Abstract
The pharmacokinetic (PK) property of fluconazole might be significantly altered in major burn patients by medical interventions and physiologic changes. In this study, our aims were to investigate fluconazole PK in burn patients using a population approach and to recommend the optimal fluconazole regimen based upon the predicted therapeutic outcome. At steady state, blood samples for PK analysis were obtained from 60 burn patients receiving between 100 and ∼400 mg fluconazole daily. A mixed-effect modeling was performed and the therapeutic outcome of antifungal therapy was predicted for 10,000 virtual patients using NONMEM (version 7.2). MIC values were sampled from the MIC distribution at the study site. An area under the free drug concentration-time curve (fAUC)/MIC measurement of >25 h was used as the criterion for therapeutic success. When the same dose was given, the plasma concentration of fluconazole was predicted to be lower in burn patients compared to the nonburn population because of the large PK parameter (clearance, volume of distribution) estimates and continuous renal replacement therapy (CRRT). This tendency was particularly predominant when the patients were within 30 postburn days. Based upon our findings, 400 mg/day fluconazole is recommended to obtain therapeutic successes in major burn patients.
INTRODUCTION
Fungal infection is a frequently found comorbidity in seriously ill patients. Accordingly, fungi (predominantly Candida spp.) have been among the most frequently isolated microorganisms in intensive care units (ICUs) (1, 2), including burn ICUs (BICUs). Because this kind of infection results in an unfavorable prognosis with more complications and a higher mortality (3), effective antifungal therapy is thought to be crucial for treatment success.
Fluconazole is one of the most important therapeutic options in antifungal therapy. To predict the effectiveness of fluconazole therapy, dose/MIC or area under the free drug concentration-time curve (fAUC)/MIC can be monitored (4). Since the pharmacokinetic (PK) characteristics of fluconazole are well known and relatively stable, either dose/MIC or fAUC/MIC seems to be a reasonable marker for predicting efficacy (4–7).
Because patients with major burns undergo intensive interventions as well as physiological changes with time, the PK property of fluconazole might be significantly altered (8). A number of factors, such as the size and depth of the burn, the presence of sepsis, serum protein levels, sex, age, renal function, time since burn injury, degree of hydration, and renal replacement therapy, may affect clearance (CL) or volume of distribution (V) of the drug in burn patients (9, 10). It was also reported that the conventional fluconazole dosage regimen can be often therapeutically unsuccessful in burn patients because their concentrations are likely to be lower than expected (11).
In this context, estimating the population PK parameters of fluconazole in burn patients is the first step in recommending alternative dosage regimens that can give optimal fAUC/MIC values for burn patients. Through this population approach, we were able to find factors affecting the PK parameters and to quantify their influence in the model. With the final population PK model and the MIC distribution of clinically isolated Candida spp., the probability of target attainment (PTA) was also assessed.
MATERIALS AND METHODS
Subjects and ethical considerations.
Among the patients admitted to the BICU of Hangang Sacred Heart Hospital in Seoul between December 2008 and May 2010 with burns ranging from 11% to 95% of total body surface area, 60 were enrolled. Those who were allergic to fluconazole, younger than <18 years, pregnant, or breastfeeding were excluded. The mean patients' age was 50.3 years, and the mean time from burn injury to fluconazole administration was 23.0 days (Table 1). The trial was designed and monitored in accordance with the good clinical practice guidelines of South Korea and with the principles of the Declaration of Helsinki. The independent institutional review board (Hangang Sacred Heart Hospital) approved the protocol before execution of the trial, and all participants gave written informed consent or assent when patients were unable to give consent.
Table 1.
Demographic and clinical characteristics of subjects
| Variable | Distributiona |
|---|---|
| Age (yr) | 50.3 (20–82) |
| Sex (male/female) | 50/10 |
| Wt (kg) | 65.6 (40.0–90.0) |
| Total body surface area burned (%) | 50.3 (11–95) |
| Sepsis (with/without) | 29/31 |
| Edema (with/without)b | 20/40 |
| APACHE II score | 7.6 (2–26) |
| Creatinine clearance (ml/min)c | 123.5 (21.6–282.7) |
| Continuous renal replacement therapy (with/without) | 15/45 |
| Time from burn injury (days) | 23.0 (7–88) |
Mean (range) for continuous variables and actual number of subject for categorical variables are presented.
Clinical diagnosis (puffy face and pitting edema in legs).
Estimated using Cockcroft-Gault equation.
Fluconazole administration and blood sampling.
Patients were intravenously infused with 100 to 400 mg of fluconazole for 10 to 40 min every 24 h for the treatment of suspected (47 cases) or confirmed (13 cases) fungal infection. They had received 5 to 17 days (mean 6.5 days) of fluconazole treatment prior to the PK sampling. Eight venous blood samples were obtained via central venous catheter at 0 (just before dosing), 3, 5, 9, 24, 27, 48, and 51 h after the initiation of infusion. Fluconazole dosing was maintained every 24 h regardless of sampling.
Plasma fluconazole assay.
Fluconazole concentrations were determined using high-performance liquid chromatography coupled with tandem mass spectrometry (LC/MS-MS). Plasma was separated immediately by centrifugation at 2,093 × g for 10 min at 4°C and stored at −70°C until assayed. Briefly, the assay method was as follows: a volume of 50 μl of plasma was mixed with 30 μl of internal standard (cephalexin, 10 μg/ml in distilled water) and deproteinized with 950 μl of 10% trichloroacetic acid (TCA). After thoroughly vortexing for 30 s, the samples were centrifuged at 17.311 × g for 10 min at 4°C. A volume of 2 μl of the supernatant was injected into an LC/MS-MS system. The analytes were separated through a Luna C18 column (100 by 2.0 mm; 5-μm particle size) at a flow rate of 0.5 ml/min by a mobile phase consisting of 0.1% formic acid and methanol with 0.1% formic acid (75:25 [vol/vol]) and detected by an electrospray positive-ionization mode of tandem mass spectrometry. Mass-to-charge ratios (m/z) in multiple-reaction monitoring (MRM) were 307.2 to 238.2 for fluconazole and 348.2 to 158.0 for the internal standard.
The lower limit of quantification (LLOQ) was 0.04 μg/ml. The coefficients of correlation (r) were greater than 0.9995 in the range of 0.04 to ∼10 μg/ml by weighted linear regression (1/concentration). The precisions (% relative standard deviation [RSD]) and mean accuracies of intra- and interdays were below 7.42% and 96.14 to ∼108.3%, respectively.
Population PK model development.
A population pharmacokinetic analysis was performed using NONMEM (version 7.2; Icon Development Solution, Ellicott City, MD).
Data set.
The data set consisted of a total of 409 fluconazole concentration observations from 60 subjects. Demographic and clinical variables such as age, sex, weight (WT), sepsis condition (SEPS), edema status (EDEM), APACHE II score (APA), creatinine CL calculated by the Cockcroft-Gault equation (CLCR), application of continuous renal replacement therapy (CRRT), total body surface areas burned (TBSA), and time after burn injury (TBI) were included as potential covariates. Because the postburn hypermetabolic response is maximized between day 7 and day 17 after injury (it decreases thereafter) and varies by subjects (12, 13), we considered TBI to be a potential covariate explaining the physiological difference. TBI was included as a categorical variable (1 for patients whose time from burn injury was within 30 days and 0 for others), and the cutoff point was determined by consideration of the time course of physiological change.
Base PK model.
The first-order conditional estimation method with the η-ε interaction option (FOCE-I) was used throughout the model building process. Both a single- and a multicompartmental model were tested to describe fluconazole distribution. The first-order kinetics was assumed for all the PK processes. Between-subject variabilities (BSV) of PK parameters were expressed in equation 1:
| (1) |
where Pi is the pharmacokinetic parameter for the ith individual, θ is the population typical value of the according parameter, and ηi is a random variable for the ith individual following a Gaussian distribution with the mean of 0 and the variance of ω2. For intraindividual variability (residual error), a combined error model of both additive and proportional characteristics was used (equation 2):
| (2) |
where COBS and CIPRED are the observed and predicted concentrations of fluconazole, respectively. The ε1 and ε2 were assumed to follow Gaussian distributions with the mean of 0 and variance of σ12 and σ22, respectively. The different models were evaluated both numerically and visually using the objective function value (OFV) for nested models, the Akaike criterion (AIC) for nonnested models, and diagnostic plots, including goodness of fit, distribution of residuals, and parameter correlations.
Covariate selection.
Demographic and clinical variables were tested as potential covariates for PK parameters. Both visual and numerical covariate screening procedures were performed before testing each covariate for model improvement. For visual screening, scatterplots for continuous variables and boxplots for discrete variables were used. A generalized additive modeling (GAM) procedure was applied as numerical screening. Variables showing a potential relationship with a certain PK parameter in one or both of the screening procedures were included in the model to be tested as covariates.
Covariates were chosen using forward selection-backward elimination with the likelihood ratio test. Because the OFV follows a chi-square distribution, a decrease in the OFV greater than 3.84 (P < 0.05; df = 1) was used as a statistical significance criterion.
Model evaluation.
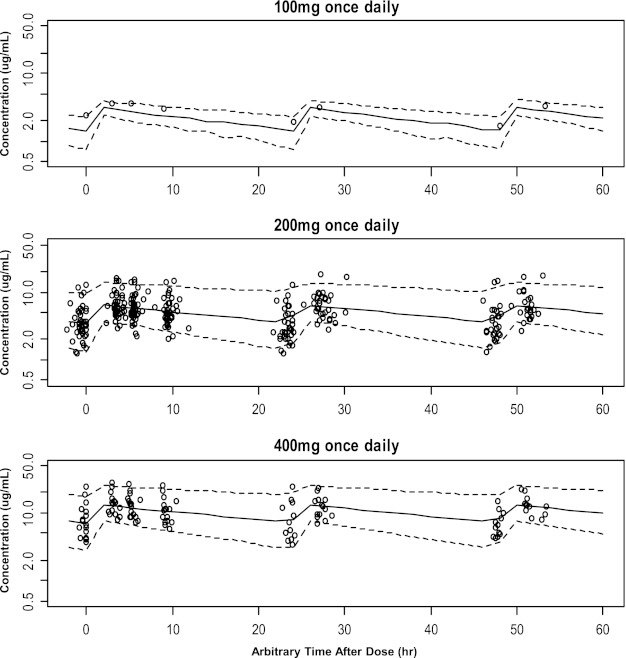
To evaluate the parameter values obtained from the final model, 1,000 bootstrap-resampled data sets from the original data set were sequentially estimated using the same final model. The median and 90% confidence intervals (CIs) (5th percentile and 95th percentile) of parameters obtained from this step were compared with the final parameter estimates. In addition, the visual-predictive check (VPC) was also performed. A total of 7,000 simulated concentrations from 1,000 virtual subjects (i.e., 7 fluconazole plasma concentrations for each subject at predefined time points) were simulated using the final model for fluconazole PK. The 90% CIs of fluconazole plasma concentrations at each observation time in the simulated data set were overlaid with the observed concentrations grouped by the dosage regimens (100, 200, or 400 mg per day).
Simulation of the fAUC/MIC.
The target values of fAUC/MIC (h) used to calculate PTA were 25 and 50. The simulation was performed for the 200-mg/day and 400-mg/day regimens. Using the final PK model, 24-h steady-state AUC values were simulated for 10,000 virtual patients with the covariate composition that was the same as that of the original data set. In this step, we actually calculated single-dose AUC to infinite time (= daily dose/CL) and used the value as the 24-h steady-state AUC because fluconazole showed linear PK in the dose range of interest. Assuming the plasma protein binding of fluconazole to be about 12% (14), fAUC was calculated as the 24-h steady-state AUC multiplied by the free fraction (0.88). A total of 10,000 MIC values were randomly sampled with replacement from the MIC profile of fluconazole-susceptible Candida spp. isolated from the BICU of Hangang Sacred Heart Hospital (not from the patients involved in the PK sampling but representing the general patient population). The MIC profile consisted of 45 observed MIC values for Candida albicans (67%), C. tropicalis (27%), and other Candida spp. (7%). The actual values of MIC were 0.25 μg/ml (15.6%), 0.5 μg/ml (40.0%), 1 μg/ml (24.4%), and 2 μg/ml (20.0%). Because the distribution profile did not appear to follow a simple statistical distribution, i.e., Gaussian, we had to use randomly sampled MIC values instead of those randomly generated from the distribution function.
However, we could not investigate the correlation target attainment and clinical outcome, because the MIC values were obtained from the general population at the study site rather than the patients involved in our study.
RESULTS
PK model.
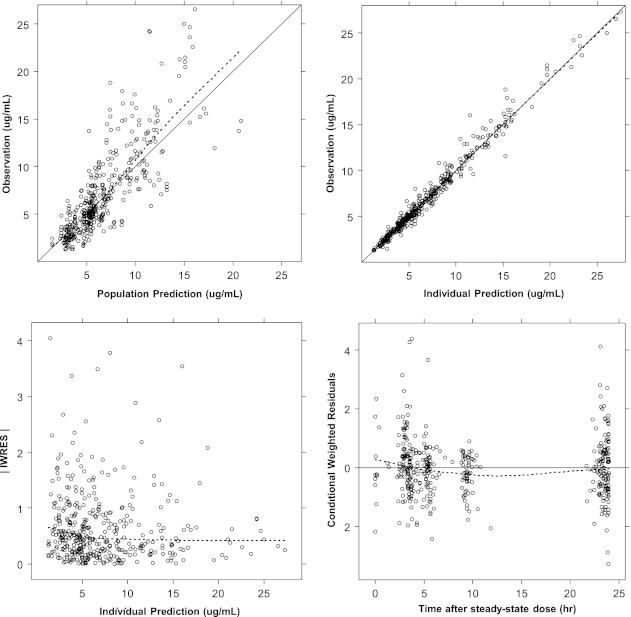
A one-compartment first-order elimination model with proportional residual error was chosen as the base PK model. In the covariate analysis, CRRT, CLCR, TBI, and SEPS were included as statistically meaningful covariates for CL and WT, EDEM, and TBI for the volume of distribution V. The covariate selection process is shown in Table 2 in decreasing order of the OFV (model improvement). The basic goodness-of-fit plot of the final PK model with all covariates is also given (Fig. 1).
Table 2.
Model development process
| Covariate | Model structure | df | OFV | ΔOFV |
|---|---|---|---|---|
| Base model | CL = θ1 · eη1, V = θ2 · eη2 | 420.246 | ||
| CRRTa to CL | 2 | 401.208 | −19.038 | |
| Without CRRT | CLRENAL = θ1 · eη1 | |||
| With CRRT | CLCRRT = θ3 · eη3 | |||
| CLCRb to CL | CLRENAL = (θ1 + CLCR · θ4) · eη1 | 1 | 385.672 | −15.536 |
| WTc to V | V = (θ2 + (WT/65) · θ5) · eη2 | 1 | 371.074 | −14.598 |
| EDEMd to V | V = (θ2 + (WT/65) · θ5 + EDEM · θ6) · eη2 | 1 | 361.849 | −9.225 |
| TBIe to CL | CLRENAL = (θ1 + CLCR · θ4 + TBI · θ7) · eη1 | 1 | 353.365 | −8.484 |
| TBI to V | V = (θ2 + (WT/65) · θ5 + EDEM · θ6 + TBI · θ8) · eη2 | 348.481 | −4.884 | |
| SEPSf to CL | CLRENAL = (θ1 + CLCR · θ4 + HMP · θ7 + SEPS · θ9) · eη1 | 1 | 343.639 | −4.842 |
Application of continuous renal replacement therapy (0, not applied; 1, applied).
Creatinine clearance estimated using Cockcroft-Gault equation.
Body weight of patient.
Presence of edema (0, not present; 1, present).
Time from burn injury (0, ≥30 days; 1, <30 days).
Presence of sepsis (0, not present; 1, present).
Fig 1.
Basic goodness-of-fit plot of final PK model. Solid line, line of identity (upper panels) or line of reference (y = 0, bottom right panel). Dotted line, line of locally weighted scatterplot smoothing (LOWESS). |IWRES|: absolute value of individual weighted residuals.
When a patient was in hypermetabolic status with a CLCR of 120, the typical value of CL was expected to be 1.75 liters/h (sum of θ1, θ4, and θ7), which was similar to that of patients receiving CRRT (1.85 liters/h). The value showed about a 29% decrease by 30 days after burn injury. For a patient within 30 days after injury, weighing 65 kg, the typical value of V was estimated to be 59.9 liters (sum of θ5 and θ8) when the patient had no edema. The edema was expected to increase V to about 13.6 liters. In the final model, when the covariates were fully included, the slope of the proportional relationship (θ2) was not successfully estimated.
The final parameter estimates and their bootstrap results (mean and 95% confidence interval [CI]) are shown in Table 3. The VPC results in Fig. 2 shown as 90% simulated CI range curves overlaid with the observed data suggest that the predictive performance of the final model was appropriate throughout the dose range studied.
Table 3.
Final parameter estimates and bootstrap results
| Parameter | Description (units) | Estimation results (estimate [% RSE])a | Bootstrap results (mean [95% CI]) |
|---|---|---|---|
| Fixed effects | |||
| CL = (1−CRRT) · (θ1 + CLCR · θ4 + TBI · θ7 + SEPS · θ9) + CRRT · θ3 | |||
| θ1 | CL intercept nonseptic, ≥30 days postburn patients without CRRT (liters/h) | 0.693 (28.3) | 0.622 (0.116–1.03) |
| θ3 | CL in patients with CRRT (liters/h) | 1.85 (4.3) | 1.86 (1.70–2.06) |
| θ4 | Proportionality constant between CL and CLCR/120 | 0.557 (28.9) | 0.593 (0.180–0.993) |
| θ7 | Increase of CL within 30 postburn days (liters/h) | 0.504 (36.3) | 0.580 (0.293–0.946) |
| θ9 | Decrease of CL due to sepsis (liters/h) | −0.369 (45.0) | −0.390 (−0.0649–−0.730) |
| V = θ2 + (WT/65) · θ5 + EDEM · θ6 + TBI · θ8 | |||
| θ2 | V intercept in nonedematous, ≥30 days postburn patients (liters) | NEb | |
| θ5 | Proportionality constant between V and WT (liters/kg) | 50.3 (6.8) | 50.6 (43.9–59.0) |
| θ6 | Increase of V due to edema (liters) | 13.6 (33.3) | 13.5 (5.35–22.6) |
| θ8 | Increase of V within 30 postburn days (liters) | 9.61 (43.1) | 9.44 (0.37–17.7) |
| Random effects (estimates, % CV) | |||
| ω12 | BSV of CL in the patients without CRRT | 35.7 (19.8) | 32.4 (24.8–40.4) |
| ω22 | BSV of V | 17.4 (36.5) | 15.9 (8.91–22.2) |
| ω32 | BSV of CL in the patients with CRRT | 16.1 (30.5) | 15.0 (9.84–20.6) |
| Residual error | |||
| σ12 | Residual error (proportional) | 0.111 (11.0) | 0.110 (0.0883–0.135) |
RSE, relative standard error.
NE: Not estimated.
Fig 2.
Visual predictive check results by dosing regimen. Solid line, median from time-concentration profile. Dotted lines, lower and upper limits of 90% confidence interval. All y axes are presented in the log scale.
Simulation of the fAUC/MIC.
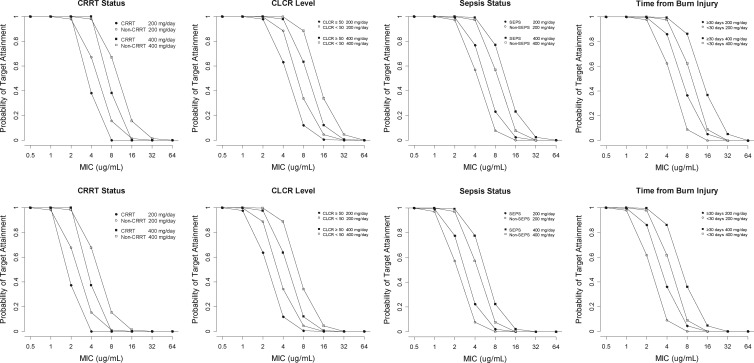
The PTA curves for daily 200-mg and 400-mg doses, where PTA values are plotted against MIC values, are shown in Fig. 3. The lower cutoff value of CLCR used in the simulation was 50 ml/min because dose reduction was generally recommended for patients with a CLCR of <50 ml/min (15).
Fig 3.
Probability of target attainment by fluconazole regimen and covariates affecting fluconazole clearance. Upper panels: target fAUC/MIC value of 25. Lower panels: target fAUC/MIC value of 50.
When the target fAUC/MIC was assumed to be 25, PTAs were almost 100% even for the daily 200-mg regimen at the MIC of ≤2 μg/ml. The PTA values at the other simulation scenarios reflected the simple proportional or reciprocal relationships with the daily dose, MIC, and target fAUC/MIC as expected (Fig. 3). The covariates increasing drug CL, such as CRRT and TBI, diminished PTA, while sepsis and lowered CLCR improved the PTA.
DISCUSSION
The results from the final PK model were similar to those in previous reports. For the parameter estimates, our model gave the values of CL and V as 1.25 liters/h and 50.3 liters for a typical burn patient (65 kg, ≥30 days after injury, without renal insufficiency, sepsis, or edema). According to the article by Boucher et al. (8), where a noncompartmental full PK analysis of 9 burn patients was performed, the parameter values in such patients were observed as 1.52 ± 0.31 liters/h and 46.8 ± 7.8 liters, respectively, which were larger than those from nonburn patients or healthy adults (16, 17). In addition, the tendency of the PK parameter change by the medical condition was reasonable. It is generally known that the CLCR and CRRT affect fluconazole CL, as indicated on the label (15). Pittrow and Penk (18) reported that fluconazole plasma levels appear to be highly variable in septicemia patients, and this could account for the relatively large standard error of the CL decrease under a septic condition (θ9).
Both CL and V were affected by the general physiologic status of a patient, judging from the fact that TBI was included as a meaningful covariate for both parameters. Among many factors, hypermetabolism, which is known to be maximized within 1 month from a burn injury, might be the most plausible explanation for the result. The glomerular filtration rate is increased by the increased cardiac output and the organ blood flow in the overt hypermetabolic period. Likewise, fluconazole CL can be influenced because urinary excretion is the major elimination pathway of fluconazole. The larger V in the first 30 postburn days seems to be caused by both the physiological change and the massive fluid therapy given as a part of medical intervention. Although the amount of daily fluid was gradually reduced, the influence seems to be sustained for more than a month nevertheless, given that the V of fluconazole was larger than those of nonburn patients or healthy adults as previously mentioned.
Even though hypoalbuminemia (mean plasma albumin level, 2.5 g/dl, despite albumin replacement in this study) is common in burn patients, the unbound fraction of fluconazole does not seem to be increased because only a small portion (12%) of fluconazole molecules is bound to plasma protein (19). In addition, hypoalbuminemia appeared to exert little influence on CL and V in this study.
Recommended target fAUC/MIC levels vary between publications. Some suggest 25, with evidence from animals and clinical trials (20, 21), while others suggest 50 (56.8 as AUC/MIC) based upon a patient-based observational study (4). Meanwhile, the EUCAST fluconazole rationale document (14) specified that the target value should be 100 based upon the result of a Monte Carlo simulation. Regarding this issue, Mouton (22) summarized that a fAUC/MIC ratio of around 25 is needed for fungistatic effect, 50 for a clinically better outcome, and 100 for a high probability of cure for Candida strains with MICs up to 2 mg/liter. Therefore, selection of the maintenance dose can be at the discretion of physicians.
In addition to the MIC, patient characteristics should also be considered, particularly when the medical conditions causing increase of fluconazole CL (e.g., CRRT, no sepsis, and time <30 days after injury) are combined. In this situation, maintenance with 200 mg/day fluconazole might be insufficient regardless of the target fAUC/MIC.
In conclusion, 400 mg/day fluconazole is recommended in major burn patients infected by susceptible strains (MIC, ≤2 μg/ml), especially for those recently (within 30 days) injured or receiving CRRT.
ACKNOWLEDGMENT
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A084589).
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. Lipman J, Saadia R. 1997. Fungal infections in critically ill patients. BMJ 315:266–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pittet D, Wenzel RP. 1995. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 155:1177–1184 [DOI] [PubMed] [Google Scholar]
- 3. Nguyen MH, Peacock JE, Jr, Morris AJ, Tanner DC, Nguyen ML, Snydman DR, Wagener MM, Rinaldi MG, Yu VL. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617–623 [DOI] [PubMed] [Google Scholar]
- 4. Pai MP, Turpin RS, Garey KW. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aoyama T, Hirata K, Hirata R, Yamazaki H, Yamamoto Y, Hayashi H, Matsumoto Y. 2011. Population pharmacokinetics of fluconazole after administration of fosfluconazole and fluconazole in critically ill patients. J. Clin. Pharm. Ther. doi:10.1111/j.1365-2710.2011.01297.x [DOI] [PubMed] [Google Scholar]
- 6. Berl T, Wilner KD, Gardner M, Hansen RA, Farmer B, Baris BA, Henrich WL. 1995. Pharmacokinetics of fluconazole in renal failure. J. Am. Soc. Nephrol. 6:242–247 [DOI] [PubMed] [Google Scholar]
- 7. Debruyne D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33:52–77 [DOI] [PubMed] [Google Scholar]
- 8. Boucher BA, King SR, Wandschneider HL, Hickerson WL, Hanes SD, Herring VL, Canada TW, Hess MM. 1998. Fluconazole pharmacokinetics in burn patients. Antimicrob. Agents Chemother. 42:930–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debruyne D, Ryckelynck JP. 1993. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 24:10–27 [DOI] [PubMed] [Google Scholar]
- 10. Verbeeck RK, Musuamba FT. 2009. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur. J. Clin. Pharmacol. 65:757–773 [DOI] [PubMed] [Google Scholar]
- 11. Rayatt S, Wienbren M, Clarke J. 2000. Fluconazole use in burns patients. Burns 26:109–110 [DOI] [PubMed] [Google Scholar]
- 12. Hannon RA, Pooler C, Porth C. 2009. Porth pathophysiology: concepts of altered health states. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 13. Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. 2000. Persistence of muscle catabolism after severe burn. Surgery 128:312–319 [DOI] [PubMed] [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. EUCAST fluconazole rationale document version 1.0. 2007. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Fluconazole_rationale_document_1.0_final.pdf.
- 15. Pfizer 2011. Diflucan (fluconazole tablets). Pfizer Inc., New York, NY: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019949s051lbl.pdf [Google Scholar]
- 16. Chin T, Fong IW, Vandenbroucke A. 1990. Pharmacokinetics of fluconazole in serum and cerebrospinal fluid in a patient with AIDS and cryptococcal meningitis. Pharmacotherapy 10:305–307 [PubMed] [Google Scholar]
- 17. Foulds G, Brennan DR, Wajszczuk C, Catanzaro A, Garg DC, Knopf W, Rinaldi M, Weidler DJ. 1988. Fluconazole penetration into cerebrospinal fluid in humans. J. Clin. Pharmacol. 28:363–366 [DOI] [PubMed] [Google Scholar]
- 18. Pittrow L, Penk A. 1999. Special pharmacokinetics of fluconazole in septic, obese and burn patients. Mycoses 42:S87–S90 [DOI] [PubMed] [Google Scholar]
- 19. Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 50:99–110 [DOI] [PubMed] [Google Scholar]
- 20. Andes D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235–247 [DOI] [PubMed] [Google Scholar]
- 22. Mouton JW. 2007. Pharmacokinetics and pharmacodynamics of azoles, p 327–354 In Nightingale CH, Ambrose PG, Drusano DL, Murakawa T. (ed), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed Informa Healthcare, New York, NY [Google Scholar]