Abstract
Bacterial pathogens have specific virulence factors (e.g., toxins) that contribute significantly to the virulence and infectivity of microorganisms within the human hosts. Virulence factors are molecules expressed by pathogens that enable colonization, immunoevasion, and immunosuppression, obtaining nutrients from the host or gaining entry into host cells. They can cause pathogenesis by inhibiting or stimulating certain host functions. For example, in systemic Staphylococcus aureus infections, virulence factors such as toxic shock syndrome toxin 1 (TSST-1), staphylococcal enterotoxin A (SEA), and staphylococcal enterotoxin B (SEB) cause sepsis or toxic shock by uncontrolled stimulation of T lymphocytes and by triggering a cytokine storm. In vitro, these superantigens stimulate the proliferation of human peripheral blood mononuclear cells (PBMC) and the release of many cytokines. NVC-422 (N,N-dichloro-2,2-dimethyltaurine) is a broad-spectrum, fast-acting topical anti-infective agent against microbial pathogens, including antibiotic-resistant microbes. Using mass spectrometry, we demonstrate here that NVC-422 oxidizes methionine residues of TSST-1, SEA, SEB, and exfoliative toxin A (ETA). Exposure of virulence factors to 0.1% NVC-422 for 1 h prevented TSST-1-, SEA-, SEB-, and ETA-induced cell proliferation and cytokine release. Moreover, NVC-422 also delayed and reduced the protein A- and clumping factor-associated agglutination of S. aureus cultures. These results show that, in addition to its well-described direct microbicidal activity, NVC-422 can inactivate S. aureus virulence factors through rapid oxidation of methionines.
INTRODUCTION
Virulence factors (VFs) are molecules expressed and secreted by bacteria that play several important roles in pathogen infection, including colonization of a niche in the host, adhesion to cells, evasion of the host's immune response, inhibition of the host's immune response, entry into and exit out of cells (if the pathogen is an intracellular one), and obtaining nutrition from the host (1).
Staphylococcus aureus expresses several VFs that compromise the effectiveness of neutrophils and macrophages, which are the first line of defense against infection. S. aureus secretes proteins that inhibit complement activation and neutrophil chemotaxis, lyse neutrophils, or neutralize antimicrobial defensin peptides. Alpha-hemolysin from S. aureus assembles from a water-soluble, monomeric species to a membrane-bound heptamer on the surface of target cells, creating water-filled channels that lead to cell death and lysis (2). Furthermore, S. aureus expresses several types of superantigens (SAGs) that corrupt the normal humoral immune response, resulting in immunosuppression (3). Examples of staphylococcal superantigens include toxic shock syndrome toxin 1 (TSST-1) and enterotoxins, of which there are six antigenic types (named staphylococcal enterotoxin A [SEA], SEB, SEC, SED, SEE, and SEG) (4). TSST-1 is a pyrogenic superantigen known to cause profound disturbances in the homeostasis of the immune system, including massive proliferation of T cells and uncontrolled release of proinflammatory cytokines. TSST-1, as well as other superantigens, binds nonspecifically to major histocompatibility complex class II (MHC-II) in the antigen-presenting cells and T-cell receptors bearing specific Vβ elements on the receptors.
The exfoliative (epidermolytic) toxins of S. aureus are the causative agents of the staphylococcal scalded-skin syndrome (SSSS), a blistering skin disorder that predominantly affects children (5). S. aureus protein A helps to inhibit phagocytic engulfment and acts as an immunological disguise. Mutants of S. aureus lacking protein A are more efficiently phagocytosed in vitro, and mutants in infection models have diminished virulence (6). Two clumping factors, designated ClfA (clumping factor A) and ClfB (clumping factor B) have been identified in S. aureus. Both factors are related surface-associated proteins that bind fibrinogen and mediate adherence of bacteria to immobilized fibrinogen, to blood clots, to conditioned biomaterial ex vivo, and to thrombi on damaged heart valves (7, 8). VFs are often highly resistant to acid, heat, and protease digestion. Once secreted, they can cause a pathogenic response even after bacteria have been killed. The ability of an antimicrobial compound to effectively inactivate VFs would attenuate the virulence of pathogens and mitigate the course of infection even at sublethal concentrations or short exposure time.
As part of the innate immune system, leukocytes are capable of generating and releasing reactive oxygen species. During oxidative burst, neutrophils selectively generate hypochlorous acid to destroy invading microbial pathogens. In a secondary reaction, hypochlorous acid reacts with taurine, a semiessential amino acid. The resulting N-chlorotaurine (NCT) is a well-known antimicrobial and anti-inflammatory oxidant (9). Furthermore, NCT has shown significant bactericidal and fungicidal properties with relatively low toxicity compared to other oxidants and the inactivation of bacterial virulence at sublethal concentrations (10). Unfortunately, NCT has a relatively short solution half-life, decomposing by hydrolytic dehydrochlorination. NVC-422 (N,N-dichloro-2,2-dimethyltaurine), an NCT analog with a long shelf life at room temperature, is a fast-acting anti-infective against a broad-spectrum of pathogens, including bacterial strains that are multiresistant against antibiotics. NVC-422 was active against S. aureus, including methicillin-resistant S. aureus (MRSA), in vitro as well as in an impetigo phase 2 clinical trial (11, 12). In this study, we demonstrated the direct impact of NVC-422 on several VFs of S. aureus.
MATERIALS AND METHODS
Materials.
Staphylococcal enterotoxin A (SEA), toxic shock syndrome toxin 1 (TSST-1), as well as methionine were purchased from Sigma-Aldrich (St. Louis, MO). Staphylococcal enterotoxin B (SEB) was purchased at List Biological Laboratories (Campbell, CA), and exfoliative toxin A (ETA) was purchased from Toxin Technologies (Sarasota, FL). Normal human peripheral blood mononuclear cells (PBMC) were obtained from AllCells (Emeryville, CA) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% glutamine, and 1% penicillin/streptomycin from Invitrogen (Carlsbad, CA) incubated at 37°C in 5% CO2 and at 90% relative humidity. NVC-422 was synthesized as described previously (12).
LC-MS.
For each of the virulence factor (VF) samples, the lyophilized stock was first dissolved in PBS (20 mM phosphate-buffered saline, pH 7.4; Mediatech) to a concentration of 20 μg/ml. Two aliquots of a 50-μl VF solution were then mixed with 25 μl of 0.3% (12 mM) NVC-422 and PBS (control). The final VF and NVC-422 concentrations were ∼5 μM and 0.1% (4 mM), respectively. After incubating for 15 or 60 min at 37°C, the sample was quenched by adding 15 μl of 6× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Life Technologies) loading buffer containing 0.6 M dithiothreitol (DTT; Sigma-Aldrich). The samples were then incubated at 75°C for 5 min and loaded on a 10 to 20% Tris-glycine minigel (Invitrogen), and electrophoresis was performed at 200 V for 60 min. Gels were then stained with Coomassie stain. Finally, standard tryptic digestion protocol was performed to obtain samples for liquid chromatography-mass spectrometry (LC-MS) experiments, in which Trypsin-Gold (Promega, Madison, WI) was used for peptide digestion. To avoid undesirable oxidation of cysteines by air oxygenation, digested SEB samples were further treated with 10 mM TCEP [tris(2-carboxyethyl)phosphine] and then with 100 mM iodoacetamide (Sigma-Aldrich) to reduce and alkylate cysteines; this step was not necessary with TSST-1, as this VF does not have cysteines. LC-MS analysis was performed to detect protein modifications induced by NVC-422. Digested peptide samples were separated using a Jupiter C4 column (150 by 2 mm; nominal diameter, 5 μm; pore size, 300 Å; Phenomenex), and mass-to-charge (m/z) ratios were detected on an Agilent 6410 LC triple-quadrupole mass spectrometer in positive mode. Product ions were further obtained on selected peptides by the tandem MS method with fragmentation and collision energies of 120 V and 30 V.
Cell proliferation assay.
The 10 nM and 200 pM solutions of all VFs were incubated for 1 h with 0.1% (4 mM) or 0.01% (0.4 mM) NVC-422 (final concentrations) in PBS at room temperature. A solution of 40 μg/ml phytohemagglutinin A (Sigma-Aldrich) was used as a positive control and treated the same way as the VFs. Excess NVC-422 was inactivated by adding an equal volume of 20 mM methionine in 20 mM PBS, pH 7, and incubating for 1 h at room temperature. Controls with methionine alone and NVC-422 inactivated with methionine before addition to the system (inactivation controls) were performed, and it was shown that methionine had no effect on the activity of VFs. A 10-μl aliquot of the NVC-422/methionine-treated VFs were used to activate 100,000 PBMC to a final volume of 110 μl. The final concentrations of the VFs were 500 and 10 pM. The final concentration of PHA (phytohemagglutinin) was 1 μg/ml. After incubation of the cells for 48 h at 37°C in 5% CO2 and at 90% relative humidity, cell proliferation was measured by adding 10 μl diluted bromodeoxyuridine (BrdU) labeling reagent (Roche Applied Sciences, Indianapolis, IN) and incubating for an additional 24 h. Incorporation of BrdU was determined according to the manufacturer's directions by measuring the A370/A492 ratio. All test conditions were carried out at least in triplicate in 96-well plates.
Cytokine release assay.
VFs were treated with NVC-422 and PBMC were stimulated as described above. After incubation for 72 h, cells were pelleted by centrifugation and the supernatant was carefully transferred to a new plate. Cytokine (interleukin 2 [IL-2], IL-4, IL-6, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor [GM-CSF], tumor necrosis factor alpha [TNF-α], and gamma interferon [IFN-γ]) release was determined using the magnetic bead-based immunoassay Bio-Plex Pro 8-plex group I on a Bio-Plex 200 suspension array system (Bio-Rad, Hercules, CA) with the appropriate controls according to manufacturer's instructions.
Clumping factor and protein A.
Slidex Staph Plus (bioMérieux) was applied to test the impact of NVC-422 on the surface VFs clumping factor and protein A. The test contained blue latex particles covered with fibrinogen and monoclonal antibodies against protein A, which led to agglutination upon the addition of S. aureus. S. aureus ATCC 25923 (final inoculum, 1 × 109 CFU/ml) was grown in tryptic soy broth overnight, washed twice in saline (pH 7), and mixed with NVC-422 at a final concentration of 1.38% (55 mM) in 4 ml 0.1 M phosphate buffer (pH 7.1). Controls without NVC-422 were performed in parallel, as well as a negative control with mock particles according to the manufacturer. After a 15-min incubation time at 20°C, the samples were washed and resuspended in 1 ml saline (pH 7). Subsequently, 20 μl of the treated bacteria were mixed with one drop of the test or control reagents according to the test prescription. Agglutination on glass slides was monitored for 10 min.
RESULTS
Methionine oxidation in TSST-1.
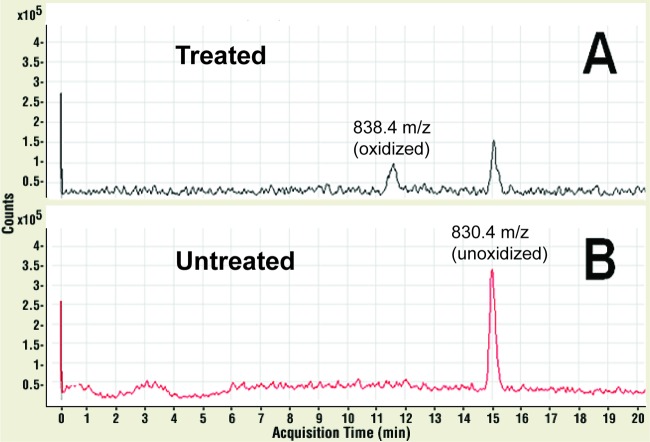
To investigate the molecular impact of NVC-422 in inactivating TSST-1 and to investigate the mechanism of action, we have performed an LC-MS analysis of chemical modifications in TSST-1 after trypsin digestion and treatment with NVC-422. The LC-MS total ion chromatogram (TIC) showed several peaks which correspond to the various fragments of the digested TSST-1. Importantly, when the TICs of the untreated and NVC-422-treated samples were compared, a clear shift in the retention time of one specific peak was observed (from 15 min to 11.5 min, respectively) (Fig. 1). The 15-min peak in the untreated sample had m/z values of 830.4 and 554.0, which were consistent with the predicted m/z values for 2+ and 3+ ions of the peptide I156TMNDGSTYQSDLSK170. The 11.5-min peak in the 0.1% NVC-422-treated sample had m/z values of 838.4 and 559.4. These values are consistent with the m/z values of 2+ and 3+ of the same peptide with an additional mass of 16, the molecular weight of oxygen. Notably, an LC-MS/MS (tandem mass spectrometer) analysis indicated that the additional mass of m/z +16 was consistent with the oxidation of Met158 in the peptide to form a methionine sulfoxide functionality. This observation was consistent with the chemistry of NVC-422, where methionine was chlorinated to form a chlorosulfonium ion, which is followed by hydrolysis of this intermediate to give a methionine sulfoxide.
Fig 1.
LC-MS/MS product ion chromatograms of tryptic digested TSST-1 peptide ions with an m/z value of 838.4 (t = 11.5 min) (A) and 830.4 (t = 15 min) (B). Untreated SEB and NVC-422-treated SEB samples are shown in red and black, respectively. Six micromolars TSST-1 (6 μM) was incubated in 0.1% (4 mM) NVC-422 in 20 mM PBS (pH 7) buffer at 37°C for 15 min.
Methionine oxidation in SEB.
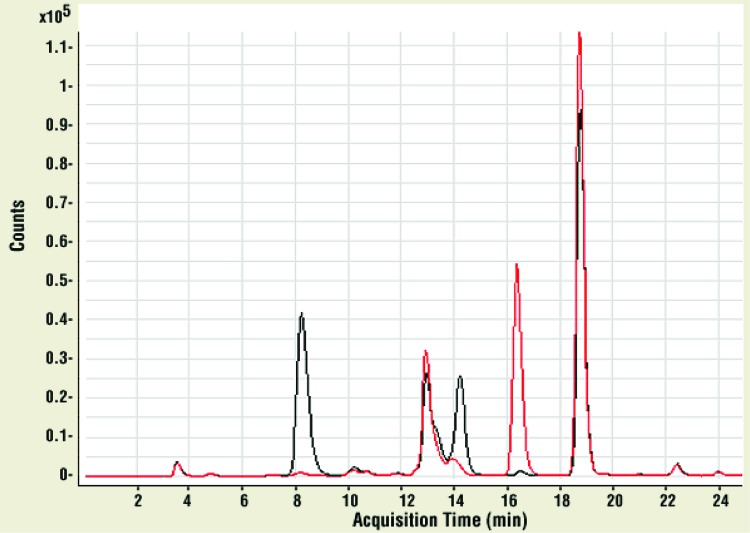
As with TSST-1, LC-MS experiments were performed with superantigen SEB. In Fig. 2, the LC-MS in selected ion monitoring mode (LC-MS SIM) chromatogram is shown for SEB, which contained two cysteine (reduced and alkylated as described above) and eight methionine residues. Three peaks corresponding to methionine-containing peptides were clearly identified. In the untreated SEB sample, these peptide fragments had retention times of 18.8, 16.3, and 12.7 min (with m/z values of 535.82+ from the fragment F17TGLM21ENM24K25, 596.32+ from the fragment Y213LM215M216YNDNK221, and 641.93+ from the fragment T112C113M114YGGVTEHNGNQLDK128, respectively). In the NVC-422-treated sample, these peaks had partially shifted and split, due to oxidation. The 18.8-min peak shifted to 14.2 min (543.92+, one methionine oxidized in fragment) and 10.1 min (551.82+, both methionines oxidized in fragment). The 16.3-min peak shifted to 13.3 min (604.32+, one methionine oxidized) and 8.1 min (612.32+, both methionines oxidized). The 12.7-min peak shifted to 10.7 min (647.43+, the only methionine in this fragment was oxidized). The m/z values indicated that these changes were consistent with methionine oxidation. Only the peptides with two methionines showed double oxidation (m/z +32), suggesting that we observe two sulfoxides rather than one unoxidized methionine and one sulfone. Methionine oxidation was also verified in the two peptides with Met21/24 and Met215/216 using LC-MS/MS (Fig. 2). Taken together, these results indicate that NVC-422 oxidizes methionine residues in VFs such as TSST-1 and SEB.
Fig 2.
LC-MS SIM chromatograms of tryptic in-gel digested SEB peptide ions containing fragments F17TGLM21ENM24K25, Y213LM215M216YNDNK221, and T112C113M114YGGVTEHNGNQLDK128. Untreated SEB is shown in red, and 5 μM SEB incubated in 0.1% (4 mM) NVC-422 in 20 mM PBS (pH 7) buffer at 37°C for 15 min is shown in black.
Effect of NVC-422 on superantigen-induced PBMC proliferation.
Proliferation of T cells and uncontrolled release of proinflammatory cytokines are the hallmarks of the disturbance of the immune system caused by many bacterial superantigens. Oxidation of TSST-1, SEB, and SEA by 0.1% NVC-422 suggests that, in addition to killing microbial pathogens, NVC-422 can also alter and potentially inactivate bacterial VFs. To evaluate this hypothesis, we tested if pretreatment of TSST-1, SEA, SEB, and ETA with NVC-422 prevents activation and proliferation of PBMC. All of these untreated superantigens induced PBMC proliferation at a concentration of 500 and 10 pM, except for 10 pM ETA (Fig. 3). Preincubation with 0.1% (4 mM) NVC-422 prevented SAG-induced cell proliferation. Preincubation with 0.01% (0.4 mM) NVC-422 completely prevented proliferation induced by 10 pM TSST-1 and 500 pM ETA and partially that induced by 10 pM SEA and SEB (Fig. 3).
Fig 3.
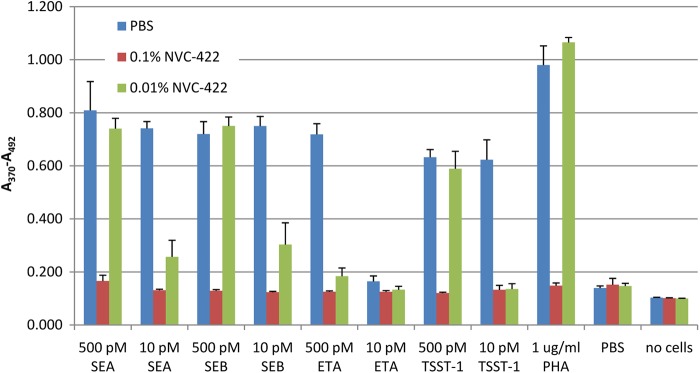
Effect of bacterial VFs pretreated for 1 h at room temperature with NVC-422 or PBS upon PBMC cell proliferation, as measured by BrdU incorporation. The average of triplicate experiments with the standard deviation from one representative example of three or more experiments is shown.
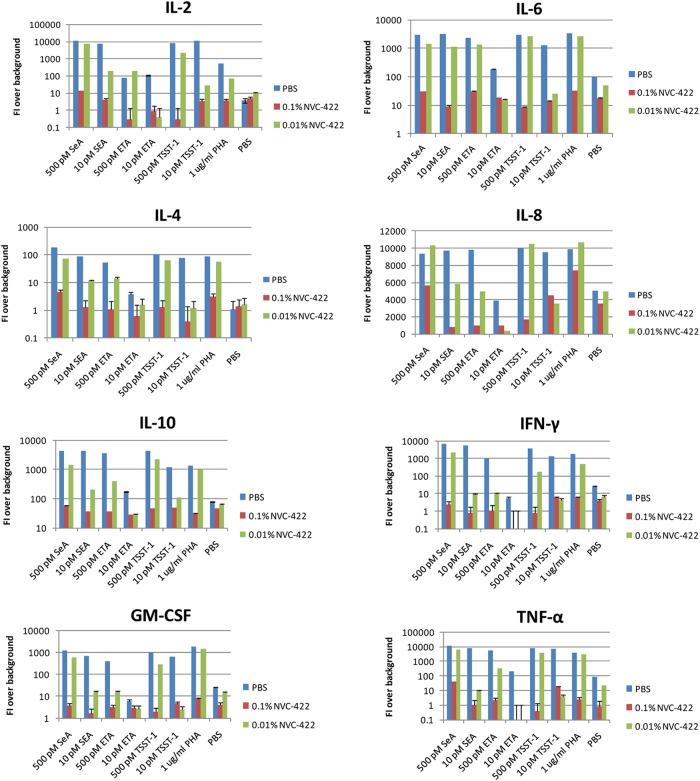
Effect of NVC-422 on superantigen-induced cytokine release.
Pretreatment with 0.1% (4 mM) NVC-422 for 1 h prevented superantigens from activating PBMC and releasing cytokines (IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, TNF-α, and IFN-γ). Pretreatment with 0.01% (0.4 mM) NVC-422 showed a partial inhibitory effect, indicating a dose response (Fig. 4). Taken together with its inhibitory activity on superantigen-induced PBMC proliferation, these data suggest that NVC-422 can oxidize and inactivate bacterial VFs that act as SAGs.
Fig 4.
Effect of bacterial VFs pretreated for 1 h at room temperature with NVC-422 or PBS upon cytokine release. The average of triplicate experiments with the standard deviation from one representative example of three or more experiments is shown.
Impact on clumping factor and protein A.
We next investigated whether NVC-422 can also inactivate bacterial VF that have other mechanisms of action, such as S. aureus protein A and clumping factor. These two VFs are expressed on the bacterial surface and bind to immunoglobulins or cause clumping of human plasma, respectively. To this end, we determined if treatment of S. aureus with NVC-422 affects agglutination. Untreated S. aureus cultures induce clumping of fibrinogen-coated beads within 1 to 2 min. In contrast, agglutination of NVC-422-treated S. aureus was delayed by 3.5 to 4.5 min. Moreover, only minimal clumping occurred with NVC-422-treated samples, while it was markedly larger with the controls.
In summary, we show that NVC-422, in addition to its well-established bactericidal effect, can also inactivate VFs in situ, such as protein A and clumping factor on the bacterial surface, and purified SAGs, such as TSST-1, SEA, SEB, and ETA.
DISCUSSION
Local and distant diseases are caused by toxins produced by organisms present at the infection site. For example, staphylococcal scalded-skin syndrome (SSSS) is produced by the exfoliative toxin A or B (ETA or ETB), staphylococcal toxic shock syndrome by TSST-1. Attenuation of virulence is desirable for antimicrobial agents since it mitigates the course of infection even at sublethal concentrations or incubation times. The active chlorine compound N-chlorotaurine (NCT) has been shown to attenuate the virulence of S. aureus and Streptococcus pyogenes after sublethal incubation times in the mouse peritonitis model (9, 13). Moreover, secretory aspartyl proteinases of Candida albicans were found to be downregulated by sublethal concentrations of NCT, and gliotoxin of Aspergillus fumigatus is inactivated by this compound (14, 15). This was connected with a lag of regrowth (9, 13, 14) and with chlorination of the surface of the pathogens (16). Attenuation of virulence is an important benefit, especially in topical indications where rapid washout may occur, such as eye or skin infections.
A novel, stable analog of NCT, NVC-422 (N,N-dichloro-2,2-dimethyltaurine), is a broad-spectrum, fast-acting anti-infective against microbial pathogens, including antibiotic-resistant strains (11, 12, 17). Previously, it was shown that NVC-422 efficiently oxidizes essential sulfur-containing functional groups in viral proteins, namely, Cys thiols and Met thioethers. These reactions lead to protein conformational changes to disrupt protein assembly and to lose enzymatic functions (17). In this study, we demonstrate that NVC-422 rapidly inactivates a number of bacterial VFs by oxidation of sulfur-containing amino acids, in particular, methionine residues, which can lead to inactivation of bacterial toxins and other VFs by changing their protein conformations. In TSST-1, there are two Met residues (Met33 and Met158), of which Met158 was identified to be oxidized by NVC-422 in this study. The major consequence of the methionine oxidation in TSST-1 is that the conformation of TSST-1 would be changed. As the protein-protein interactions between the superantigen TSST-1 and the immune receptors are highly dependent on the conformations of these proteins, the change in the protein conformation of TSST-1 would result in lowering, if not loss, of binding to the immune receptors.
In SEB, the peptide with Met215 and Met216 (second peptide with retention time of 16.3 min) exhibits complete oxidation in either one or both methionines. The crystal structure of SEB (available in the PDB database under the accession number 3SEB) indicates that Met215 and Met216 are highly exposed to the surface of the protein, suggesting that these Met residues are more vulnerable to oxidation by NVC-422 (18). This would be critical in toxins that act as superantigens, such as TSST-1, SEA, and SEB, which tightly bind to the immune receptors. Since some identified methionines were only partially oxidized, it is likely that methionines that are less exposed to the protein surface can be more protected from NVC-422-induced oxidation.
In this study, we have demonstrated that preincubation of TSST-1, SEA, SEB, and ETA with 0.1% NVC-422 (and, to a lesser extent, 0.01%) inactivates these VFs measured as loss of ability to cause PBMC proliferation and cytokine release. For representative VFs, we have shown that this inactivation is obviously connected with oxidation of methionine residues. Due to its basic underlying mechanism of thiol or thioether oxidation, we expect that NVC-422 also inactivates further surface bacterial proteins critical for bacterial survival, such as those involved in respiratory chain and metabolite transport. This multitarget mechanism of action explains the rapid bactericidal activity of NVC-422 without the development of resistance in multiple passage studies (19). Furthermore, NVC-422 has microbicidal activity against Gram-positive and Gram-negative bacteria, fungi, and viruses, making it an attractive candidate for the treatment of bacterial and viral conjunctivitis, impetigo, and urinary catheter blockage and encrustation. These indications are currently being evaluated in clinical trials.
Footnotes
Published ahead of print 3 December 2012
REFERENCES
- 1. Casadevall A, Pirofski LA. 1999. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 67:3703–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gouaux E, Hobaugh M, Song L. 1997. alpha-Hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 6:2631–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958 [DOI] [PubMed] [Google Scholar]
- 4. Llewelyn M, Cohen J. 2002. Superantigens: microbial agents that corrupt immunity. Lancet Infect. Dis. 2:156–162 [DOI] [PubMed] [Google Scholar]
- 5. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. 1999. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin. Microbiol. Rev. 12:224–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodyear CS, Silverman GJ. 2003. Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 197:1125–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Entenza JM, Foster TJ, Ni Eidhin D, Vaudaux P, Francioli P, Moreillon P. 2000. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 68:5443–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francois P, Schrenzel J, Stoerman-Chopard C, Favre H, Herrmann M, Foster TJ, Lew DP, Vaudaux P. 2000. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J. Lab. Clin. Med. 135:32–42 [DOI] [PubMed] [Google Scholar]
- 9. Nagl M, Hess MW, Pfaller K, Hengster P, Gottardi W. 2000. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob. Agents Chemother. 44:2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottardi W, Nagl M. 2010. N-chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob. Chemother. 65:399–409 [DOI] [PubMed] [Google Scholar]
- 11. Iovino SM, Krantz KD, Blanco DM, Fernandez JA, Ocampo N, Najafi A, Memarzadeh B, Celeri C, Debabov D, Khosrovi B, Anderson M. 2011. NVC-422 topical gel for the treatment of impetigo. Int. J. Clin. Exp. Pathol. 4:587–595 [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Belisle B, Bassiri M, Xu P, Debabov D, Celeri C, Alvarez N, Robson MC, Payne WG, Najafi R, Khosrovi B. 2011. Chemical characterization and biological properties of NVC-422, a novel, stable N-chlorotaurine analog. Antimicrob. Agents Chemother. 55:2688–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagl M, Hengster P, Semenitz E, Gottardi W. 1999. The postantibiotic effect of N-chlorotaurine on Staphylococcus aureus. Application in the mouse peritonitis model. J. Antimicrob. Chemother. 43:805–809 [DOI] [PubMed] [Google Scholar]
- 14. Nagl M, Gruber A, Fuchs A, Lell CP, Lemberger EM, Borg-Von Zepelin M, Wurzner R. 2002. Impact of N-chlorotaurine on viability and production of secreted aspartyl proteinases of Candida spp. Antimicrob. Agents Chemother. 46:1996–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reeves EP, Nagl M, O'Keeffe J, Kelly J, Kavanagh K. 2006. Effect of N-chlorotaurine on Aspergillus, with particular reference to destruction of secreted gliotoxin. J. Med. Microbiol. 55:913–918 [DOI] [PubMed] [Google Scholar]
- 16. Gottardi W, Nagl M. 2005. Chlorine covers on living bacteria: the initial step in antimicrobial action of active chlorine compounds. J. Antimicrob. Chemother. 55:475–482 [DOI] [PubMed] [Google Scholar]
- 17. Yoon J, Jekle A, Najafi R, Ruado F, Zuck M, Khosrovi B, Memarzadeh B, Debabov D, Wang L, Anderson M. 2011. Virucidal mechanism of action of NVC-422, a novel antimicrobial drug for the treatment of adenoviral conjunctivitis. Antiviral Res. 92:470–478 [DOI] [PubMed] [Google Scholar]
- 18. Papageorgiou AC, Tranter HS, Acharya KR. 1998. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 A resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J. Mol. Biol. 277:61–79 [DOI] [PubMed] [Google Scholar]
- 19. D'Lima L, Friedman L, Wang L, Xu P, Anderson M, Debabov D. 2012. No decrease in susceptibility to NVC-422 in multiple-passage studies with methicillin-resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli. Antimicrob. Agents Chemother. 56:2753–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]