Abstract
Pdr5 is a major ATP-binding cassette (ABC) multidrug transporter regarded as the founding member of a fungal subfamily of clinically significant efflux pumps. When these proteins are overexpressed, they confer broad-spectrum ultraresistance. To better understand the evolution of these proteins under selective pressure, we exposed a Saccharomyces cerevisiae yeast strain already overexpressing Pdr5 to a lethal concentration of cycloheximide. This approach gave mutations that confer greater resistance to a subset of transport substrates. One of these mutations, V656L, is located in intracellular loop 2 (ICL2), a region predicted by structural studies with several other ABC transporters to play a critical role in the transmission interface between the ATP hydrolysis and drug transport domains. We show that this mutation increases drug resistance, possibly by altering the efficiency with which the energy from ATP hydrolysis is used for transport. Val-656 is a conserved residue, and an alanine substitution creates a nearly null phenotype for drug transport as well as reduced ATPase activity. We posit that despite its unusually small size, ICL2 is part of the transmission interface, and that alterations in this pathway can increase or decrease resistance to a broad spectrum of drugs.
INTRODUCTION
The World Health Organization lists antimicrobial resistance as a major global concern that threatens advancements in medicine, national security, and economic development (http://www.who.int/drugresistance/en/). Multidrug resistance remains a major clinical problem in the treatment of cancer and pathogenic infection (1). This phenomenon is attributable, in part, to the overexpression of ATP-binding cassette (ABC) efflux pumps that were initially identified and characterized in mammalian cells (2–5). Multidrug resistance was observed in Saccharomyces cerevisiae yeast in 1973, when it was attributed to a single-gene alteration (6), but the cloning and characterization of the efflux pumps mediating this phenomenon occurred much later (7–12).
Pdr5 is a highly promiscuous ABC transporter. Overexpression leads to hyperresistance for all known substrates (13–15). This efflux pump has a basic structure found in full-length members of this superfamily. It has two nucleotide-binding domains and two transmembrane domains (TMDs) made up of six alpha helices, each of which is connected by intra- and extracellular loops. In several significant ways, however, Pdr5 departs from the canonical architecture of ABC transporters. It has a deviant ATP-binding site that lacks an obvious catalytic residue in the Walker B motif; it also contains deviant sequences in the Walker A, signature region (C-loop) and Q-loop motifs. Furthermore, in contrast to most ABC transporters, the intracellular loops (ICLs) are very short. ICL2 and ICL4 are predicted to have only 10 and 7 residues, respectively (16). Important studies with Tap1/Tap2 antigen transporter (17) and P-gp (18) using cross-linking of cysteine-modified residues provide evidence that the X-loop, Q-loop, and intracellular loops are critical for establishing a transmission interface suggested by the atomic structure of the Sav1866 multidrug transporter (19). In vivo evidence for a similar interface was established in Pdr5 by using suppressor mutations (20, 21). In this transporter, a signal transmission-deficient mutation in transmembrane helix 2 (TMH2) (S558Y) was suppressed by second-site mutations in ICL1 (S597T, S597I) and the Q-loop region (N242K, E244G, D246Δ). These residues were implicated in intradomain signaling from the Sav1866 atomic structure.
Here we present new observations of the role of ICLs in the evolution of a highly asymmetric transporter; this investigation arose from a seemingly different issue concerning the evolution of ABC efflux pumps. Although overexpression of transporters creates hyperresistance to xenobiotic substrates, increasing the concentration beyond the 50% inhibitory concentration (IC50) will kill even overexpressing cells. However, mutations that show even greater resistance than the original overexpressing strain can be selected. It is of great interest to understand how this occurs. We sought to determine whether such changes are limited to further increases in expression or to changes in the drug-binding pocket. We isolated mutants with increased resistance in an overexpressing strain on plates containing an otherwise lethal (5.4 μM) dose of cycloheximide (cyh). One of these mutations, V656L, is in ICL2 and confers increased resistance to Pdr5 substrates of diverse structure and function. We present evidence suggesting a novel mechanism of increased drug resistance in which a mutation in the transmission interface increases the efficiency by which the energy of ATP hydrolysis is used to power drug efflux. We also show that Val-656 is a conserved residue and that a V656A mutation has a phenotype characterized by markedly reduced transport and ATPase activity.
MATERIALS AND METHODS
Chemicals.
All chemicals except 5-fluororotic acid (5-FOA) and G-418, which we obtained from Research Products International (Mt. Prospect, IL), aprotinin, which we purchased from Fisher Scientific (Waltham, MA), and climbazole, cyprodinil, cerulenin, cyproconazole, tebuconazole, and imazalil, which we obtained from LKT laboratories (St. Paul, MN), were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals except cyh, which was dissolved in water, and 5-FOA and G-418, which were dissolved in sterilized medium, were dissolved in dimethyl sulfoxide (DMSO). [125I]Iodoazidoarylprazosin (IAAP) was purchased from PerkinElmer Life Sciences (Waltham, MA).
Yeast strains and plasmids.
All yeast strains used in this study are isogenic and are derived from R-1, a stock lacking all plasma membrane ABC transporters. It also contains a PDR1-3 mutation, which results in overexpression of PDR5 when the gene is inserted into its chromosomal location following transformation. This strain offers other advantages for genetic and biochemical analyses, which are described in detail elsewhere (20, 21) and include the ability to easily construct stocks with two copies of either wild-type (WT) or mutant PDR5 genes, thus resulting in more-robust biochemical assays. All culturing of strains was carried out at 30°C. The pSS607-integrating plasmid (22) was used for all site-directed mutagenesis, as previously described. This plasmid has a WT PDR5 gene under the transcriptional control of its own upstream region as well as a ura3-selectable marker. Strains containing two copies of either WT or a mutant PDR5 gene were used for ATPase and IAAP photoaffinity labeling experiments. The rationale for using these strains and the method for generating them were previously described (20).
Yeast transformation.
Integrative transformation of pSS607 into the Δpdr5 strain and its isogenic derivatives was performed with a Sigma-Aldrich lithium acetate transformation kit.
Determination of IC50s.
We determined the IC50s for Pdr5 transport substrates by incubating cells in 2 ml yeast extract-peptone-dextrose (YPD) broth containing various concentrations of drug. Cultures were started with an initial inoculum of 0.5 × 105 cells and incubated at 30°C for 48 h in a shaking water bath. The cell concentration was determined with a spectrophotometer at 600 nm. The percent growth relative to the untreated culture was calculated.
Isolation of mutants with increased resistance to cyh.
Ten 2-ml cultures of YPD were inoculated with 104 cells of the yeast strain JG2015. This strain was produced by transforming R-1 with the integrating plasmid pSS607, which contains a WT copy of PDR5. The strain also contains a G-418 resistance cassette that replaces the PDR5 open reading frame (ORF), although the PDR5 upstream and downstream sequences remain. This arrangement permits several important genetic manipulations, which are described elsewhere (20, 21). The cultures were grown for 48 h at 30°C before plating 107 cells on YPD medium containing 5.4 μM cyh. The plates were incubated for 96 h, and resistant colonies were picked for further testing.
Site-directed mutagenesis.
Mutations were introduced into pSS607 with a QuikChange Lightning site-directed mutagenesis kit (Life Technologies, Grand Island, NY). Mutant primers were designed with a genomics program provided by Agilent Technologies (Santa Clara, CA; www.genomics.agilent.com). The mutant plasmids were introduced into XL-Gold Escherichia coli by transformation, as described in the QuikChange instruction manual. Plasmid DNA was extracted from the transformants with an IBI miniprep kit (MidSci, St. Louis, MO) and sequenced commercially to confirm the presence of the mutation in the plasmid (Retrogen, San Diego, CA). The mutant plasmid DNA was introduced into R-1 with a Sigma-Aldrich yeast transformation kit. Following genetic testing to confirm that the construct was correctly inserted (21), genomic DNA was extracted from yeast transformants using a Qiagen Puregene Yeast/Bact. kit (Qiagen, Valencia, CA), and the pdr5 gene was amplified by PCR using 80 to 120 ng purified chromosomal DNA per reaction. The PCR product was purified, and the entire coding region was sequenced commercially (Retrogen and SeqWright, Houston, TX) to confirm that only the desired alteration was present.
Preparation of purified plasma membrane vesicles.
Purified plasma membrane (PM) vesicles were prepared from 750 ml of exponentially growing yeast cells containing two copies of either WT or mutant PDR5. Following harvesting by centrifugation, the pellets were weighed and resuspended in homogenization buffer (50 mM Tris [pH 7.5], 2.5 mM EDTA, and a protease cocktail containing 1 mM phenylmethylsulfonate, 10 μl/ml aprotinin, 10 μg/ml tosyl phenylalanyl chloromethyl ketone [TPCK] and tosyllysine chloromethyl ketone [TLCK], and 1 μg/ml pepstatin and leupeptin) in a 2:1 ratio of buffer volume to pellet weight. The cells were lysed with glass beads in a bead beater (Biospec, Bartlesville, OK) using five 30-s intervals of lysing followed by 30 s of cooling as directed in the accompanying product manual. Whole cells and glass beads were pelleted at 1,000 × g for 7.5 min in an SS34 rotor at 4°C. The supernatant was spun at 100,000 × g in a Ti50 rotor for 1 h at 4°C to pellet the crude membranes. Following this, the crude membrane pellet was resuspended in 2 ml resuspension buffer (10 mM Tris, 0.5 mM EDTA, 10% glycerol with protease inhibitors) using a 7-ml Dounce homogenizer. The resuspension was applied to a discontinuous sucrose gradient made up of equal volumes of 53.5% (wt/vol) and 43.5% (wt/vol) sucrose. Following centrifugation for 5 h at 100,000 × g in an SW28 swinging bucket rotor, the purified PM vesicles were collected from the interface. The sucrose was diluted out by addition of resuspension buffer and centrifugation for 1 h at 100 × g in a Ti50 rotor. The pellets were resuspended in 2 to 3 ml of resuspension buffer, and 75-μl aliquots were frozen in 1.5-ml Eppendorf tubes at −80°C. The amount of protein in solubilized vesicles was determined with a bicinchoninic acid (BCA) protein determination kit (Thermo Scientific, Rockford, IL).
Gel electrophoresis and Western blotting.
The presence and amount of Pdr5 in PM vesicles were confirmed by Western blotting. Proteins were separated by electrophoresis at 150 V on 7.5% Tris-acetate gels (Life Technologies, Grand Island, NY) before being transferred to a nitrocellulose membrane at constant current (400 mA, 1 h). The blots were blocked for 30 min in phosphate-buffered saline (PBS) buffer containing 1% Tween 20 and 5% nonfat milk. We obtained antibodies from Santa Cruz Biotechnology (Santa Cruz, CA). The Pdr5 antibody was diluted 1:500; the Pma1 antibody was diluted 1:2,000. Following overnight incubation at 4°C in primary antibodies, the blots were washed 3 times for 15 min in PBS plus 1% Tween 20. Following this, the blots were incubated in PBS with nonfat milk and 1% Tween containing a 1:10,000 dilution of donkey anti-goat IgG horseradish peroxidase antibody. The filters were washed 3 times for 5 min in PBS with 1% Tween 20. The chemiluminescent signal was developed by addition of 10 ml enhanced chemiluminescence (ECL) reagent (Life Technologies) for 1 min before exposure to X-ray film for 0.5 to 1 min. The ratio of Pdr5 to Pma1 signals was calculated with Image J software (rsbweb.nih.gov/ij/) using scans with a resolution of 1,200 dpi.
R6G transport assay.
Rhodamine 6G (R6G) transport assays were carried out with 5 μM R6G. Cells were grown overnight in SD medium supplemented with uracil and histidine to a concentration of ∼107 cells/ml. The cells were washed with 0.05 M HEPES buffer (pH 7.0) and concentrated to one-tenth of the starting volume in HEPES buffer. For each sample, 106 cells were incubated in 0.05 M HEPES containing 5 μM R6G for 90 min at 30°C in a final volume of 100 μl. Following loading, the cells were spun down, the supernatant was removed, and the pellets were resuspended in 0.05 M HEPES and 1 mM glucose for 30 min. Following efflux, the cells were pelleted, the supernatant was removed, and the cells were resuspended in 400 μl HEPES for analysis by fluorescence-activated cell sorting. R6G fluorescence was measured with a Becton, Dickinson (Franklin Lakes, NJ) FACSort with an excitation wavelength of 488 nm and an emission wavelength of 620 nm. The data were analyzed with a CellQuest program. Retained fluorescence is expressed in arbitrary units.
ATPase assays.
We assayed the Pdr5-specific ATPase activity with purified PM vesicles in an ATPase assay buffer containing 100 mM MOPS (morpholinepropanesulfonic acid) (pH 7.4), 50 mM KCl, 5 mM NaN3, 2 mM EGTA (pH 7.0), 2 mM dithiothreitol (DTT), and 10 mM MgCl2 at 35°C for 8 min as previously described (22).
Cross-linking IAAP to Pdr5.
Purified membranes, prepared from double-copy-bearing yeast cells (30 μg protein/125 μl), were incubated with the competing substrate clotrimazole (clo) for 5 min in ATPase buffer, followed by incubation with IAAP (3.5 nM) at room temperature for 5 min under subdued light. The samples were photo-cross-linked for 10 min with 365-nm UV light in an ice water bath, followed by electrophoresis on 7% NuPAGE gels (Life Technologies). The gels were quantified as described previously (20). The dried gels were also exposed to X-ray film to create an autoradiogram of the samples.
RESULTS
The V656L mutation appeared in two distinct genetic screens.
We recovered the V656L mutation in two separate genetic screens, the first of which is illustrated in Fig. S1 in the supplemental material. We suppressed S558Y drug hypersensitivity by a series of second-site mutations, including several near or in the Q-loop, a region required for intradomain communication (20, 21). One of these mutants was N242K. When N242K is not coupled to S558Y, it is also drug hypersensitive (21). Therefore, it can also serve as a target for suppressor isolation. This tactic recovered a V656L mutation that exhibited greater resistance than the wild-type (WT) residue to cyh, although it was not extensively characterized (21).
We carried out the second screen for our present study, exposing cells overexpressing WT Pdr5 (JG2015) to cyh at the MIC for the WT strain on solid medium (5.4 μM). The selected mutations were dominant. Therefore, standard complementation testing was not useful for determining whether these lie in PDR5. This was necessary because we expected to observe hyperresistant mutations both in and outside Pdr5. The genetic approach for distinguishing these possibilities in the JG2015 strain was recently described (20, 21). We identified five putative mutants in PDR5, and we estimate that they arose with a frequency of ∼10−8.
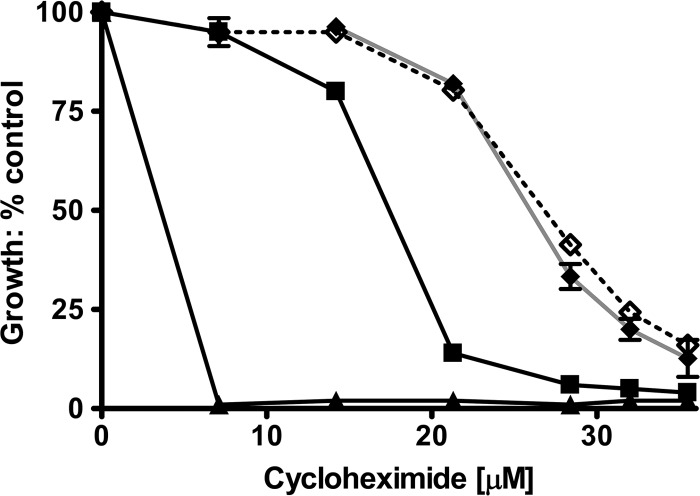
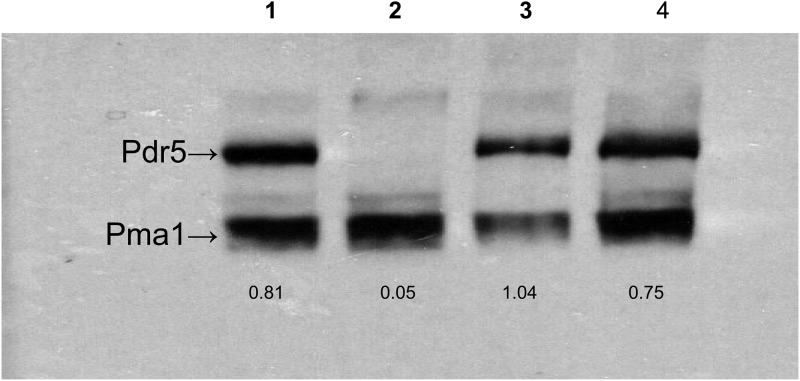
We recovered the PDR5 gene from these mutants by PCR amplification and sequenced the DNA (Retrogen, San Diego, CA). Each mutant had a single-base-pair substitution resulting in a missense mutation. One of these was V656L. The other mutations were P596L in ICL1, L1367F in TMH12, A670S in TMH5, and I1063M in nucleotide-binding domain 2 (NBD2). In this study, we focus on Val-656 because of its location in a region strongly implicated in signal transmission by structural and cross-linking studies. We therefore recreated the V656L mutation in pSS607 and placed it into the Δpdr5 strain by yeast transformation. We compared these strains to the original chromosomal mutants. The data in Fig. 1 show that the cyh killing curves for the plasmid and chromosomal mutations are statistically indistinguishable. A two-way analysis of variance (ANOVA) comparing each of the mutant curves to the WT curve indicated that the likelihood that they are the same is <0.0001. We used the plasmid reconstruction in all of the subsequent experiments. Figure 2 shows a representative Western blot for the mutant strains used in this study. The V656L mutant protein is found at WT levels in purified plasma membrane (PM) vesicles. We determined the ratio of Pdr5 to Pma1 signals for all of the strains. This value allowed us to make a quantitative comparison between the WT and the mutants analyzed. The amount of Pdr5 protein in the V656L mutant was 0.89 ± 0.13 times that of the WT. Therefore, the increased resistance of the former cannot be attributed to an increased level of the transporter in the PM.
Fig 1.
The V656L mutant increases resistance to cyh. We constructed V656L in pSS607 and placed it in R-1 (Δpdr5 strain) by yeast transformation. Following this, the original chromosomal mutation and the plasmid reconstruction were compared to WT and Δpdr5 isogenic controls for their relative resistances to cyh in liquid culture as described in Materials and Methods. All plots in this study were made using GraphPad Prism (San Diego, CA). n = 3. ▲, Δpdr5; ■, WT; ◆, chromosomal V656L mutation; ♢, plasmid-reconstructed V656L mutation.
Fig 2.
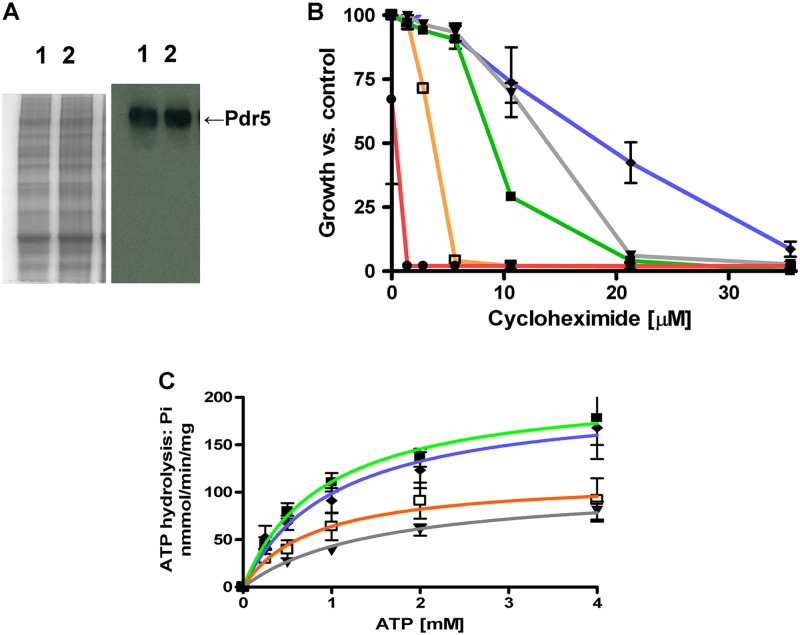
Expression of the V656L and V656A mutants is similar to that of the WT. PM vesicles were solubilized in SDS-PAGE sample buffer at 37°C for 30 min. We used 10 μg of protein to produce the Western blot. Each is a representative example from three independent experiments. Lane 1, WT; lane 2, Δpdr5 strain; lane 3, V656A mutant; lane 4, V656L mutant. Values are the ratios of the Pdr5 signal to the Pma1 signal, computed from Image J software as described in the experimental procedures.
Val-656 is one of four conserved residues in ICL2.
According to the bioinformatic analysis performed by Rutledge et al. (16) to build their atomic model of Pdr5, ICL2 is remarkable for its relatively short size. It is predicted to contain only 10 amino acids (654 to 663); in contrast, ICL1 is made up of 21 residues. They also observed two loops with analogous sizes in the second half of Pdr5.
We performed a sequence alignment using the Swiss Protein Database alignment tool (www.unitprot.org).We compared Pdr5 to 17 members of the Pdr5 subfamily, with amino acid identities ranging from 30.7% to 74.4%. These sequences were initially selected for use by Rutledge et al. (16) as part of an atomic modeling study. The alignment that we obtained is found in Fig. S2 in the supplemental material. The first and last amino acids in the loop are a highly conserved arginine and either a serine or threonine residue, respectively. The third position has a medium-to-large hydrophobic residue (V, I, L, M, F) followed by a small nonpolar one (G or A) in the fourth. All of the residues located at the third position can be interchanged as a result of a single-nucleotide substitution. ICL4 is located in TMD2 and is predicted to comprise only seven amino acids (22), suggesting a correspondence to ICL2 because of its size similarity. In contrast to ICL2, however, none of the residues in ICL4 are conserved. This indicates that ICL2 and ICL4 may be functionally distinct. A recent report clearly demonstrates that ICL4 is necessary for drug resistance, although its role is unknown (23).
Val-656 is an essential residue for Pdr5-mediated drug transport.
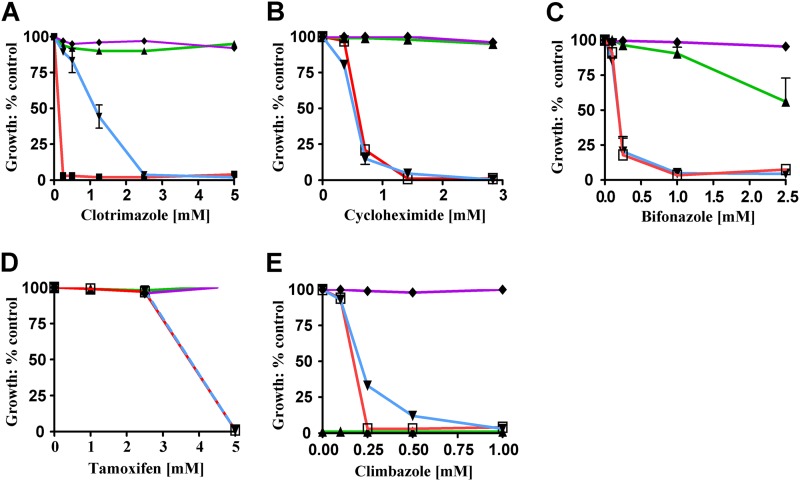
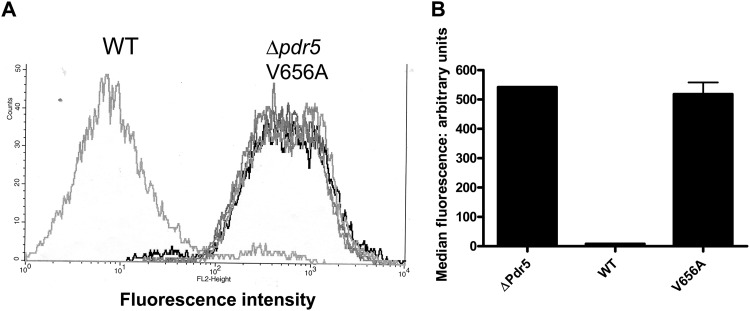
To determine whether Val-656 is essential for Pdr5 function, we used site-directed mutagenesis to create a V656A mutation and introduced it into the Δpdr5 strain. Ordinarily, a valine-to-alanine change is structurally neutral and a single-base-pair substitution can interchange these residues. However, no alanines are found in position 3 of ICL2 in the alignment sequences. This strongly suggested that such a substitution would be unfavorable, as indeed it is. The level of the V656A mutant protein in purified PM vesicles was, if anything, slightly higher (1.3 ± 0.1 times) than the level of the WT (Fig. 2). The relative cyh, clo, bifonazole, tamoxifen, and climbazole resistances of the V656A mutant were compared to those for the isogenic WT (JG2015) and Δpdr5 strains (Fig. 3A to E). The V656A and Δpdr5 mutations were equally hypersensitive to cyh, tamoxifen, bifonazole, and climbazole. Although the V656A mutant showed significantly more resistance to clo than did Δpdr5, it was considerably more sensitive than the WT (Fig. 3A). We also tested the ability of the V656A mutant to efflux R6G in a whole-cell assay. These data demonstrate that the V656A mutant and the Δpdr5 control were equally deficient in R6G efflux (Fig. 4).
Fig 3.
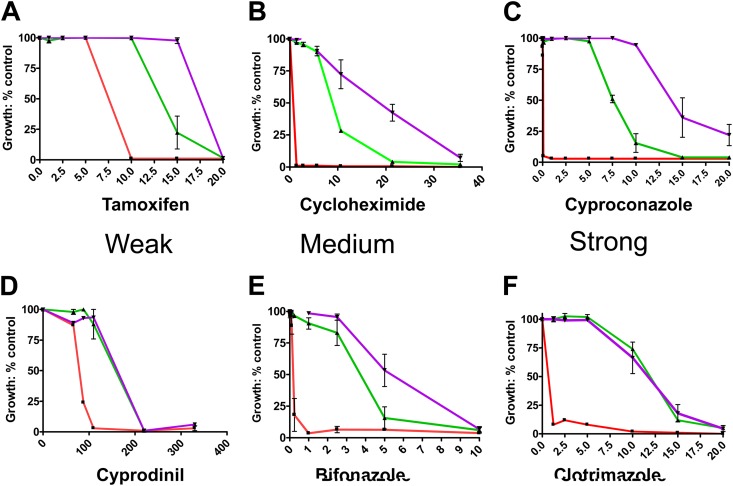
The V656A mutant exhibits substantial drug hypersensitivity. The sensitivity of the V656A mutant (▼, blue line) was compared to those of the isogenic controls (□ and red, Δpdr5 strain; ▲ and green, WT; ◆ and violet, V656L) for relative resistance to clo (A), cyh (B), bifonazole (C), tamoxifen (D), and climbazole (E) in liquid culture as described in the experimental procedures. All concentrations are in μM units. Error bars denote standard errors. n = 3.
Fig 4.
R6G transport is impaired in the V656A mutant. (A) Histogram plots from a single experiment using two independent cultures of the Δpdr5 and V656A strains and a single culture of the isogenic WT strain (JG2015). The assay is described in Materials and Methods. (B) Representation of data pooled from the experiment in panel A and an additional independent experiment.
ATPase activity is significantly reduced in the V656A mutant.
A hallmark of loss-of-function mutations in the signal transmission interface of Pdr5 analyzed thus far is a reduction—but not an elimination—of ATPase activity. This feature does not fully reflect the severity of the mutation. For instance, although the E244G (deviant Q-loop) and S558Y mutants both showed ATPase activity that is 3 to 4 times lower than that of the WT (mutants, ∼50 nmol/min/mg; WT, ∼200 nmol/min/mg), the E244G mutant retained significant transport capability and the S558Y mutant was phenotypically null (20, 21).
We performed similar experiments with the V656A mutant (Fig. 5A). Purified plasma membrane vesicles exhibited a reduced but significant level of ATPase activity. The Vmax obtained for three independent membrane preparations was 42 nmol/min/mg. This value is similar to those observed with the transmission interface mutants S558Y, E244G, and Q951G. The Km values were 1.6 mM for the WT and 0.8 mM for the V656A mutant, values within the range seen routinely in our experiments (for example, the WT data in Fig. 9C indicate a Km of 0.9 mM).
Fig 5.
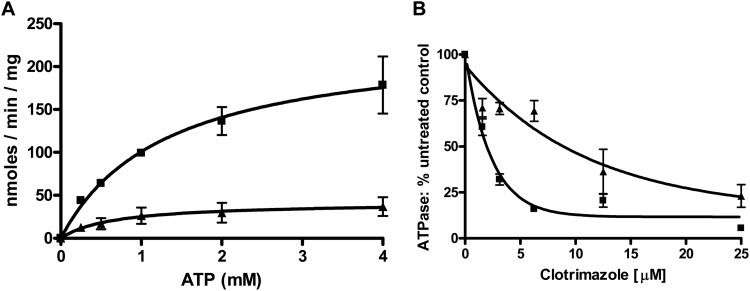
ATPase activity of the V656A mutant. ATPase was measured as described in the experimental procedures using 16 μg of PM vesicle protein from double-copy strains (see reference 23). Reactions with a final volume of 100 μl were initiated by addition of ATP and incubated at 35°C for 8 min before being terminated with 2.5% (final volume) SDS. The nonspecific activity (<5%) present in the Δpdr5 strain is subtracted prior to calculating the activity. (A) Activity as a function of ATP concentration. Data represent the averages of at least two independent vesicle preparations per strain, and 3 independent experiments were performed. (B) Activity in the presence of clotrimazole (3.1 to 50 μM). Standard ATPase conditions were used. PM vesicles were incubated at room temperature for 5 min in the presence of clo before initiation of the reactions with the addition of ATP to 3 mM. ■, WT; ▲, V656A mutant. n = 3.
Fig 9.
V656L suppresses the cyh hypersensitivity of E244G. (A) Colloidal blue-stained gel (30 μg of solubilized protein) and Western blot (10 μg of solubilized protein) of the E244G mutant (lane 1) and the E244G, V656L double mutant (lane 2), prepared as described in Fig. 3. (B) Plots of growth versus cyh concentration. Experiments were performed in liquid culture as described in Materials and Methods. The conditions for testing strains in liquid culture are found in Materials and Methods. ● and red, Δpdr5 strain; ■ and green, WT; □ and orange, E244G mutant; ◆ and violet, V656L mutant; ▼ and gray, V656L, E244G double mutant. (C) ATPase activity of the E244G, V656L double mutant was compared to those of the single E244G mutant and isogenic control strains using the assay conditions described in Fig. 5. ■, WT; □, E244G mutant; ◆, V656L mutant; ▼, V656L, E244G double mutant.
V656A mutant ATPase is significantly more resistant to clo than the WT enzyme.
Although Pdr5 ATPase is not stimulated by drug substrates, some, such as R6G, clo, and ketoconazole, are inhibitors (22, 24). The residual ATPase activity found in the S558Y mutant is considerably more resistant to allosteric inhibition by clo than is the ATPase activity found in the WT (20). This strongly suggested that the inhibitory signal is not being properly transmitted in the mutant. To determine whether ICL2 is required for allosteric inhibition of ATPase, we tested the residual V656A mutant ATPase activity for its susceptibility to clo. The results show that the mutant ATPase exhibits significantly greater clo resistance than does the WT protein (Fig. 5B). The IC50 (clo) observed for the WT in this particular set of experiments was 2.2 μM, which is similar to the WT values obtained in the experiments described later in Fig. 9. In contrast, the mutant enzyme had a value of 9.9 μM. A two-way ANOVA indicated that the probability that the two curves are the same was less than 0.0001. These results strongly suggest that both transport and ATPase inhibition are highly dependent on Val-656.
V656A mutant drug binding is similar to that for WT Pdr5.
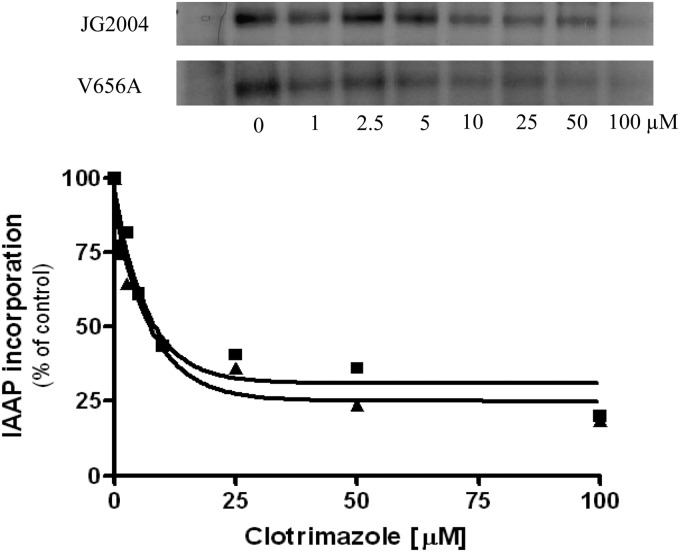
The crystal structure of mouse P-gp showed that binding of its drug substrates takes place in the TMDs (25). It may be that the ICLs play an indirect role in drug binding and that the V656A mutant is deficient in this process. To find out, we compared the abilities of the WT and the V656A mutant to bind IAAP in the presence of the competing substrate clo (Fig. 6). Previous work in our laboratory demonstrated that both R6G and clo show a concentration-dependent inhibition of IAAP photoaffinity labeling (20, 26). This is significant because R6G is a competitive inhibitor of clo efflux (see Fig. S3 in the supplemental material). Substrates such as tripropyltin chloride or tritylimidazole that do not block R6G or clo efflux do not inhibit IAAP labeling (26). In addition, Pdr5 mediates IAAP efflux in a whole-cell transport assay (Z. Sauna and J. Golin, unpublished data). In the present experiments, we found that IAAP binds to the WT Pdr5 and the V656A mutant and yields a signal of similar intensity. Furthermore, the addition of clo yielded inhibition curves (percent IAAP incorporation at a specific dose) that were indistinguishable from each other. In each case, the IC50 was about 15 μM. Thus, it is unlikely that V656A alters the transporter-drug substrate-binding interaction to a degree that would yield the observed null phenotype.
Fig 6.
The V656A mutant exhibits WT drug-binding behavior. We carried out photoaffinity labeling with IAAP in the presence and absence of clo as described in Materials and Methods. (A) Each lane contains 10.8 μg of solubilized PM vesicle protein. After electrophoresis, the gels were dried and exposed to an X-ray film. The radioactivity incorporated into the pdr5 band was quantified using a phosphorimager and analyzed using GraphPad Prism. The autoradiogram described above shows IAAP binding to the WT pdr5 and the mutant pdr5 in the presence and absence of clo. The isogenic strain lacking Pdr5 (_pdr5) was used as a control in the experiment. WT and V656A mutant strains were analyzed in the same experiments. (B) Plots of the experiment shown in panel A. ■, WT; ▲, V656A mutant. We performed this experiment an additional time with nearly identical results.
Response of the V656L mutant to Pdr5 substrates of varied strength.
The Western blot in Fig. 2 demonstrates that the increased resistance of the V656L mutant to cyh is not attributable to a further increase in the expression of Pdr5. We considered several models to account for the observation that V656L increases resistance to cyh. The first, known as the kinetic selection model, was proposed by Ernst et al. (24) to explain the behavior of an H-loop mutation in Pdr5 that reduced R6G transport but exhibited WT resistance to other transport substrates. Pdr5 has a high basal ATPase activity that is not stimulated by drugs. Thus, hydrolysis may well be constitutive and independent of drug substrate stimulation. This model posits that mutations can alter the rate of switching from a drug-binding to a drug-effluxing conformation. While precise measurements of on- and off-rates of drugs would be required to rigorously establish or reject the model, this leads to a specific phenotypic prediction. If the Pdr5 pump is altered so that relative to the WT protein the mutant is more frequently in the drug-binding (according to the Sav1866 model, the inward-facing conformation) rather than the drug-effluxing state (the outward-facing model), modest or poor Pdr5 substrates that normally interact slowly will have a greater opportunity for binding.
The first model makes a specific prediction about substrate behavior: the V656L mutant exerts its strongest effect on substrates that interact relatively poorly with Pdr5. We therefore tested the response of the V656L mutant to a set of 10 transport substrates (Table 1). We determined the relative strength of each substrate by calculating its IC50 in isogenic PDR5 and Δpdr5 strains and calculating the PDR5/Δpdr5 ratio. These data are summarized in Table 1, and six plots are shown in Fig. 7, in which they are arranged according to relative substrate strength. The IC50 indices form a continuum ranging from tamoxifen (1.7) to cyproconazole (106). We determined the IC50s for the V656L, WT, and Δpdr5 strains. The V656L mutant conferred greater resistance to eight compounds. These did not cluster according to the IC50 index but were evenly distributed. Furthermore, we observed a similar increase in resistance for each one. Thus, the V656L mutant is 1.4 to 2.1 times more resistant to the compounds than the WT. These results cast doubt on the kinetic selection model as an explanation for the observed ultraresistance of the V656L mutant, although it remains an important possibility for the H-loop mutant described by Ernst et al. (24).
Table 1.
Enhancement of drug resistance in the V656L mutant is independent of substrate strength
| Compound | WT/Δpdr5 IC50 ratio | IC50 (μM) |
V656L mutant/WT IC50 ratio | ATPase IC50 (μM) | |
|---|---|---|---|---|---|
| WT | V656L mutant | ||||
| Tamoxifen | 1.7 | 13 | 18 | 1.4 | Incomplete |
| Cyprodinil | 2.0 | 140 | 140 | 1.0 | 200 |
| Cerulenin | 10 | 14 | 28 | 2.0 | No inhibition |
| Imazalil | 10 | 4.3 | 7.7 | 1.8 | Incomplete |
| Cycloheximide | 17 | 7.5 | 16 | 2.1 | No inhibition |
| Bifonazole | 19 | 3.7 | 5.7 | 1.5 | 5.3 |
| Climbazole | 40 | 14 | 28 | 2.0 | Incomplete |
| Clotrimazole | 75 | 9.7 | 9.7 | 1.0 | 2.5 |
| Tebuconazole | 80 | 6.2 | 9.5 | 1.5 | No inhibition |
| Cyproconazole | 106 | 7.5 | 15 | 2.0 | No inhibition |
Fig 7.
Resistance of the V656L mutant to Pdr5 transport substrates. We show plots of growth versus substrate concentration for six of the 10 compounds tested. Conditions for testing substrates in liquid culture are found in Materials and Methods. These vary in their substrate strength. (A to C) Data from substrates ranging from weak (tamoxifen) to strong (cyproconazole), to which the V656L mutant (purple plots) confers increased resistance. Strength is determined by comparing the IC50s of the WT (green) and Δpdr5 mutant (red) plots. (D to F) Data from substrates ranging from weak (cyprodinil) to strong (clo) that cause significant (>50%) inhibition of ATPase activity (see Fig. 9). All concentrations are in μM units. n = 3.
We then considered a second and a third explanation for the behavior of the V656L mutant. The second asserts that Val-656 affects the level of Pdr5 ATPase. Increased resistance is due to an increased ATPase activity available for transport. A third model is that these mutants alter the transmission interface, causing a more efficient use of energy from rather than an increase in ATP hydrolysis.
Both of these models predict that if a transport substrate such as clo eliminates this activity before or at the WT IC50 for growth, the V656L mutant will not increase resistance. We therefore looked at the effect of substrates on ATPase activity.
Effect of Pdr5 transport substrates on ATPase activity.
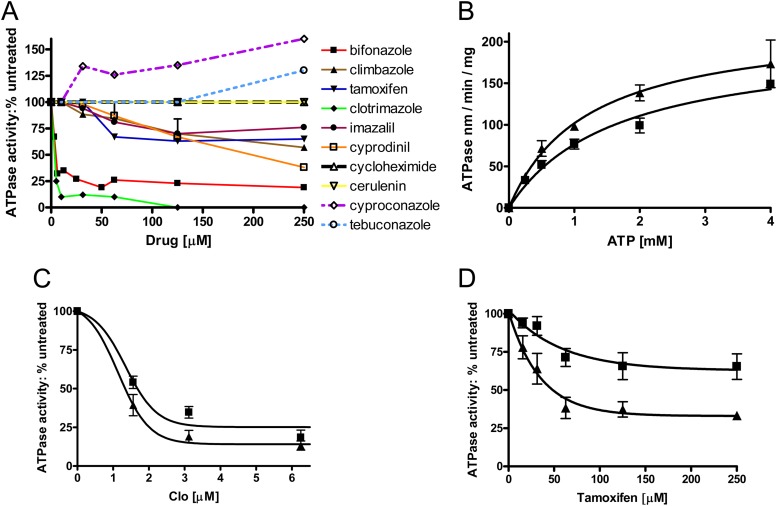
We tested the effect of the same set of compounds on the WT Pdr5-mediated ATPase activity (Fig. 8A). Three of these, clo, bifonazole, and cyprodinil, caused strong (>50%) inhibition of WT ATPase activity. Although bifonazole and clo are similar in structure, cyprodinil is much smaller and it is not an imidazole derivative. The V656L mutant did not show an increase in resistance to cyprodinil or clotrimazole. The remaining transport substrates had a weak (≤30% inhibition) effect on ATPase activity or none at all. It would appear, then, that strong substrate inhibition of ATPase activity is seen with only a minority of the transport substrates. In some instances, an extremely weak stimulation of ATPase occurred at very high transport substrate concentrations. The V656L mutant showed increased resistance to all of the substrates that leave ATPase activity largely unaffected. However, it also exhibited increased resistance to bifonazole, which shows substantial inhibition of ATPase activity. Though this was initially unexpected, we observed that the IC50 for killing in WT whole cells is 3.7 μM while that of the V656L mutant (5.7 μM) is very close to the IC50 for ATPase inhibition by this drug (5.3 μM). This suggests that as long as the IC50 for allosteric inhibition is above the IC50 for killing of WT cells, the V656L mutation can promote increased resistance. An exact correlation between inhibition in vesicles and killing in whole cells cannot be made, however, without determining the effective intracellular concentration of the drug in question. Our observations are thus consistent with the second and third models, which invoke either an increase in or a more efficient utilization of ATPase activity. We next attempted to distinguish these possibilities.
Fig 8.
Effect of substrates on ATPase activity. (A) We performed assays of ATPase activity with purified PM vesicles from the WT double-copy strain in the presence of transport substrates as described in Fig. 5 using 3 mM ATP in each reaction. Plots for each compound are shown. At least two curves were generated for each compound. (B) ATPase activity of the V656L mutant. ATPase activities were determined as described in Fig. 5. Data represent determinations from two independent PM vesicle preparations from each strain. (A) ATPase activity as a function of ATP concentration (0 to 3 mM). n = 3. Error bars indicate the standard error. (C) The effect of clo (1.78 to 12.5 μM) on ATPase activity is shown using 3 mM ATP in each reaction. ■, WT; ▲, V656L mutant. Inhibition curves are the averages of 3 independent experiments, including two different PM preparations of the V656L mutant. (D) Effect of tamoxifen on V656L mutant ATPase. The conditions are identical to those described in panel C except that tamoxifen was added to the reaction mixes.
ATPase activity of the V656L gain-of-function mutation.
Suppressor mutations located very near (N242K) or in a Pdr-specific residue defining the Q-loop that were isolated for their ability to restore resistance to the transmission interface-deficient mutant S558Y do not increase ATPase levels. Rather, they apparently improve the efficiency with which the signal from ATP hydrolysis is transmitted, and they restore noncompetitive inhibition of the Pdr5 ATPase by clo. Presumably, if the V656L mutant were to increase drug resistance without a concomitant increase in ATPase activity, we would have additional evidence that Val-656 and, therefore, ICL2 play a role in transmitting the signal from the nucleotide-binding domains to the TMDs. We compared the ATPase activity of the V656L mutant to that of WT Pdr5 in purified plasma membranes (Fig. 8B). The Vmax of the V656L mutation (∼200 nmol/min/mg) fell well within the range of WT values (216 nmol/min/mg in these experiments) but was certainly not greater. The Km values for the WT and mutant were 1.6 mM and 1.2 mM, respectively. The increased resistance that we observed is therefore not attributable to greater ATPase activity.
The ATPase activity of the V656L mutant is slightly more sensitive to clo than is that of the WT (Fig. 8C). According to a two-way ANOVA, the two curves are probably not equivalent (P = 0.007); however, the IC50s are nearly the same. Any effect of V656L is therefore very small. The effects of climbazole, tamoxifen, and cyproconazole on V656L mutant ATPase activity were also tested. Climbazole and cyproconazole had identical effects on V656L mutant and WT ATPase activity (see Fig. S4 in the supplemental material). The V656L mutant ATPase activity, however, was significantly more sensitive to tamoxifen than was that of the WT (Fig. 8D). The IC50 was about 32 μM, and the inhibition leveled out to about 70%. In V656L mutant cells, the IC50 (tamoxifen) was about 18 μM (Table 1), a value not terribly different considering that the comparison is between measurements in PM vesicles and whole cells, which may not be equivalent. This observation may explain why the enhancement in resistance observed in the V656L mutant compared to the WT (∼1.4×) was less than that obtained with some of the other compounds.
The V656L and V656A mutations therefore create opposite phenotypes that are consistent with altered signal transmission. The V656L mutation causes increased resistance to many drugs but increased sensitivity to allostery, at least in the case of tamoxifen. In contrast, the V656A mutant is markedly drug hypersensitive but more resistant to allosteric inhibition by clo.
The V656L mutant also suppresses E244G, a mutation in the residue defining the pdr-specific Q-loop.
These data are consistent with the idea that Val-656 is part of the transmission interface. To test this idea further, we created an E244G, V656L double mutant to detect increased drug resistance and/or ATPase activity. E244G is the deviant Q-loop residue that is completely conserved in the Pdr5 subfamily. Recent work in our laboratory established its role in the transmission interface (20). When E244G is present in an otherwise WT strain, the result is reduced ATPase activity and increased drug sensitivity.
The Western blot in Fig. 9A shows that Pdr5 is present at similar levels in the PM vesicles of each strain. The data in Fig. 9B clearly demonstrate that the V656L mutant suppresses the significant cyh hypersensitivity of the E244G transmission interface mutant. In fact, the resistance observed in the suppressor strain is similar to that of the WT. The resistance of the double mutant is about four times that observed with the E244G mutant. At issue, however, is whether the suppression is attributable solely to better use of the residual ATPase activity in the E244G mutant or to an actual increase in the level of hydrolysis (Fig. 9C). Significantly, the ATPase activity of the double mutant was no different than the activity observed for the E244G mutant, which was in the range of 60 to 75 nmol/min/mg.
DISCUSSION
Multidrug resistance is often caused by overexpression of ABC efflux pumps, and it is a major problem in the clinical treatment of cancers and infection. Furthermore, Pdr5 defines an ABC subfamily found only in fungi; many of these are of agricultural significance. It is important to determine whether a further increase in resistance can be obtained under continued selective pressure in strains that are already overexpressing multidrug transporters. We found that mutations in the Pdr5 gene imparting resistance to cyh are recovered with a frequency of roughly 10−8. The V656L mutation leads to an increase in resistance to a broad spectrum of Pdr5 substrates that is 1.5 to 2 times that of the WT. The enhancement is not dependent on substrate strength. Although the increased resistance may seem modest, numerous chemotherapeutic and antifungal agents are toxic if the dose is doubled. Microevolution often works incrementally but effectively.
V656L is located in the very short ICL2 of TMD1. Thus, this residue is not in the drug-binding pocket, as might be expected, and this alteration does not result in an increased steady-state expression level of Pdr5 in purified PM vesicles. Rather, Val-656 is one of four conserved residues in ICL2. An alanine substitution has an effect that is entirely different from that of V656L. The V656A mutation is nearly phenotypically null to all Pdr5 substrates tested, although its drug-binding capability is intact. It is plausible that although valine and alanine are biochemically similar, the larger size of the former is required for interaction between ICL2 and the NBD(s). In fact, the behavior of the V656A mutant resembles that of the transmission-deficient mutant S558Y, which lies in TMH2. As was the case with the S558Y mutant, the noncompetitive inhibition of Pdr5-specific ATPase by clo is significantly diminished in the V656A mutant. These data show that the signals for drug transport and allosteric ATPase inhibition both use ICL2. V656L's suppression of the E244G and N242K signaling deficiencies in the Q-loop region also strongly implies a role for Val-656 and ICL2 in the transmission interface. This is further supported by the observation that although the V656L mutant increases resistance in either a WT or E244G mutant background, it does so without raising ATPase activity. These observations indicate that in the WT transporter, the energy from hydrolysis is not used to full capacity. From the standpoint of evolution, mutations that improve the efficiency of energy utilization should have a strong selective advantage in the presence of a xenobiotic agent. Thus, multidrug transporters can evolve to greater resistance by manipulating signal transmission. This is a novel mechanism of multidrug resistance not previously described. In the case of the V656L mutant, this results in additional resistance to a broad spectrum of Pdr5 substrates that appears limited only in cases in which ATPase activity is reduced by allosteric inhibition. The behavior of mutants in this study further emphasizes that it is impossible to link ATPase activity levels with the resistance phenotype in Pdr5.
The high-resolution structure of the Sav1866 homodimer shows physical contact between ICL2 and the X-loop and Q-loop regions of the opposite monomer. Cross-linking studies with other eukaryotic transporters also lend additional credence to a transmission interface arrangement in a trans orientation (17, 18). Sav1866 and P-gp were used heavily by Rutledge et al. (22) to construct a homology model of Pdr5. As a result, the Pdr5 interface is modeled in a trans conformation with ICL2 in close proximity to the Q-loop of NBD2. This model was extremely challenging to build, however, because there are big structural differences and relatively low amino acid identity between Pdr5 and these transporters. In Table S1 in the supplemental material, we list the residues of the Pdr5 transmission interface as defined by suppressor mutations. ICL1, ICL2, and the Q-loop region are all represented in the collection. All of these lie in the same half of the transporter. Our original collection of suppressors restored resistance to a transmission-deficient S558Y mutant. Ser-558 is located at the extracellular end of TMH2, which is admittedly a good distance from the NBDs, so the interaction between these residues may be very indirect. The V656L and E244G residues, however, are presumably in much closer proximity to each other, and their observed interaction strengthens our contention that unlike other eukaryotic ABC transporters, ICL2 of Pdr5 (represented by Val-656) interacts with the cis Q-loop. We do not mean to imply that Val-656 and Glu-244 are necessarily a physically interacting pair in the WT, as their chemistry makes that implausible. It is possible that V656L and E244G create a new hydrophobic interaction that restores the signal to the interface. Alternatively, these mutant residues may never be in physical contact although they clearly are part of the same transmission pathway.
Although the E244G (Q-loop NBD1), Q951G (Q-loop NBD2), and V656A substitutions are deficient in intradomain communication, the Q-loop deficiencies show some drug specificity. For instance, the E244G mutant exhibits almost WT clo resistance (20). We also observed that the E244G mutant has R6G transport capability that is indistinguishable from that of the WT (J. Mehla, unpublished data). In contrast, an E244G, Q951G double mutant exhibits strong hypersensitivity to clo and cyh (20). Therefore, the Q-loop residues are overlapping in function. The broad, striking hypersensitivity of the V656A mutant and the absence of an obvious consensus sequence in ICL4 or ICL3 suggest the possibility that there is no equivalent residue in TMD2.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by PHS grant GM07721 and NSF grant 1048838 to J.G. and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
This article is dedicated to the memory of Seth Aryeh Golin Weisman (1981 to 2012), who isolated the first mutants with increased resistance in the summer of 2003 and continued to insist on the importance of their characterization.
Footnotes
Published ahead of print 17 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02133-12.
REFERENCES
- 1. Borges-Walmsey MJ, McKeegan K, Walmsey AR. 2003. Structure and function of efflux pumps that confer resistance to drugs. Biochem. J. 376:313–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fojo AT, Whang-Peng J, Gottesman MM, Pastan I. 1985. Amplification of DNA sequences in human multidrug-resistant KB carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 82:7661–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gros P, Croop J, Roninson I, Varshavsky A, Housman DE. 1986. Isolation and characterization of DNA sequences amplified in multidrug resistant human cells. Proc. Natl. Acad. Sci. U. S. A. 83:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roninson IB, Abelson HT, Housman DE, Howell N, Varshavsky A. 1984. Amplification of specific DNA sequences correlates with multidrug resistance in Chinese hamster cells. Nature 309:626–628 [DOI] [PubMed] [Google Scholar]
- 5. Van der Bliek AM, Van der Velde-Koerts, Ling TV, Borst P. 1986. Overexpression and amplification of five genes in a multidrug resistant Chinese hamster ovary cell line. Mol. Cell. Biol. 6:1671–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rank GH, Bech-Hansen NJ. 1973. Single nuclear gene inherited cross-resistance and collateral sensitivity to 17 inhibitors of mitochondrial function in Saccharomyces cerevisiae. Mol. Gen. Genet. 126:93–102 [DOI] [PubMed] [Google Scholar]
- 7. Leppert G, McDevitt R, Falco SC, Van Dyk T, Ficke MB, Golin J. 1990. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics 125:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. 1994. Pdr5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator Pdr1. J. Biol. Chem. 269:2206–2214 [PubMed] [Google Scholar]
- 9. Hirata D, Yano K, Miyahara K, Miyakawa R. 1994. Saccharomyces cerevisiae Ydr1, which encodes a member of the ATP-binding cassette superfamily, is required for multidrug resistance. Curr. Genet. 26:285–294 [DOI] [PubMed] [Google Scholar]
- 10. Servos J, Haase E, Brendel M. 1993. Gene SNQ2 of Saccharomyces cerevisiae, which confers resistance to 4-nitoquinoline-N-oxide and other chemicals, encodes a 169 kDa protein homologous to ATP-dependent permeases. Mol. Gen. Genet. 236:214–218 [DOI] [PubMed] [Google Scholar]
- 11. Bissinger PH, Kuchler K. 1994. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J. Biol. Chem. 269:4180–4186 [PubMed] [Google Scholar]
- 12. Katzmann DJ, Hallstrom TC, Voet M, Wysock W, Golin J, Volckaert G, Moye-Rowley WS. 1995. Expression of an ATP-binding cassette transporter gene YOR1 is required for oligomycin resistance in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:6875–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyers S, Schauer W, Balzi E, Wagner M, Goffeau A, Golin J. 1992. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr. Genet. 21:431–436 [DOI] [PubMed] [Google Scholar]
- 14. Golin J, Ambudkar SV, Gottesman MM, Habib AD, Sczepanski J, Ziccardi W, May L. 2003. Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J. Biol. Chem. 278:5963–5969 [DOI] [PubMed] [Google Scholar]
- 15. Rogers B, Decottignies A, Kolaczkowski M, Caravajal E, Balzi E, Goffeau A. 2001. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 3:207–214 [PubMed] [Google Scholar]
- 16. Rutledge R, Esser L, Ma J, Xia D. 2011. Toward and understanding of the mechanism of action of the yeast multidrug resistance transporter Pdr5p: a molecular modeling study. J. Struct. Biol. 173:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oancea G, O'Mara ML, Drew-Bennett W, Tielman P, Abeles R, Tampe R. 2009. Structural arrangement of the transmission interface of the antigen ABC transport complex TAP. Proc. Natl. Acad. Sci. U. S. A. 106:5551–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zolnerciks JK, Wooding C, Linton KJ. 2007. Evidence for a Sav1866-like architecture for the human multidrug transporter P-glycoprotein. FASEB J. 21:3937–3948 [DOI] [PubMed] [Google Scholar]
- 19. Dawson RJ, Locher KP. 2006. Structure of a bacterial multidrug ABC transporter. Nature 443:180–185 [DOI] [PubMed] [Google Scholar]
- 20. Sauna ZE, Bohn SS, Rutledge R, Dougherty MP, Cronin S, May L, Xia D, Ambudkar SV, Golin J. 2008. Mutations define cross talk between the N-terminal nucleotide-binding domain and transmembrane helix-2 of the yeast multidrug transporter Pdr5: possible conservation of a signaling interface for coupling ATP hydrolysis to drug transport. J. Biol. Chem. 283:35010–35022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ananthaswamy N, Rutledge R, Sauna ZE, Dine E, Nelson E, Xia D, Golin J. 2010. The signaling interface of the yeast multidrug transporter Pdr5 adopts a cis conformation and there are functional overlap and equivalence of the deviant and canonical Q-loop residues. Biochemistry 49:4440–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golin J, Kon ZN, Wu CP, Martello J, Hanson L, Supernavage S, Ambudkar SV, Sauna Z. 2007. Complete inhibition of the Pdr5p multidrug efflux pump ATPase activity by its transport substrate clotrimazole suggests GTP as well as ATP may be used as an energy source. Biochemistry 46:18109–18119 [DOI] [PubMed] [Google Scholar]
- 23. Guo X, Li J, Wang T, Liu Z, Chen X, Li Y, Gu Z, Mao X, Guan W, Li Y. 2012. A mutation in intracellular loop 4 affects the drug efflux activity of the yeast multidrug transporter Pdr5p. PLoS One 7:e29520 doi:10.1371/journal.pone.0029520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ernst R, Kueppers P, Klein CM, Schwarzmueller T, Kuchler K, Schmitt L. 2008. A mutation of the H-loop selectively affects rhodamine transport by the yeast ABC transporter Pdr5. Proc. Natl. Acad. Sci. U. S. A. 105:5069–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aller S, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Urbatsch IL, Chang G. 2009. Structure of P-glycoprotein reveals a molecular basis for polyspecific drug binding. Science 323:1718–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanson L, May L, Tuma P, Keeven J, Mehl P, Ferenz M, Ambudkar SV, Golin J. 2005. The role of hydrogen bond acceptor groups in the interaction of substrates with Pdr5, a major yeast drug transporter. Biochemistry 44:9703–9713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.