model was used to perform PK analyses because β-lactams have a small Vd and low protein binding and are essentially excreted by the kidneys. Assuming that the steady state was reached and considering the exponential elimination of drugs in one-compartment models, PK variables, such as Vd, total CL, elimination constant (ke), and elimination half-life (t1/2), could be calculated from concentrations measured at T0 and T2 (11, 12, 25).
Serum concentrations obtained from hypothetical antibiotic dosage regimens.
On the basis of the serum concentrations measured at T0 and T2 in each patient for a given dose, projected concentrations were calculated with the following formula: projected concentration = measured concentration × new dose/given dose. Using the known CL for each patient, the projected CT for hypothetical new dosage regimens could be calculated.
For all obese patients, based on measured serum concentrations obtained with doses equal to or greater than SDRs, we calculated the concentrations that would have been obtained if SDRs and/or increased drug regimens based on the correction formula for weight had been applied. We therefore obtained a mixture of measured and projected serum concentrations for SDRs and for increased dosage regimens based on the proposed correction for weight.
Clinical breakpoints, PK, and pharmacodynamic (PD) criteria.
For each TDM, the patient was classified as having an insufficient, an adequate, or an overdosed serum concentration. β-Lactam therapy was considered adequate when the serum concentration remained greater than 4 but less than 8 times the target MIC (4× MIC < T < 8× MIC, where T is the target MIC) during an optimal period of time corresponding to 70% of the dose interval for CEF, 50% for TZP, and 40% for MEM. The drug concentration at the end of the optimal period of time (CT) was calculated. A CT of <4× MIC was defined as an insufficient serum concentration, and a CT of >8× MIC was defined as an overdose. We used clinical breakpoints for Pseudomonas aeruginosa, as defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; version 2.0) (13) as our target MICs. The sensitivity thresholds for P. aeruginosa are ≤8 μg/ml for CEF, ≤16 μg/ml for TZP, and ≤2 μg/ml for MEM. Adequate CTs were, therefore, 32 to 64 μg/ml for CEF, 64 to 128 μg/ml for TZP, and 8 to 16 μg/ml for MEM.
Risk factors for inadequate serum drug concentrations.
We evaluated the adequacy of treatment when SDRs were administered for all serum drug levels and CTs were obtained. Risk factors for inadequate (insufficient or overdosed) β-lactam serum concentrations were looked for. The risk factors considered were weight, BMI, APACHE II score on admission, early TDM (TDM performed during the first 48 h of antibiotic therapy), use of vasopressors, serum creatinine levels, specific antibiotic administered, SOFA score, and CRRT on the day of TDM.
We calculated the interindividual coefficient of variation (CV) of CTs and the probability of achieving target CT values for other MICs that can be found in ICU-isolated Gram-negative bacteria. We also calculated the total daily dose (with a change only in the frequency of drug administration) that would have been needed in order to attain PD targets. The same calculations were performed for the subgroups of patients receiving and not receiving CRRT.
Selection criteria for the control group of nonobese patients.
Each TDM from the cohort of obese patients was matched with one TDM obtained from a nonobese (BMI less than or equal to 25 kg/m2) critically ill patient also diagnosed with sepsis in order to account for the high interpatient and intrapatient variability of β-lactam PKs during sepsis. We used an institutional database in which all TDMs for β-lactams in the ICU during the study period were recorded. Five criteria were used to match the TDMs: (i) type of antibiotic (CEF, TZP, or MEM), (ii) renal function (patients receiving CRRT were matched with patients receiving CRRT at time of TDM, and patients not receiving CRRT were matched with patients with the same creatinine clearance based on a 24-h urine collection), (iii) SOFA score, (iv) age, and (v) gender. In case of a lack of agreement on the five criteria, the type of antibiotic used and renal function were considered necessary conditions for patient selection.
Comparison between the cohorts of obese patients and nonobese patients.
Antibiotic choice, criteria for treatment with CRRT, data collection, serum samples for TDM, PK analyses, and calculations for interindividual CV and optimal antibiotic daily dose were performed in the same way as described for the cohort of obese patients. Patients in the control group received only SDRs. Because CRRT was identified as an independent risk factor for overdose and a protective factor against insufficient serum concentrations in the obese patient cohort (see Results), the two cohorts of patients were separated into subgroups of those with CRRT and those without CRRT, and the same analyses were performed.
Evaluation of β-lactam dose adjustment based on a correction formula for weight in the obese patient cohort.
The adequacy of serum drug levels to treat “difficult-to treat” pathogens obtained with increased dosage regimens based on a correction formula for weight was compared to adequacy when SDRs were administered to the cohort of obese patients. The same analysis was performed for the subgroups of obese patients receiving CRRT and those not receiving CRRT.
Statistical analysis.
Statistical analyses were performed using IBM SPSS Statistics, version 19, for Windows. Descriptive statistics were performed for all study variables. A Kolmogorov-Smirnov test was used to verify the normality of distribution of continuous variables. Discrete variables are expressed as counts (and percentages), and continuous variables are expressed as medians (and ranges). Categorical data were compared using a chi-square test or Fisher's exact test, as appropriate. Continuous variables were compared using a Mann-Whitney U test. We performed univariable and multivariable analyses using generalized estimating equation (GEE) regression models, with “Logit” as the link function, to identify risk factors for inadequate (either insufficient or overdosage) β-lactam serum concentrations. All tests were two-sided, and P value less than 0.05 were considered significant.
RESULTS
Obese cohort. (i) Patients and samples.
We studied 49 obese critically ill patients. Their demographic and clinical characteristics are presented in Table 1. A total of 68 TDMs were performed (12 for CEF, 19 for TZP, and 37 for MEM), corresponding to a median of 1 (range, 1 to 5) TDM per patient. Fifty-eight serum drug concentrations were obtained in patients treated with an SDR and 10 were obtained in those treated with a dose greater than the SDR. TDM was performed a median of 3 (range, 1 to 17) days after antibiotic therapy was begun. The demographic, biological, and clinical characteristics of the obese patients the day of TDM are presented in Table 2.
Table 1.
Demographic, biological, and clinical characteristics of all patients
| Variablea | Value for the cohort |
P valueb | |
|---|---|---|---|
| Obese patients (n = 49) | Nonobese patients (n = 59) | ||
| Age (yr [range]) | 59 (24–79) | 57 (19–91) | 0.326 |
| No. of male/female patients (%) | 27 (55)/22 (45) | 41 (70)/18 (30) | 0.123 |
| Weight (kg [range]) | 116 (80–178) | 61 (37–80) | <0.001 |
| BMI (kg/m2 [range]) | 40 (30–60) | 22 (15–25) | <0.001 |
| No. of medical/surgical admissions to ICU (%) | 23 (47)/26 (53) | 34 (58)/25 (42) | 0.268 |
| Comorbidity (no. of patients [%]) | |||
| COPD | 13 (27) | 12 (20) | 0.448 |
| Cardiomyopathy | 23 (47) | 23 (39) | 0.405 |
| Diabetes | 26 (53) | 19 (32) | 0.029 |
| Chronic renal insufficiency (serum creatinine >2.5 mg/dl) | 14 (29) | 9 (15) | 0.094 |
| Liver cirrhosis | 6 (12) | 7 (12) | 0.952 |
| Immunosuppressive drugs | 6 (12) | 27 (46) | <0.001 |
| Malignancy | 2 (4) | 14 (24) | 0.005 |
| Apache II score on ICU admission (range) | 18 (8–32) | 18 (8–36) | 0.367 |
| ICU length of stay (days [range]) | 13 (3–60) | 14 (2–65) | 0.929 |
| Overall ICU mortality (no. of patients [%]) | 16 (33) | 24 (41) | 0.390 |
| Overall hospital mortality (no. of patients [%]) | 21 (43) | 28 (48) | 0.633 |
| Infection sites (no. of patients [%]) | |||
| Lungs | 18 (37) | 35 (59) | 0.019 |
| Abdomen | 15 (31) | 16 (27) | 0.689 |
| Skin | 13 (27) | 5 (9) | 0.018 |
| Urinary tract | 3 (6) | 3 (5) | 0.999 |
| Unknown | 1 (2) | 2 (3) | 0.999 |
| Identified pathogens (no. of patients [%]) | |||
| P. aeruginosa | 14 (29) | 16 (27) | 0.867 |
| Enterobacteriaceae | 14 (29) | 20 (34) | 0.553 |
| S. aureus | 9 (18) | 8 (14) | 0.495 |
| Nonfermenting Enterobacteriaceae | 9 (18) | 6 (10) | 0.220 |
| Streptococcus sp. | 6 (12) | 9 (15) | 0.653 |
| Unknown | 7 (14) | 11 (19) | 0.545 |
ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; Apache II score, acute physiology and chronic health evaluation II score.
Boldface indicates significant P values.
Table 2.
Clinical characteristics of all patients in case control study on day of therapeutic drug monitoring
| Variablea | TDM in obese patients (n = 68)c | TDM in nonobese patients (n = 68)c | P valued |
|---|---|---|---|
| SOFA score (range) | 10 (1–19) | 8 (1–20) | 0.259 |
| Creatinine clearance (ml/min [range])b | 67 (10–257) | 63 (8–271) | 0.976 |
| CRRT (no. of samples [%]) | 34 (50) | 34 (50) | 1.00 |
| Ultrafiltrate (ml/h [range]) | 2,750 (1,100–4,500) | 2,000 (1,000–5,500) | 0.262 |
| Ultrafiltrate/kg (range) | 22 (11–39) | 35 (14–149) | <0.001 |
| Early TDM (no. of samples [%]) | 29 (43) | 25 (37) | 0.483 |
| Mechanical ventilation (no. of samples [%]) | 32 (47) | 47 (69) | 0.009 |
| Use of vasopressors (no. of samples [%]) | 49 (72) | 32 (47) | 0.003 |
SOFA, sequential organ failure assessment score; CRRT, continuous renal replacement therapy.
Only for patients not receiving CRRT (n = 34).
n, number of TDMs.
Boldface indicates significant P values.
(ii) PK data and PD analysis: serum drug concentrations obtained with SDRs and risk factors for inadequate drug levels.
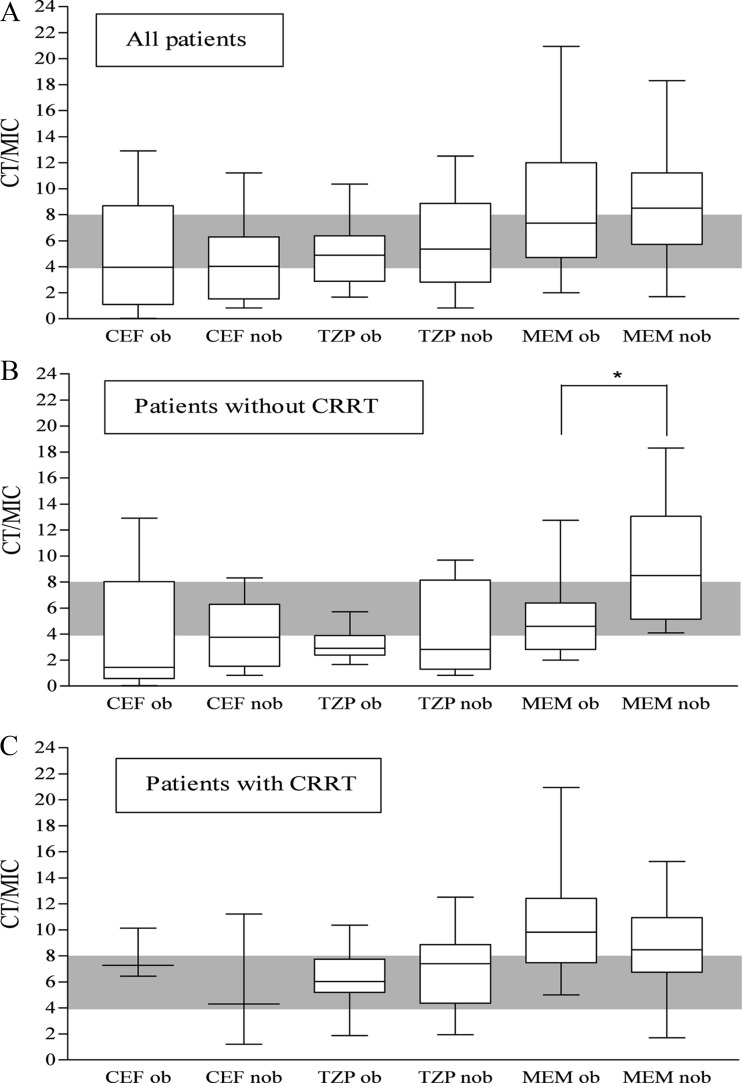
Median total doses of CEF, TZP, and MEM were 6 (range, 2 to 6) g, 16 (range, 12 to 16) g, and 3 (range, 2 to 3) g, respectively. The CTs obtained with SDRs are shown in Fig. 1. Only 25%, 47%, and 49% of drug levels for CEF, TZP, and MEM, respectively, attained adequate levels. The interindividual CVs were 92%, 50%, and 54% for CEF, TZP, and MEM, respectively. Univariable analysis followed by multivariable analysis identified early TDM as an independent predictor against overdosed serum concentrations. CRRT on the day TDM was performed was identified as an independent predictor against insufficient serum drug concentrations and as a risk factor for overdosed serum drug concentrations (Table 3). There were significantly fewer instances of inadequate drug levels among the CRRT-receiving patients compared to those not receiving CRRT (2/34 [5.9%] insufficient receiving CRRT versus 18/34 [52.9%] insufficient not receiving CRRT, P < 0.001). There were furthermore more instances of overdosed drug levels among the CRRT-receiving patients compared to those not receiving CRRT (15/34 [44.1%] overdosed receiving CRRT versus 3/34 [8.8%] overdosed not receiving CRRT, P = 0.002).
Fig 1.
Serum drug concentrations obtained in obese and nonobese patients when standard drug regimens were administered. (A) All TDM (therapeutic drug monitoring) events. (B) TDMs obtained when patients were not receiving CRRT (continuous renal replacement therapy). (C) TDMs obtained when patients were receiving CRRT. CT/MIC ratio, the drug concentration at the end of the optimal period of time/MIC (clinical breakpoints for Pseudomonas aeruginosa, as defined by EUCAST, were used [13]); CEF cefepime or ceftazidime; TZP, piperacillin-tazobactam; MEM, meropenem; ob, obese; nob, nonobese. Gray shading indicates adequate serum concentrations for difficult-to-treat pathogens. *, P = 0.003.
Table 3.
Risk factors for inadequate β-lactam serum concentrations when standard drug regimens were administered to obese patients
| Variablea | Risk analysis of insufficient serum concn by methodb |
Risk analysis of overdosed serum concn by methodb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||||
| T < 4× MIC | T > 4× MIC | P value | P value | OR (95% CI) | T < 8× MIC | T > 8× MIC | P value | P value | OR (95% CI) | |
| Weight (kg [range]) | 116 (100–130) | 115 (108–126) | 0.297 | 115 (100–129) | 118 (105–130) | 0.406 | ||||
| Body mass index (kg/m2 [range]) | 36 (33–47) | 41 (37–46) | 0.207 | 38 (34–43) | 43 (37–51) | 0.120 | ||||
| SOFA score on day of TDM (range) | 9 (1–13) | 10 (2–19) | 0.035 | 9 (1–18) | 11 (2–19) | 0.174 | ||||
| APACHE II score on admission (range) | 16 (8–28) | 20 (8–32) | 0.015 | 19 (8–32) | 20 (15–25) | 0.223 | ||||
| CRRT (no. of samples [%]) | 2 (10) | 32 (67) | 0.002 | 0.002 | 0.06 (0.01–0.36) | 19 (38) | 15 (83) | 0.006 | 0.010 | 7.3 (1.6–33.4) |
| Serum creatinine (mg/dl [range]) | 1.0 (0.4–2.9) | 1.3 (0.6–8.3) | 0.406 | 1.1 (0.4–3.8) | 1.4 (0.8–8.1) | 0.040 | ||||
| Early TDM (no. of samples [%]) | 9 (45) | 20 (42) | 0.932 | 25 (50) | 4 (22) | 0.020 | 0.042 | 0.3 (0.09–0.96) | ||
| Use of vasopressors (no. of samples [%]) | 13 (65) | 43 (90) | 0.051 | 41 (82) | 15 (83) | 0.976 | ||||
| Mechanical ventilation (no. of samples [%]) | 12 (60) | 22 (46) | 0.253 | 27 (54) | 7 (39) | 0.269 | ||||
| β-lactam administered (no. of samples [%]) | ||||||||||
| Meropenem | 6 (30) | 31 (65) | 0.042 | 24 (48) | 13 (72) | 0.497 | ||||
| Piperacillin-tazobactam | 8 (40) | 11 (23) | 0.725 | 17 (34) | 2 (11) | 0.274 | ||||
| Cefepime or ceftazidime | 6 (30) | 6 (13) | —c | 9 (18) | 3 (17) | —c | ||||
APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; TDM, therapeutic drug monitoring.
T values are for the time period specified in the description of the variable. OR, odds ratio; CI, confidence interval.
Used as reference.
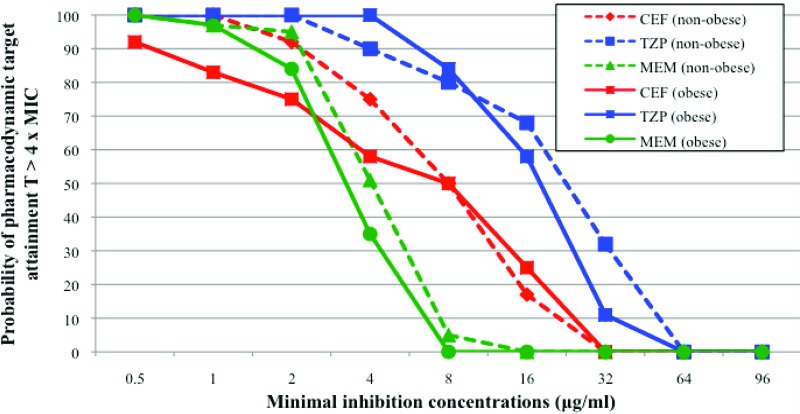
PK variables are presented in Table 4. The median total daily doses needed to reach PD targets are shown in Table 5. The probability of target CT attainment at various MICs when SDRs were administered to the obese cohort is shown in Fig. 2. Serum concentrations reached the PK/PD targets to treat infections caused by bacteria with MICs of the drugs corresponding to the clinical breakpoints for P. aeruginosa in 50%, 58%, and 84% of patients treated with CEF, TZP, and MEM, respectively.
Table 4.
Pharmacokinetic variables in all patients
| Variable and populationa | Value for the indicated drugb |
|||||
|---|---|---|---|---|---|---|
| MEM |
TZP |
CEF |
||||
| Mean (range) | P | Mean (range) | P | Mean (range) | P | |
| Vd (liters) | ||||||
| Obese | 40.0 (8.0–191.0) | 29.6 (14.3–51.3) | 24.0 (15.3–149.2) | |||
| Nonobese | 27.9 (5.4–205.2) | 0.103 | 21.3 (1.3–165.7) | 0.068 | 21.4 (13.1–48.9) | 0.488 |
| Indexed Vd (liters/kg) | ||||||
| Obese | 0.3 (0.1–1.6) | 0.3 ((0.1–0.4) | 0.3 (0.1–1.6) | |||
| Nonobese | 0.5 (0.1–3.0) | 0.001 | 0.3 (0.0–2.7) | 0.003 | 0.4 (0.2–0.8) | 0.65 |
| CL (ml/min) | ||||||
| Obese | 101.0 (6.4–487.6) | 90.2 (31.5–271.6) | 51.0 (7.4–174.3) | |||
| Nonobese | 77.0 (35.0–390.0) | 0.424 | 53.5 (13.8–547.0) | 0.093 | 47.0 (1.3–181.0) | 0.686 |
| CL with CRRT (ml/min) | ||||||
| Obese | 75.5 (6.4–188.9) | 74.4 (44.4–271.6) | 27.7 (10.5–39.7) | |||
| Nonobese | 61.8 (38.5–390.0) | 0.685 | 53.5 (13.8–115.2) | 0.178 | 25.0 (19.7–101.3) | 0.827 |
| CL without CRRT (ml/min) | ||||||
| Obese | 168.0 (26.7–487.6) | 167.1 (31.5–256.6) | 73.4 (7.4–174.3) | |||
| Nonobese | 102.0 (35.0–275.0) | 0.209 | 85.0 (21.7–547.0) | 0.529 | 49.8 (1.3–151.0) | 0.965 |
| t1/2 (h) | ||||||
| Obese | 3.7 (1.3–115.5) | 3.2 (1.6–18.2) | 3.0 (0.6–28.9) | |||
| Nonobese | 4.3 (1.0–15.1) | 0.841 | 3.8 (1.0–15.2) | 0.397 | 9.1 (1.3–115.5) | 0.157 |
| t1/2 with CRRT (h) | ||||||
| Obese | 5.5 (2.3–115.5) | 2.5 (1.8–7.7) | 2.4 (1.7–23.1) | |||
| Nonobese | 4.8 (2.0–15.1) | 0.617 | 2.9 (1.3–9.0) | 0.491 | 15.8 (13.1–30.6) | 0.513 |
| t1/2 without CRRT (h) | ||||||
| Obese | 1.6 (1.3–57.8) | 3.0 (1.6–18.2) | 5.5 (0.6–28.9) | |||
| Nonobese | 1.3 (1.0–8.3) | 0.448 | 3.5 (1.0–15.2) | 0.674 | 5.0 (1.3–115.5) | 0.251 |
Vd, volume of distribution; CL, clearance; t1/2, half-life; CRRT, continuous renal replacement therapy.
Boldface indicates significant P values.
Table 5.
Total daily doses necessary to reach pharmacodynamic targets for all patients
| Drug | Minimum median dose (range) by cohort and/or treatment (g)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total population |
With CRRT |
Without CRRT |
|||||||
| Obese | Nonobese | P value | Obese | Nonobese | P value | Obese | Nonobese | P valueb | |
| MEM | 2 (1–9) | 2 (1–7) | 0.433 | 1 (1–2) | 1 (1–7) | 0.272 | 3 (1–5) | 2 (1–3) | 0.011 |
| TZP | 12 (6–76) | 12 (4–76) | 0.422 | 8 (4–76) | 12 (4–16) | 0.921 | 24 (8–40) | 20 (4–76) | 0.674 |
| CEF | 4 (2–18) | 10 (2–30) | 0.419 | 2 (2–4) | 6 (2–12) | 0.127 | 12 (2–24) | 12 (2–30) | 0.757 |
CRRT, continuous renal replacement therapy.
Boldface indicates a significant P value.
Fig 2.
Probability of attaining the target CT of >4× MIC for various MICs when standard dosage regimens of CEF, TZP, and MEM were administered in obese and nonobese patient cohorts.
Nonobese control group. (i) Patients and samples.
Sixty-eight TDMs (12 for CEF, 19 for TZP, and 37 for MEM) were used as a match from a cohort of 59 nonobese critically ill patients. A median of 1 (range, 1 to 3) TDM per patient was performed a median of 3 (range, 1 to 29) days after antibiotic therapy was begun. TDMs between groups were well matched (Tables 1 and 2).
(ii) PK data and PD analysis: serum drug concentrations obtained with SDRs.
Median total doses of CEF, TZP, and MEM were similar to those administered to the cohort of obese patients: 6 (range, 2 to 6) g, 16 (range, 12 to 16) g, and 3 g, respectively. We observed a similar large variability in serum concentrations between patients in both cohorts, as shown by the high CVs for the three antibiotics (54% versus 43% for MEM, 50% versus 60% for TZP, and 92% versus 74% for CEF for obese versus nonobese patients, respectively). The CTs obtained with SDRs in the nonobese control group are shown in Fig. 1. No differences between the two cohorts were observed for the three antibiotics.
Because CRRT was identified as a risk factor for higher serum concentrations in obese patients, we compared adequacy of treatment between obese and nonobese patients not receiving CRRT and then between those receiving CRRT (Fig. 1B and C). In patients receiving CRRT, adequacy of treatment and CTs were similar for all three antibiotics. In contrast, in patients treated with MEM and not receiving CRRT, lower concentrations were observed in obese patients than in nonobese patients (Fig. 1B). Therefore, more obese patients had concentrations that did not reach therapeutic targets than nonobese patients (6/17 [35%] versus 0/17 [0%]; P = 0.02). No differences were observed for CEF and TZP.
Few differences were observed in PK variables when the two groups of patients were compared (Table 4). The absolute Vds of all three drugs were similar between obese and nonobese patients, but when normalized for weight, Vds were significantly higher for MEM and TZP in nonobese individuals. The CL values and t1/2s of all antibiotics were similar in both cohorts. No significant differences were observed between the two cohorts concerning the probability of target CT attainment for various MICs (Fig. 2). Total daily doses necessary to reach PD targets were similar in both obese and nonobese patients for all antibiotics, except for in the obese patient population treated with MEM and not receiving CRRT: greater doses of MEM were needed for obese patients than for the nonobese population (Table 5).
Impact of correction formula for weight.
If increased dosage regimens based on the correction formula for weight had been administered to the obese patient cohort, daily doses of CEF, TZP, and MEM would have been 8 (range, 2 to 8) g, 20 (range, 16 to 24) g, and 4 (range, 2 to 5) g, respectively, corresponding to an increase in the daily dose administered of 33%, 25%, and 33% for CEF, TZP, and MEM, respectively. Because increased dosage regimens were always rounded up or down to the closest unitary dose of the antibiotic, 12 dosage regimens (6 for CEF, 2 for TZP, and 4 for MEM) were not increased by the correction formula for weight. The adequacy of treatment obtained for all obese patients (including subgroups of patients treated with and without CRRT) with increased dosage regimens based on the proposed correction formula for weight was not significantly different than the adequacy of treatment obtained with SDRs (for all patients, 30/68 [44%] versus 28/68 [41%]; P = 0.729) (data not shown for subgroups of patients).
DISCUSSION
This is the first study to evaluate β-lactam levels in critically ill, obese patients and to compare them to a nonobese critically ill cohort. In the obese population, SDRs of β-lactams resulted in insufficient serum concentrations in one-third of the patients and in overdosed, potentially toxic serum concentrations in one-fourth. CRRT was identified as a risk factor for higher serum concentrations for CEF, TZP, and MEM. Dosage regimens based on a correction formula for weight only slightly increased serum drug concentrations but had no impact on adequacy of treatment.
We have compared the obese patient cohort to a well-matched nonobese critically ill patient cohort. Both groups showed marked variability in PK variables and serum concentrations for all three antibiotics. Antibiotic serum concentrations were similar in the two groups of patients except for patients treated with MEM. In these patients, lower serum concentrations were observed in obese than in nonobese patients not receiving CRRT. In contrast, this difference was not observed if patients received CRRT. Indeed, a less effective dialysis, shown by lower ultrafiltrate per kg of body weight leading to more drug accumulation in obese patients, could explain this observation. Because of technical difficulties or device limitations, the median ultrafiltrate rate ranged from 2,000 to 2,750 ml/h, resulting in lower than recommended rates in some patients, especially if they were overweight. Because half of the TDMs analyzed were performed while patients received CRRT, less effective dialysis may have had an impact on serum concentrations observed in the total obese patient population (14).
No significant differences in PK parameters were observed between the two cohorts. Because absolute Vds were similar between obese and nonobese patients, the Vd indexed for weight, as expected, was increased in nonobese patients compared to the value in obese patients. Indeed, β-lactams are hydrophilic molecules, and adipose tissue is composed of only 30% water, thus explaining these findings. There was, however, a trend for a higher CL and total Vd in obese patients receiving MEM without CRRT than in nonobese patients. The combined effect of these two variables, even if not significant when analyzed independently, may explain the lower serum concentrations observed in these patients. For CEF and TZP, the cohorts may have been too small to demonstrate differences between the two groups.
Concordant with the rest of our results, obese patients receiving MEM without CRRT were the only patients needing higher doses than the nonobese cohort to attain PD targets. However, the total daily dose needed for this subgroup of patients was not greater than the current SDR. The probabilities of PD target attainment for pathogens like P. aeruginosa that are difficult to treat were no different between the obese and nonobese patient cohorts. SDRs were adequate only for the treatment of very susceptible pathogens in both obese and nonobese patients.
Our obese patient population was representative of obese critically ill patients with high severity scores, high frequency of infections due to P. aeruginosa, and high mortality rates. Even though our obese cohort was well matched with a nonobese cohort, more obese patients received vasopressors on the day of TDM than in the nonobese group, and more nonobese patients benefitted from mechanical ventilation than the obese cohort. These factors may have influenced the PKs of the drugs as both factors influence Vd and CL (2). However, in the subgroup of patients treated with MEM and not receiving CRRT, no differences were observed between obese and nonobese patients concerning treatment with vasopressors and mechanical ventilation on the day of TDM (data not shown). Therefore, these factors do not explain the lower serum concentrations observed in this subgroup of patients.
There are no reports on β-lactams in critically ill obese patients and very few series on β-lactams in noncritically ill obese patients. Studies on prophylaxis with narrow-spectrum cephalosporins showed that dosages needed to be doubled to reach target concentrations in obese patients (15, 16, 26). A dose of 2 g twice daily of cefepime was insufficient to reach PD targets (17). Serum concentrations of ertapenem were lower in obese patients than in nonobese patients (18). Finally, the average steady-state serum concentration of TZP in a very obese septic patient was less than the concentration obtained in a healthy, nonobese population (19). Previous studies on general populations of critically ill patients have already shown that SDRs of β-lactams are inadequate in general populations of critically ill patients (3, 12). Therefore, obesity alone is unlikely to explain the insufficient drug levels measured in our critically ill patients but may have aggravated the inadequacy of β-lactam serum concentrations, particularly in the case of MEM.
A correction dosage formula for weight has been proposed for use in obese patients, based on a correction factor of 0.30 to take into account the lower distribution of β-lactams in adipose tissue (4). This correction formula induced small increases of daily doses (33%, 25%, and 33% for TZP, CEF, and MEM, respectively) and only minor modifications in serum concentrations without impact on the adequacy of treatment.
There are several limitations to our study. First of all, the study is retrospective: serum concentrations were performed only in selected patients and not in all patients. Second, results are more robust for MEM than for TZP and CEF because of larger groups of patients. More patients are needed in the TZP and CEF groups before any conclusions can be made. Finally, because CRRT was a risk factor for higher serum concentrations in the obese patient cohort, probably because of lower efficacy of dialysis, there is reason to believe that there are two populations of obese critically ill patients: those who receive CRRT and those who do not receive CRRT. Because of the great variability of serum concentrations observed in critically ill patients and the impact of CRRT on these concentrations, it is challenging to show differences between the two groups of patients.
Finally, our definitions of adequate, insufficient, and overdosed serum concentrations are not based on a consensus. However, the target of 4× the MIC of a drug for the given pathogen for the specified time periods has been extensively discussed (11, 12). We defined the upper limit target as 8× the MIC for the specified time periods (40% of the time for MEM, 50% of the time for TZP, and 70% of the time for CEF) based on in vitro data showing the absence of better efficacy with concentrations greater than 4× to 5× the MIC for the pathogen (20, 21) and reported toxicity, mainly neurological, in the case of high serum concentrations (22–24).
In conclusion, this is the very first case-control study comparing serum concentrations of β-lactams in obese to those in nonobese critically ill patients. No major differences between obese and nonobese critically ill patients were found. Sepsis appears to alter PK parameters of β-lactams and, thus, serum concentrations much more than obesity in itself does. Given the large variability in serum concentrations observed in obese patients with severe sepsis, the impact of the efficacy of CRRT, and the absence of validated correction formulas for weight or sepsis, we recommend that until results from large prospective PK studies are available, TDM should be performed routinely in obese, critically ill patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Karen Pickett for her suggestions after careful reading of the text. We also thank Hassane Njimi for the statistical calculations.
Footnotes
Published ahead of print 12 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01083-12.
REFERENCES
- 1. Gonçalves-Pereira J, Povoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit. Care 15:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851 [DOI] [PubMed] [Google Scholar]
- 3. Roberts JA, Ulldemolins M, Roberts M, McWhinney B, Ungerer J, Paterson D, Lipman J. 2010. Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int. J. Antimicrob. Agents 36:332–339 [DOI] [PubMed] [Google Scholar]
- 4. Wurtz R, Itokazu G, Rodvold K. 1997. Antimicrobial dosing in obese patients. Clin. Infect. Dis. 25:112–118 [DOI] [PubMed] [Google Scholar]
- 5. Levy M, Fink M, Marshall J, Abraham E, Angus D, Cook D, Cohen J, Opal S, Vincent JL, Ramsay G. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 6. Cockcroft D, Gault M. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 7. Green B, Duffull S. 2004. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br. J. Clin. Pharmacol. 58:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent JL. 2009. Le manuel de réanimation, soins intensifs et médecine d'urgence, 3rd ed Springer, Paris, France [Google Scholar]
- 9. Knaus W, Draper E, Wagner D, Zimmerman J. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829 [PubMed] [Google Scholar]
- 10. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart C, Suter P, Thijs L. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/ failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22:707–710 [DOI] [PubMed] [Google Scholar]
- 11. Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent JL, Jacobs F. 2011. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care. 15:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, Jacobs F. 2010. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care. 14:R126 doi:10.1186/cc9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Committee on Antimicrobial Susceptibility Testing 2012. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf
- 14. Valtonen M, Tiula E, Takkunen O, Backman JT, Neuvonen PJ. 2001. Elimination of the piperacillin/tazobactam combination during continuous venovenous haemofiltration and haemodiafiltration in patients with acute renal failure. J. Antimicrob. Chemother. 48:881–885 [DOI] [PubMed] [Google Scholar]
- 15. Edmiston C, Jr, Krepel C, Kelly H, Larson J, Andris D, Hennen C, Nakeeb A, Wallace J. 2004. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery 136:738–747 [DOI] [PubMed] [Google Scholar]
- 16. Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE., Jr 2011. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet. Gynecol. 117:877–882 [DOI] [PubMed] [Google Scholar]
- 17. Rich B, Keel R, Ho V, Turbendian H, Afaneh C, Dakin G, Pomp A, Nicolau D, Barie P. 2012. Cefepime dosing in the morbidly obese patient population. Obes. Surg. 22:465–471 [DOI] [PubMed] [Google Scholar]
- 18. Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS., Jr 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob. Agents Chemother. 50:1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman D, Scheetz MH, Adeyemi O, Montevecchi M, Nicolau D, Noskin G, Postelnick MJ. 2007. Serum piperacillin/tazobactam pharmacokinetics in a morbidly obese individual. Ann. Pharmacother. 41:1734–1739 [DOI] [PubMed] [Google Scholar]
- 20. Craig WA, Redington J, Ebert SC. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl C):29–40 [DOI] [PubMed] [Google Scholar]
- 21. Williamson R, Tomasz A. 1985. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur. J. Biochem. 151:475–483 [DOI] [PubMed] [Google Scholar]
- 22. Chapuis T, Giannoni E, Majcherczyk PA, Chioléro R, Schaller MD, Berger MM, Bolay S, Décosterd LA, Bugnon D, Moreillon P. 2010. Prospective monitoring of cefepime in intensive care unit adult patients. Crit. Care. 14:R51 doi:10.1186/cc8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatellier D, Jourdain M, Mangalaboyi J, Ader F, Chopin C, Derambure P, Fourrier F. 2002. Cefepime-induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure. Intensive Care Med. 28:214–217 [DOI] [PubMed] [Google Scholar]
- 24. Naeije G, Lorent S, Vincent JL, Legros B. 2011. Continuous epileptiform discharges in patients treated with cefepime or meropenem. Arch. Neurol. 68:1303–1307 [DOI] [PubMed] [Google Scholar]
- 25. Wolff F, Deprez G, Seyler L, Taccone F, Hites M, Gulbis B, Vincent JL, Jacobs F, Cotton F. 2013. Rapid quantification of six β-lactams to optimize dosage regimens in severely septic patients. Talanta 103:153–160 [DOI] [PubMed] [Google Scholar]
- 26. Forse RA, Karam B, MacLean LD, Christou NV. 1989. Antibiotic prophylaxis for surgery in morbidly obese patients. Surgery 106:750–756 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.