Abstract
Avermectins are a family of macrolides known for their anthelmintic activities and traditionally believed to be inactive against all bacteria. Here we report that members of the family, ivermectin, selamectin, and moxidectin, are bactericidal against mycobacterial species, including multidrug-resistant and extensively drug-resistant clinical strains of Mycobacterium tuberculosis. Avermectins are approved for clinical and veterinary uses and have documented pharmacokinetic and safety profiles. We suggest that avermectins could be repurposed for tuberculosis treatment.
TEXT
Antibacterial drug discovery is a costly endeavor with a very limited probability of success (1). The need for new therapies is especially acute in the case of tuberculosis (TB). While Mycobacterium tuberculosis is notoriously resistant to most antibiotics and new drugs with novel modes of action are urgently needed, such compounds have proven to be rare and difficult to identify (2). An alternative approach to generate new TB treatment options in a timely and cost-effective manner is “repurposing,” i.e., identifying new applications for existing, clinically approved drugs (alone or in combination) with known pharmaceutical properties (3). In a recent study, we validated the concept that drugs that do not inhibit M. tuberculosis at clinically relevant concentrations might be introduced for TB therapy if they could be administered within a synergistic combination (4).
In the course of this screening program, we found that selamectin, a commonly used anthelminthic veterinary drug of the avermectin family, effectively inhibited mycobacterial growth in agar and liquid cultures. The avermectins were discovered in the mid-1970s in an antinematode screening program led by Kitasato Institute and the Merck, Sharp, and Dohme (MSD) laboratories (5). The antimycobacterial activity we discovered was surprising because the avermectins were thought to be only effective against helminths, insects, and arachnids and to be inactive against flatworms, protozoa, bacteria, and fungi (5–9). However, we could not identify literature with specific information describing the (lack of) antibacterial activity of the avermectins. In all probability, these negative data remained proprietary information and were never published. We therefore examined the antibacterial effectiveness of four avermectins (doramectin, ivermectin, moxidectin, and selamectin) (Fig. 1) against representative Gram-positive and Gram-negative bacteria (Table 1). Inhibitory effects were not observed on any of these bacteria at concentrations as high as 256 μg/ml using the bacterial growth indicator MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]. The avermectins were then tested for their inhibitory activities against various Mycobacterium species using the same MTT assay (Table 1). This assay has been previously used to test drug sensitivity of M. tuberculosis, and the results are entirely consistent with other well-established methods based on nitrate reductase (12), resazurin (12), [3H]glycerol uptake (13), or the gold standard proportion method of viable CFU after treatment (12, 14). All four avermectins inhibited growth of Mycobacterium bovis BCG and M. tuberculosis laboratory strains (H37Rv, CDC 1551, and Erdman) at concentrations ranging from 1 to 8 μg/ml. Three of the four also inhibited growth of Mycobacterium smegmatis within this concentration range. All four avermectins were less active against Mycobacterium avium; doramectin had lower levels of activity against all Mycobacterium species. Recent reports have shown that the antimycobacterial activity of some drugs is dependent on the presence of glycerol in the assay medium, leading to the identification of leads that lack activity in vivo (15, 16). When the activities of ivermectin, moxidectin, and selamectin were assayed in the absence of glycerol against M. tuberculosis, only a slight decrease (twofold) in their MICs was observed, demonstrating a glycerol-independent mode of action for the avermectins. In summary, the avermectins were found to be active against each of the four Mycobacterium species tested, while all were inactive against bacteria belonging to diverse related and unrelated taxa. Although these structurally related compounds had detectable differences in their activities against the four Mycobacterium species, our results suggest that mycobacteria share an unknown essential target for avermectins.
Fig 1.
Avermectins used in this study. Images were obtained from ChemSpider.
Table 1.
Antimicrobial activities of four avermectins against bacterial species, including multidrug-resistant M. tuberculosis clinical isolatesa
| Species | Strainb | Drug resistance profilec |
In vitro MIC90 (μg/ml) for: |
|||
|---|---|---|---|---|---|---|
| Ivermectin | Selamectin | Moxidectin | Doramectind | |||
| Escherichia coli | O157:H7 | WT | >256 | >256 | >256 | >256 |
| Acinetobacter baumannii | ATCC 1960 | WT | >256 | >256 | >256 | >256 |
| Pseudomonas aeruginosa | PA01 (H103) | WT | >256 | >256 | >256 | >256 |
| S. lividans | 1326 | WT | >256 | >256 | >256 | >256 |
| R. jostii | RHA1 | WT | >256 | >256 | >256 | >256 |
| Kocuria rhizophila | WT | >256 | >256 | >256 | >256 | |
| Staphylococcus aureus | ATCC 25923 | WT | >256 | >256 | >256 | >256 |
| M. smegmatis | mc2155 | WT | 8 | 4 | 4 | 128 |
| M. avium | ATCC 25291 | WT | >128 | 16 | >128 | >128 |
| M. bovis | BCG Pasteur | WT | 4 | 4 | 4 | 8 |
| M. tuberculosis | H37Rv | WT | 6 | 3 | 3 | 8 |
| M. tuberculosis | CDC 1551 | WT | 4–8 | 1 | 2 | 4–8 |
| M. tuberculosis | Erdman | WT | 8 | 2 | 2–4 | 8 |
| M. tuberculosis | 1254 | 8 | 2–4 | 4 | 8–16 | |
| M. tuberculosis | H37Rv mc24977 | INH | 6 | 1.5 | 3 | ND |
| M. tuberculosis | CI5447 | INH | 1.5 | 1.5 | 0.8–1.5 | ND |
| M. tuberculosis | CI5297 | INH | 6 | 3 | 6 | ND |
| M. tuberculosis | CI5305 | INH | 3 | 1.5 | 1.5 | ND |
| M. tuberculosis | H37Rv mc25857 | INH, RIF | 3 | 1.5 | 3 | ND |
| M. tuberculosis | H37Rv mc25858 | INH, RIF | 3 | 1.5 | 3 | ND |
| M. tuberculosis | V2475 | INH, RIF | 1.5 | 1.5 | 3 | ND |
| M. tuberculosis | CI5058 | INH, RIF | 6 | 6 | 6 | ND |
| M. tuberculosis | CI5324 | INH, RIF | 6 | 3 | 3 | ND |
| M. tuberculosis | CI5400 | INH, RIF | 3 | 3 | 3 | ND |
| M. tuberculosis | CI5072 | INH, RIF, SM | >24 | 3–6 | 3 | ND |
| M. tuberculosis | CI5358 | INH, EMB, ETH | 3 | 1.5 | 3 | ND |
| M. tuberculosis | CI5459 | INH, EMB, ETH | 3 | 1.5–3 | 1.5 | ND |
| M. tuberculosis | CI12081 | INH, RIF, SM, EMB, ETH | >24 | 3 | 3 | ND |
| M. tuberculosis | KZN11 | INH, RIF, SM, EMB | 3 | 3 | 3 | ND |
| M. tuberculosis | KZN5 | INH, RIF, SM, EMB, ETH, KM, PZA | 6 | 3 | 3 | ND |
| M. tuberculosis | KZN6 | INH, RIF, SM, EMB, ETH, KM, PZA | 6 | 6 | 3 | ND |
| M. tuberculosis | KZN12 | INH, RIF, SM, EMB, ETH, KM, PZA | 3 | 3 | 1.5 | ND |
| M. tuberculosis | KZN14 | INH, RIF, SM, EMB, ETH, KM, PZA | 6–12 | 6 | 3–6 | ND |
| M. tuberculosis | KZN15 | INH, RIF, SM, EMB, ETH, KM, PZA | 6 | 3 | 3 | ND |
| M. tuberculosis | KZN16 | INH, RIF, SM, EMB, ETH, KM, PZA | 3 | 1.5 | 1.5 | ND |
| M. tuberculosis | BC-DS1 | 4–16 | 2 | 4 | ND | |
| M. tuberculosis | BC-DS3 | 8–16 | 1–2 | 2 | ND | |
| M. tuberculosis | BC-DS4 | 64 (8) | 1–2 | 2 | ND | |
| M. tuberculosis | BC-DS5 | >128 (8) | 2–4 | 2–4 | ND | |
| M. tuberculosis | BC-MDR2 | INH, RIF, PZA, SM, RBT | >128 (8) | 2 | 2–4 | ND |
| M. tuberculosis | BC-MDR3 | INH, RIF, RBT | 1–2 | 0.5–1 | 1–2 | ND |
| M. tuberculosis | BC-MDR4 | INH, RIF, EMB, PZA, RBT | 4–16 | 2 | 2–4 | ND |
| M. tuberculosis | BC-MDR5 | INH, RIF, PZA, SM, PAS, RBT | 4–16 | 2 | 2–4 | ND |
Mycobacterial strains were assayed in liquid 7H9 media containing 0.2% glycerol and 10% albumin-dextrose-saline. Nonmycobacterial strains were assayed in LB medium. The MTT assay was used to determine the concentration that inhibited growth by 90%. Values in parentheses indicate 50% growth inhibition (MIC50). The M. smegmatis strain was incubated for 3 days, the M. avium, M. bovis BCG, and M. tuberculosis strains were incubated for 7 days, and the nonmycobacterial strains were incubated overnight before the addition of MTT.
Strains V2475 and 1254 have been described previously (10, 11). CI strains are clinical isolates from Mexico. KZN strains are clinical isolates from KwaZulu-Natal, South Africa. BC strains are clinical isolates from British Columbia, Canada.
WT, wild type (no resistance mutations); EMB, ethambutol; ETH, ethionamide; INH, isoniazid; KM, kanamycin; RBT, rifabutin; RIF; rifampin; PAS, p-amino salicylate; PZA, pyrazinamide; SM, streptomycin.
ND, not determined.
In order to investigate the potential inclusion of avermectins in the limited repertoire of TB drugs that might be used against drug-resistant strains, the activities of avermectins were also surveyed against multidrug-resistant (MDR) and extensively drug-resistant (XDR) M. tuberculosis clinical isolates from different geographical locations (Table 1). The MICs of ivermectin, selamectin, and moxidectin were similar against a panel of 27 MDR and XDR clinical isolates having elevated drug resistance profiles including first- and second-line anti-TB drugs, such as ethambutol, ethionamide, isoniazid, kanamycin, rifabutin, rifampin, p-amino salicylate (PAS), pyrazinamide, and streptomycin (Table 1). Only three multidrug-resistant (CI15072, CI12081, and BC-MDR2) and two drug-susceptible (BC-DS4 and BC-DS5) strains were less sensitive to ivermectin (MIC90 > 24 μg/ml). Nevertheless, inhibitory activity against these strains was reflected in low MIC50 values (<8 μg/ml). Importantly, the sensitivity of these strains to selamectin and moxidectin was unaffected. In summary, the avermectins were as effective against most drug-resistant M. tuberculosis clinical isolates as they were against M. tuberculosis laboratory strains.
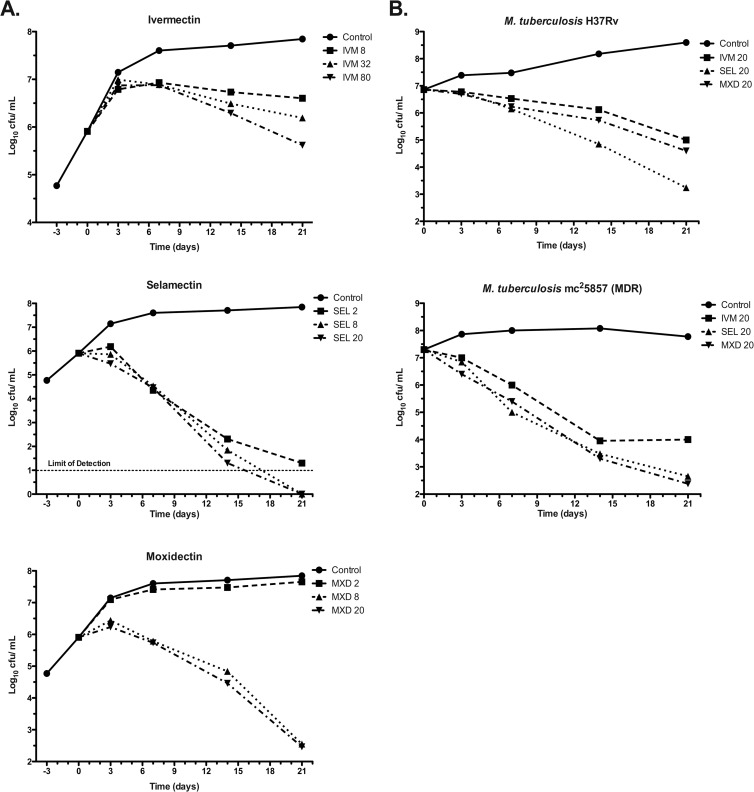
To address the question of whether avermectins are bactericidal or bacteriostatic, survival kinetic experiments were done for ivermectin, selamectin, and moxidectin (Fig. 2). Two experiments performed independently, under similar but not identical growth conditions (see the Fig. 2 legend), measured kill kinetics. In the first experiment (Fig. 2A), 21-day kill curves were performed using various concentrations of ivermectin, selamectin, and moxidectin against the laboratory M. tuberculosis strain H37Rv. Here, selamectin showed the strongest bactericidal profile. All avermectins proved to be bactericidal, reducing initial bacterial viability up to 6 orders of magnitude (the limits of CFU detection). In the comparable kill kinetic experiment (Fig. 2B) performed independently at another location, the activity of the same dose (20 μg/ml) of each of the avermectins was measured over the same time period against M. tuberculosis H37Rv and mc25857 (an MDR strain; see Table 1 for details). Results were consistent with those described above (Fig. 2A). Moreover, all avermectins tested showed promising bactericidal activity against the MDR strain, reinforcing the MIC data and suggesting potential application of the avermectins for the treatment of MDR and XDR TB patients.
Fig 2.
Time-kill kinetics of avermectins against M. tuberculosis. Two experiments were performed independently, at two different locations, under similar but not identical growth conditions. (A) Dose titration. Frozen stocks of M. tuberculosis H37Rv were cultured in 15 ml of 7H9 broth supplemented with 10% albumin-dextrose-catalase, 0.2% glycerol in standing 25-cm2 tissue culture flasks at 37°C and 5% CO2 for 3 days before the addition of avermectins. Cultures were agitated only during sampling. (B) Fixed dose strain comparison. Cultures (5 ml) of M. tuberculosis H37Rv and mc25857 (an MDR isolate) were pregrown in shaking 35-ml bottles in 7H9 broth supplemented with 10% ADC, 0.2% glycerol, and 0.05% tyloxapol to an optical density at 600 nm (OD600) of 0.8. To assess drug activity, cultures were washed once in phosphate-buffered saline (PBS), diluted (1/150) in the same medium (without tyloxapol), and grown at 37°C in shaking 15-ml conical tubes containing ivermectin, selamectin, or moxidectin at 20 μg/ml. Prior to plating, cell clumps were disrupted by sonication and viability was quantified by plating on 7H10 agar supplemented with 10% oleic acid-albumin-dextrose-catalase and 0.2% glycerol. Plates were incubated at 37°C, and colonies were counted after 2 weeks of incubation (microscopically) and reassessed after 4 weeks. Concentrations of avermectins are expressed in μg/ml. IVM, ivermectin; SEL, selamectin; and MXD, moxidectin.
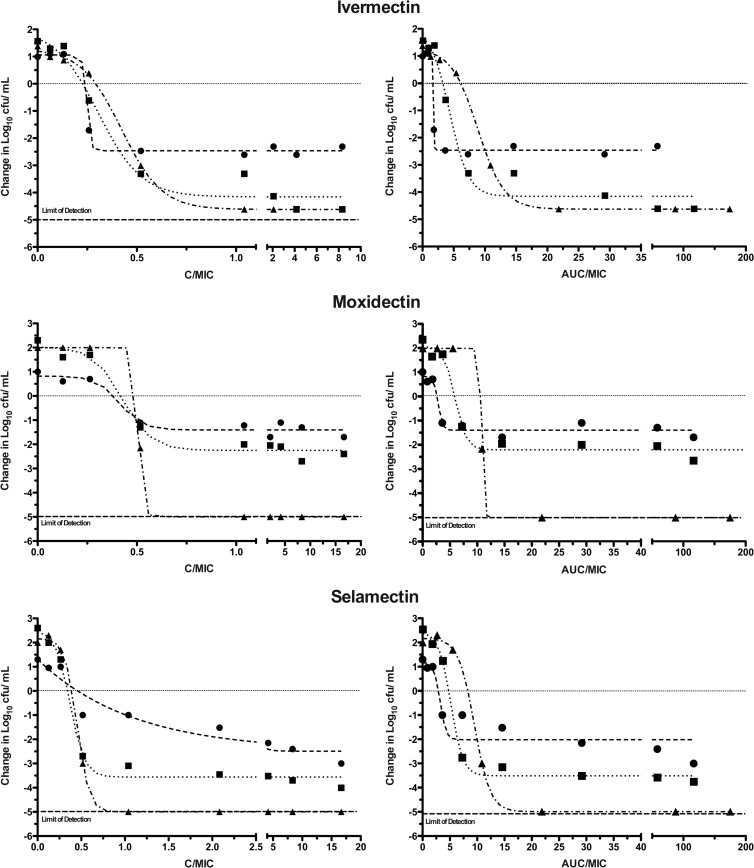
The in vitro pharmacodynamic parameters of the avermectins were further analyzed in a third experiment (Fig. 3). In vitro dose-response curves for the avermectins were obtained by plotting the change in log10 CFU of the inoculum against the drug concentration C/MIC. Using an alternative method to visualize kill kinetics, each avermectin concentration was multiplied by the time of exposure (C × Tdays) and then divided by the MIC to give the in vitro area under the concentration-time curve (AUC/MIC ratio), a standard measure of drug exposure. These analyses both showed that avermectins had exposure-dependent kill kinetics against M. tuberculosis under standard in vitro broth conditions. The AUC/MIC needed to achieve 1 log10 CFU/ml reduction varied between 2 and 4, while a bactericidal effect (4 log10 CFU/ml reduction, 99.99% killing) required AUC/MIC ratios between 12 and 14. From a TB drug treatment perspective, this value indicates the strong bactericidal effect of avermectins relative to rifampin, the standard frontline drug. The AUC at 24 h (AUC24)/MIC value required for a comparable 1 log10 CFU/ml reduction of the first-line antituberculosis drug rifampin is more than 10 times higher (18).
Fig 3.
Effect of concentration and exposure on the bactericidal activities of avermectins against M. tuberculosis. Comparison of concentration and area under the curve over MIC ratios (C/MIC and AUC/MIC, respectively) on the bactericidal activities of the avermectins. The bactericidal effect was calculated on the basis of the initial inoculum prior to the addition of avermectins. Analysis of antimycobacterial activity was performed by comparing the rates of bacterial killing determined by nonlinear regression analysis with 95% confidence limits. The r2 values on days 7 (filled circles), 14 (filled squares), and 21 (filled triangles) were 0.9959, 0.9766, and 0.9989 for ivermectin, 0.9591, 0.9827, and not available for moxidectin, and 0.88, 0.9949, and 0.9992 for selamectin, respectively. Cells were processed as described in the Fig. 2 legend for panel B but grown at 37°C in shaking 96-well plates containing 2-fold serial drug dilutions. The MIC values used for calculations were 6, 3, and 3 μg/ml for ivermectin, moxidectin, and selamectin, respectively, as calculated by the MTT assay.
Ivermectin has been used to treat human onchocerciasis and lymphatic filariasis for over 20 years (19–21). Other avermectins (doramectin, moxidectin, and selamectin) used in veterinary medicine for nematode control in pets and livestock are known to be safe and well-tolerated for these indications (19). Selamectin can be administered topically, subcutaneously, or orally in the veterinary setting to treat a number of ecto- and endoparasite conditions in dogs and cats, and it is not presently approved for human use (22). Moxidectin is also safe in humans (23), and it is currently undergoing a phase III clinical trial to compare its efficacy with ivermectin in subjects with Onchocerca volvulus infection (http://clinicaltrials.gov/ct2/show/NCT00790998). Because ivermectin is extremely well-tolerated, effective, orally active, and associated with long-term safety, Merck & Co. has donated it to patients with river blindness in needy areas throughout the world (24). Furthermore, the success of this longstanding give-away program indicates that industrial production processes are established and ivermectin could be distributed to large populations with high incidences of MDR TB, a distinct advantage for implementation of TB drug therapy programs. However, to introduce any new TB therapy (especially for MDR or XDR TB), it is essential to establish that it is equal to or better than current treatment options; the promising 2- to 8-μg/ml range of MICs for avermectins in liquid cultures is comparable to that of second-line TB drugs, ranging from 1 to 25 μg/ml against M. tuberculosis H37Rv (25). The in vitro AUC/MIC ratios of avermectins are a pharmacodynamic predictor of effective bactericidal activity in vivo. Importantly, the low exposure needed to achieve this effect (AUC/MIC ratios of 10 to 15) indicates the potential of the avermectins for TB therapy. Thus, the avermectins might be readily adopted into the limited repertoire of drugs available to treat MDR and XDR TB. However, in vivo pharmacokinetic and efficacy studies will be needed to assess their clinical application for treating TB.
The apparent specificity of avermectins for mycobacteria implies that their target is unique to this taxon of bacteria. The remarkable complexity and uniqueness of the mycobacterial cell envelope, not present in other bacteria that are resistant to avermectins (including Streptomyces lividans and Rhodococcus jostii, both related actinobacteria), suggest this structure as a potential target (26). In invertebrate nematodes, avermectins specifically bind to glutamate-gated chloride channels present in nerve and muscle cells, causing paralysis and reduced ability to reproduce (27). Avermectins are orally active and distributed to most parts of the human body, including the lungs (24, 28). Ivermectin does not bind ligand-gated chloride channels in mammals, possibly due to impermeability of the blood-brain barrier and nonspecific pumps that prevent it from reaching the central nervous system (20, 27). Interestingly, moxidectin and selamectin, compounds used extensively in animals to treat a variety of parasitic indications without reported toxicity issues, were also highly active against M. tuberculosis. The specificity of avermectins for mycobacteria would be ideal for selectively targeting the pathogen while minimizing deleterious effects on resident gut flora after oral administration.
In summary, this is the first report demonstrating the antimycobacterial activity of avermectins. Their established safety profiles in humans and animals make them potential therapeutic options for treating TB. Future work will be done to identify the mycobacterial target of avermectins, to characterize pharmacokinetic and pharmacodynamic properties in animal models using dosages that are higher than that traditionally needed to treat river blindness, and to define the synergistic drug profiles for possible applications in combinatorial TB therapies.
ACKNOWLEDGMENTS
We thank Takeshi Murakami for his mentorship and wise insights, Keerat Sidhu for technical support, Gaye Sweet for lab management and comments on the manuscript, D. Alland (University of Medicine and Dentistry of New Jersey), M. Larsen (Albert Einstein College of Medicine), A. W. Sturm (University of KwaZulu Natal, South Africa), and Patrick Tang (BC Centre for Disease Control) for the generous gifts of clinical isolates, and Julian Davies, Lindsay Eltis, Robert Hancock, and Richard Stokes for other bacterial strains. We also thank the University of British Columbia (UBC) Facility for Infectious Disease and Epidemic Research (FINDER) for technical support. Assistance from the Centre for Drug Research and Development is gratefully acknowledged.
L.E.L. was supported by a scholarship from the Canadian Natural Sciences and Engineering Research Council. This work was supported by grants from The Canadian Institute of Health Research (MOP-82745 and MOP-82855), the British Columbia Lung Association (C.J.T.), Grand Challenges Canada—Stars in Global Health (0030-01-04-01-01) (S.R.-G.), and the National Institutes of Health (AI26170 and CFAR AI-051519) (W.R.J.).
Footnotes
Published ahead of print 19 November 2012
REFERENCES
- 1. Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6:29–40 [DOI] [PubMed] [Google Scholar]
- 2. Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490 [DOI] [PubMed] [Google Scholar]
- 3. Chong CR, Sullivan DJ., Jr 2007. New uses for old drugs. Nature 448:645–646 [DOI] [PubMed] [Google Scholar]
- 4. Ramon-Garcia S, Ng C, Anderson H, Chao JD, Zheng X, Pfeifer T, Av-Gay Y, Roberge M, Thompson CJ. 2011. Synergistic drug combinations for tuberculosis therapy identified by a novel high-throughput screen. Antimicrob. Agents Chemother. 55:3861–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omura S, Crump A. 2004. The life and times of ivermectin—a success story. Nat. Rev. Microbiol. 2:984–989 [DOI] [PubMed] [Google Scholar]
- 6. Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, Kong YL, Monaghan RL, Olson G, Putter I, Tunac JB, Wallick H, Stapley EO, Oiwa R, Omura S. 1979. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 15:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell WC. 1985. Ivermectin: an update. Parasitol. Today 1:10–16 [DOI] [PubMed] [Google Scholar]
- 8. Hotson IK. 1982. The avermectins: a new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 53:87–90 [PubMed] [Google Scholar]
- 9. Nagai K, Shiomi K, Sunazuka T, Harder A, Turberg A, Omura S. 2004. Synthesis and biological evaluation of novel 4″-alkoxy avermectin derivatives. Bioorg. Med. Chem. Lett. 14:4135–4139 [DOI] [PubMed] [Google Scholar]
- 10. Ioerger TR, Koo S, No EG, Chen X, Larsen MH, Jacobs WR, Jr, Pillay M, Sturm AW, Sacchettini JC. 2009. Genome analysis of multi- and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 4:e7778 doi:10.1371/journal.pone.0007778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, Schoolnik G, Thompson CJ. 2005. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 102:12200–12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC. 2005. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 55:500–505 [DOI] [PubMed] [Google Scholar]
- 13. Gomez-Flores R, Gupta S, Tamez-Guerra R, Mehta RT. 1995. Determination of MICs for Mycobacterium avium-M. intracellulare complex in liquid medium by a colorimetric method. J. Clin. Microbiol. 33:1842–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foongladda S, Roengsanthia D, Arjrattanakool W, Chuchottaworn C, Chaiprasert A, Franzblau SG. 2002. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 6:1118–1122 [PubMed] [Google Scholar]
- 15. Pethe K, Sequeira PC, Agarwalla S, Rhee K, Kuhen K, Phong WY, Patel V, Beer D, Walker JR, Duraiswamy J, Jiricek J, Keller TH, Chatterjee A, Tan MP, Ujjini M, Rao SP, Camacho L, Bifani P, Mak PA, Ma I, Barnes SW, Chen Z, Plouffe D, Thayalan P, Ng SH, Au M, Lee BH, Tan BH, Ravindran S, Nanjundappa M, Lin X, Goh A, Lakshminarayana SB, Shoen C, Cynamon M, Kreiswirth B, Dartois V, Peters EC, Glynne R, Brenner S, Dick T. 2010. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 1:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stanley SA, Grant SS, Kawate T, Iwase N, Shimizu M, Wivagg C, Silvis M, Kazyanskaya E, Aquadro J, Golas A, Fitzgerald M, Dai H, Zhang L, Hung DT. 2012. Identification of novel inhibitors of M. tuberculosis growth using whole-cell based high-throughput screening. ACS Chem. Biol. 7:1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reference deleted.
- 18. Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geary TG. 2005. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 21:530–532 [DOI] [PubMed] [Google Scholar]
- 20. Merck & Co., Inc 2007. Stromectol (ivermectin) package insert NDA 50-742/S-022. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 21. Taylor MJ, Hoerauf A, Bockarie M. 2010. Lymphatic filariasis and onchocerciasis. Lancet 376:1175–1185 [DOI] [PubMed] [Google Scholar]
- 22. McKellar QA, Gokbulut C. 2012. Pharmacokinetic features of the antiparasitic macrocyclic lactones. Curr. Pharm. Biotechnol. 13:888–911 [DOI] [PubMed] [Google Scholar]
- 23. Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, Rock D, Chen CY, Schwertschlag US. 2003. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J. Clin. Pharmacol. 43:1108–1115 [DOI] [PubMed] [Google Scholar]
- 24. Merck & Co., Inc 2011. Merck corporate responsibility report. Mectizan donation program. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 25. Global Alliance for TB Drug Development 2008. Handbook of anti-tuberculosis agents. Tuberculosis (Edinb.) 88:85–170 [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee D. 1997. The mycobacterial cell wall: structure, biosynthesis and sites of drug action. Curr. Opin. Chem. Biol. 1:579–588 [DOI] [PubMed] [Google Scholar]
- 27. Wolstenholme AJ, Rogers AT. 2005. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 131:S85–S95 [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez Canga A, Sahagun Prieto AM, Diez Liebana MJ, Fernandez Martinez N, Sierra Vega M, Garcia Vieitez JJ. 2008. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 10:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]