Abstract
AZD5206 is a novel antimicrobial agent with potent in vitro activity against Staphylococcus aureus. We evaluated the in vitro pharmacodynamics of AZD5206 against a standard wild-type methicillin-susceptible strain (ATCC 29213) and a clinical strain of methicillin-resistant S. aureus (SA62). Overall, bacterial killing against a low baseline inoculum was more remarkable. Low dosing exposures and/or high baseline inoculum resulted in early reduction in bacterial burden, followed by regrowth and selective amplification of the resistant population.
TEXT
Staphylococcus aureus is a clinically important pathogen and a common cause of bloodstream, surgical site, skin, and skin structure infections, as well as nosocomial pneumonia (1). AZD5206 is a novel (nonfluoroquinolone) bacterial type II topoisomerase inhibitor under investigation; it exhibits potent in vitro activity against S. aureus by interfering with DNA replication. To facilitate the preclinical and clinical development of this agent, we evaluated the in vitro pharmacodynamics of AZD5206.
(This study was presented in part at the 22nd European Conference of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 31 March to 3 April 2012.)
AZD5206 powder was provided by AstraZeneca (Waltham, MA). A stock solution was prepared by dissolving the powder in dimethyl sulfoxide (DMSO). Prior to each investigation, an aliquot of the stock solution was diluted with water and subsequently Mueller-Hinton broth (MHB) (BBL, Sparks, MD). The final concentration of DMSO was <2% in all investigations. S. aureus ATCC 29213 (methicillin susceptible) (American Type Culture Collection, Manassas, VA) and a clinical (methicillin-resistant) isolate, SA62, were used. We have previously determined the MIC of AZD5206 to be 0.062 μg/ml for both isolates using a broth dilution method described by the CLSI (2). The in vitro killing of the two isolates was determined in Ca-MHB at 4× MIC. The initial inocula ranged approximately from 105 to 107 CFU/ml, and the experiment was conducted for 24 h in a shaker water bath at 35°C. Serial samples (baseline and 2, 4, 8, and 24 h) were obtained in duplicate and washed once with saline. The bacterial burden was determined by quantitative culture (50 μl) on Mueller-Hinton agar II (MHA) (BBL) after 10× serial dilutions and overnight incubation at 35°C. The absolute and reliable limits of detection were 20 CFU/ml and 400 CFU/ml, respectively.
In addition, dose ranging and fractionation studies were performed in an in vitro hollow-fiber infection model (HFIM), simulating various projected human pharmacokinetic profiles (unpublished data on file) over 72 h (3). The schematics of the HFIM have been shown previously (4). To ascertain the simulated pharmacokinetic exposures, serial samples were obtained from a circulatory loop of the HFIM on day 1 (for all dosing regimens) and day 3 (if regrowth was not detected by 48 h). The samples were assayed for AZD5206 concentration by using a validated liquid chromatography-tandem mass spectroscopy methodology (linear concentration range, 14 to 1,800 ng/ml; intraday/interday coefficient of variation, <15%). The quantification was performed using multiple reaction monitoring (MRM) mode with ion pair transitions to monitor AZD5206 and carbutamide (internal standard). The fragments for each compound detected were 465.0/203.0 (m/z) for AZD5206 and 272.0/156.0 for carbutamide. The concentration-time profiles were characterized by a one-compartment linear model. Serial samples (baseline and 4, 8, 24, 48, and 72 h) were also obtained from the hollow-fiber cartridges in duplicate. The total and resistant bacterial burdens at various times were determined by quantitative culture on drug-free and drug-supplemented (3× MIC) MHA plates, respectively. Susceptibility testing was repeated in selected isolates recovered from drug-supplemented plates to confirm resistance.
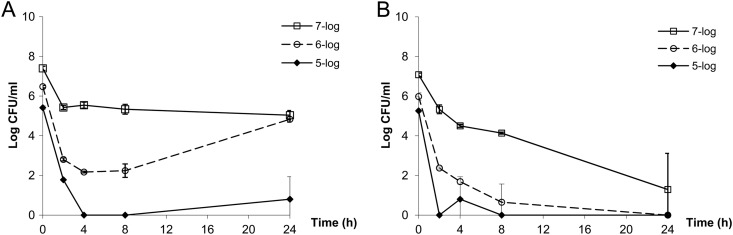
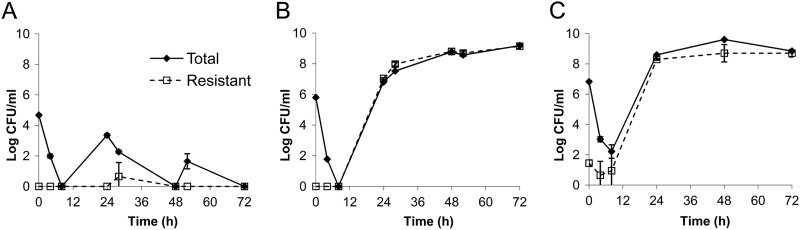
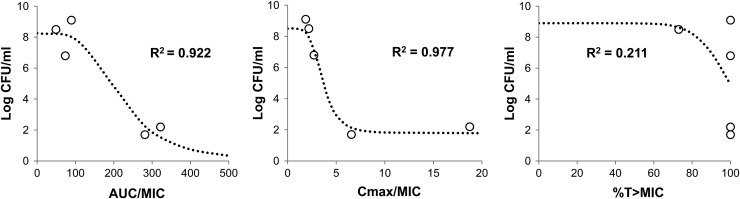
In both bacterial isolates examined, the killing of AZD5206 (at 4× MIC) against a low baseline inoculum was more remarkable than that at higher inocula (Fig. 1). Moreover, the killing activity appeared more noticeable against SA62. In the HFIM, the pharmacokinetic simulations were satisfactory (r2 > 0.90; all best-fit half-lives were within target range; data not shown) and a similar trend in bacterial killing was observed. Resistant isolates were recovered only from drug-supplemented plates at baseline from the highest-inoculum experiments; the natural mutational frequency of resistance (at 3× MIC) was approximately 3 × 10−6. Generally speaking, low dosing exposures and/or high inocula resulted in early reduction in bacterial burden, followed by regrowth and selective amplification of the resistant population by 24 h (Fig. 2). Dose fractionation studies using ATCC 29213 indicated that the microbiological burden observed at 24 h could be reasonably correlated with the AUC/MIC ratio (the area under the concentration-time curve over 24 h in the steady state divided by the MIC) and the Cmax/MIC ratio (the maximum concentration of drug in serum divided by the MIC) (Fig. 3). Furthermore, the dosing frequency appeared to have little impact on microbial response over time (i.e., growth suppression versus resistance development), suggesting that the AUC/MIC ratio was the pharmacodynamic parameter most closely linked to antimicrobial effect (Table 1). To suppress the growth of a bacterial population (approximately 106 CFU/ml at baseline) for up to 72 h, an AUC/MIC ratio of more than 70 (equivalent to a projected clinical dose of 1 g) daily would be necessary. Six random isolates were recovered from drug-supplemented plates at 72 h after regrowth was observed in different HFIM studies. Susceptibility testing (broth dilution) was repeated for all isolates recovered from drug-supplemented plates; MICs were consistently found to be elevated (≥4×) over that for the parent isolate at baseline. Less comprehensive HFIM evaluations were undertaken with SA62; a consistent overall trend was also observed (data not shown).
Fig 1.
Time-kill studies (4× MIC) at different starting inocula. (A) ATCC 29213; (B) SA62. Data are shown as means ± standard deviations.
Fig 2.
HFIM studies simulating a projected clinical dose of 1 g AZD5206 every 24 h against ATCC 29213 at different inocula: 5 log (A), 6 log (B), and 7 log (C). Data are shown as means ± standard deviations.
Fig 3.
Regression analysis of HFIM results for ATCC 29213 at 24 h. Data for a 6-log baseline inoculum are shown. Open circles depict observed bacterial burdens; dotted lines represent the best-fit inhibitory sigmoid Emax model.
Table 1.
Summary of HFIM results for ATCC 29213
| Inoculum (log) | Target simulated daily dose (g) | Dosing frequencyb | Outcome |
|---|---|---|---|
| 5 | 1 | q24h | Suppression |
| 6 | 1 | q24h | Regrowth |
| 7 | 1 | q24h | Regrowth |
| 5 | 4 | q24h | Suppression |
| 6 | 4 | q24h | Suppression |
| 7 | 4 | q24h | Regrowth |
| 6 | 1a | q24h | Regrowth |
| 6 | 1 | q12h | Regrowth |
| 6 | 1 | q8h | Regrowth |
| 6 | 4 | q24h | Suppression |
| 6 | 4 | q8h | Suppression |
Elevated MIC (≥4×) found in isolates recovered from drug-supplemented plates.
q24h, q12h, and q8h, every 24, 12, and 8 h, respectively.
Development of new and effective antimicrobial agents against resistant pathogens is urgently needed (5). In this study, we provided in-depth insights into the activity of a preclinical drug candidate under projected clinically relevant (fluctuating-concentration) exposures. Instead of solely focusing on short-term bacterial burden reduction at 24 h, we also examined the likelihood of suppressing resistance development in the extended time frame. Various key factors implicated in the emergence of resistance in in vitro infection models have been reviewed previously in detail (6). Apparently, the bactericidal activity of AZD5206 was reduced against a dense bacterial population, similar to the inoculum effect commonly reported for the beta-lactams (7). However, since this drug is a non-beta-lactam, the mechanism is unknown and unlikely to be due to the production/accumulation of beta-lactamases (8). Somewhat to our surprise, resistance developed rapidly (as early as by 24 h) when a high inoculum was exposed to a low drug exposure. In view of the mutational frequency of resistance, selective amplification of preexisting resistant mutants at baseline was a possibility. Since AZD5206 is known to interfere with DNA replication, activation of the SOS response system and induced mutagenesis could be also involved, resulting in rapid emergence of resistance, as shown previously (9). However, we could not completely rule out another mechanism(s) such as biofilm production and quorum sensing.
In summary, the antimicrobial effect of AZD5206 against S. aureus was found to be most closely linked to the daily dose (AUC/MIC ratio) but not dosing frequency. Our results could be used to support future investigations.
ACKNOWLEDGMENT
The study was supported by an unrestricted grant from AstraZeneca.
Footnotes
Published ahead of print 10 December 2012
REFERENCES
- 1. Gaynes R, Edwards JR. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854 [DOI] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2007. Performance standards for antimicrobial susceptibility testing: 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. 2006. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:2626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tam VH, Louie A, Fritsche TR, Deziel M, Liu W, Brown DL, Deshpande L, Leary R, Jones RN, Drusano GL. 2007. Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J. Infect. Dis. 195:1818–1827 [DOI] [PubMed] [Google Scholar]
- 5. Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668 [DOI] [PubMed] [Google Scholar]
- 6. Singh R, Tam VH. 2011. Optimizing dosage to prevent emergence of resistance—lessons from in vitro models. Curr. Opin. Pharmacol. 11:453–456 [DOI] [PubMed] [Google Scholar]
- 7. Mizunaga S, Kamiyama T, Fukuda Y, Takahata M, Mitsuyama J. 2005. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 56:91–96 [DOI] [PubMed] [Google Scholar]
- 8. Craig WA, Bhavnani SM, Ambrose PG. 2004. The inoculum effect: fact or artifact? Diagn. Microbiol. Infect. Dis. 50:229–230 [DOI] [PubMed] [Google Scholar]
- 9. Singh R, Ledesma KR, Chang KT, Tam VH. 2010. Impact of recA on levofloxacin exposure-related resistance development. Antimicrob. Agents Chemother. 54:4262–4268 [DOI] [PMC free article] [PubMed] [Google Scholar]