Abstract
Due to the limited number of antifungals and the emergence of resistance, new therapies against invasive aspergillosis are needed. We show that calcineurin inhibitors are active in vitro against both azole- and echinocandin-resistant Aspergillus fumigatus strains. The heat shock protein 90 (Hsp90) inhibitor geldanamycin had modest activity when used alone, but its combination with caspofungin or tacrolimus (FK506) resulted in fungicidal activity against azole-resistant strains. Targeting the Hsp90-calcineurin axis is a promising alternative strategy against azole-resistant A. fumigatus strains.
TEXT
Invasive aspergillosis (IA) is one of the most frequent infectious causes of death in immunocompromised patients. The triazole voriconazole is the first-line antifungal agent against IA (1), but azole resistance in Aspergillus fumigatus has emerged over the last decade, with a prevalence as high as 5 to 13% in some countries (2–4). The most common azole resistance mechanism consists of mutations in the cyp51A gene involved in ergosterol biosynthesis (4), with increasing evidence to support its association with treatment failure (2, 3). The echinocandins are an optional second-line therapy for IA (5). Echinocandin resistance has been well documented in clinical isolates of Candida albicans and results mainly from mutations in two specific regions of the fks1 gene encoding β-1,3-glucan synthesis (6), but it has been rarely documented in A. fumigatus (7, 8). Laboratory strains of A. fumigatus with reduced susceptibility to echinocandins have been generated by point mutations of the Af-fks1 gene (9, 10), suggesting that the same mechanism of resistance may develop in A. fumigatus.
Targeting intracellular signaling pathways involved in stress response represents a novel strategy to counteract the growing problem of antifungal resistance (11). Our recent work demonstrated the key role of calcineurin (12–15) and that of heat shock protein 90 (Hsp90) (16) in the cell wall integrity of A. fumigatus. The calcineurin inhibitors cyclosporine (CsA) and tacrolimus (FK506) have in vitro antifungal activity and a positive interaction with the echinocandin caspofungin against A. fumigatus (17, 18). Similar effects were recently reported for the Hsp90 inhibitor geldanamycin (16). In this study, we investigated the role of calcineurin or Hsp90 inhibition as an alternative antifungal strategy against A. fumigatus azole- and echinocandin-resistant strains.
In vitro antifungal activity of three triazoles, caspofungin, FK506, and geldanamycin, was assessed for each drug alone and in combinations against the wild-type AF293 strain and various A. fumigatus clinical or laboratory isolates with multi-azole or pan-echinocandin resistance. Multi-azole-resistant clinical isolates were obtained from the Regional Mycology Laboratory of Manchester (RMLM) (a gift from David Denning) (2), with all harboring various defined mutations of the cyp51A gene with resistance to triazoles according to the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antibiotic Susceptibility Testing (EUCAST) epidemiological cutoff values (≥1 μg/ml for voriconazole and itraconazole and ≥0.25 μg/ml for posaconazole) (19, 20). A laboratory-generated pan-echinocandin-resistant strain harboring the S678P substitution in Fks1p (EMFR-S678P) (a gift from David Perlin) was also tested (10).
Antifungal susceptibility testing was performed according to CLSI standards (21), and checkerboard dilutions were used for drug combinations. Antifungal activity was assessed visually and classified as follows: no activity, morphological abnormalities (hyphal blunting and impaired branching) with less than 25% growth reduction, 25 to 50% growth reduction, 50 to 75% growth reduction, 75 to 90% growth reduction, and >90% growth reduction. The minimal effective concentration (MEC) was defined as the lowest concentration of the drug producing morphological abnormalities and a substantial reduction of hyphal growth (22), and the MIC was defined as the lowest concentration achieving near-complete (>90%) growth inhibition. Antifungal checkerboard interactions were assessed by the fractional inhibitory concentration index (FICI), which is the sum of the individual fractional inhibitory concentrations (FIC) of each drug (MEC or MIC of the drug in combination divided by the MEC or MIC of the drug alone) and classified as synergistic (≤0.5), indifferent (>0.5 to 4), or antagonistic (>4) (23). In the visual absence of growth, a fraction of the liquid medium containing 100 conidia (defined on the basis of the original inoculum) was plated on glucose minimal medium (GMM) agar and incubated at 37°C for 72 h, with viability expressed as the percentage of growing colonies and fungicidal activity defined as ≥97% killing of the inoculum (<3% growing colonies). Growth on solid medium was also assessed after inoculation of 5,000 conidia on MOPS (morpholinepropanesulfonic acid)-buffered RPMI 1640 agar plates containing a defined dose of each drug.
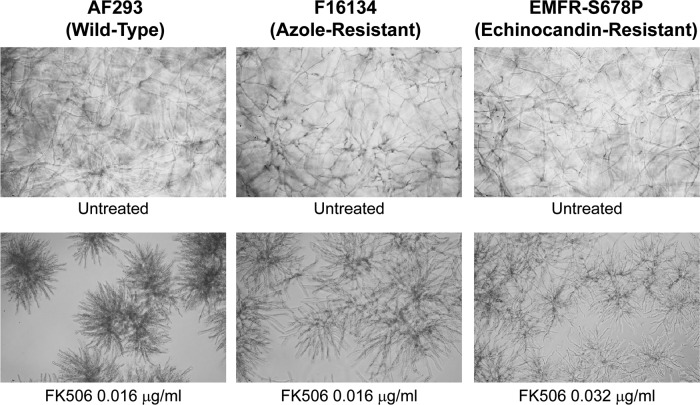
Results of antifungal susceptibility testing for caspofungin, FK506, geldanamycin, and three triazoles are shown in Table 1. The MECs for caspofungin were within one dilution among the azole-resistant strains and the wild-type AF293 strain (0.5 to 1 μg/ml). At these concentrations, a growth reduction of about 25 to 75% was observed, while higher concentrations did not result in improved activity. FK506 showed antifungal activity with an MEC of 0.016 μg/ml for AF293 and similar values (0.016 to 0.032 μg/ml) for most azole-resistant strains and the echinocandin-resistant strain. At these concentrations, hyphal growth was substantially blunted, with extensive branching as previously described (15) (Fig. 1). The maximal hyphal-growth-blunting effect of FK506 was reached at 0.1 μg/ml for all strains (see Fig. 3, row C). We did not find any correlation between the specific cyp51A mutation and susceptibility to FK506 in the azole-resistant strains. To determine if this calcineurin inhibition antifungal activity was unique to FK506, we also treated the resistant strains with CsA and found antifungal activity (MEC = 2 μg/ml). The Hsp90 inhibitor geldanamycin had modest antifungal activity against AF293 and the resistant strains at a concentration of 4 to 5 μg/ml (hyphal growth reduction ≤ 50%). Higher geldanamycin concentrations resulted in the formation of drug precipitates and were inactive.
Table 1.
Antifungal susceptibility testing of caspofungin, FK506, geldanamycin, and three triazoles against the wild-type AF293 and various clinical and laboratory A. fumigatus resistant strains
| Strain [amino acid substitution]a (reference) | MIC (μg/ml)b |
MEC (μg/ml)c |
||||
|---|---|---|---|---|---|---|
| ITZ | VCZ | POS | CSP | FK506 | GDA | |
| AF293 [wild type] | 0.25 | 0.25 | 0.25 | 0.5 | 0.016 | 4 |
| akuBKU80 (24)d | 0.25 | 0.25 | 0.25 | 0.5 | 0.032 | 4 |
| F16134 [M220K] (2) | >8 | 4 | >8 | 0.5 | 0.016 | 4 |
| F6919 [M220K] (2) | >8 | 2 | 2 | 1 | 0.016 | 4 |
| F14532 [M220T] (2) | >8 | 1 | 0.5 | 1 | 0.032 | 4 |
| F14946 [G138C] (2) | >8 | >8 | >8 | 0.5 | 0.064 | 5 |
| F12760 [G138C] (2) | >8 | 0.125 | 8 | 1 | 0.032 | 5 |
| F7075 [G54E] (2) | >8 | 1 | >8 | 1 | 0.032 | 5 |
| F12636 [G54E] (2) | >8 | 0.125 | 1 | 1 | 0.016 | 4 |
| F14403 [G54R] (2) | >8 | 0.5 | >8 | 0.5 | 0.016 | 4 |
| F16216 [L98H+TR] (2) | >8 | 8 | 2 | 1 | 0.016 | 4 |
| F12776 [Y431C] (2) | >8 | 4 | 1 | 1 | 0.1 | 5 |
| F13747 [G434C] (2) | >8 | 4 | 1 | 1 | 0.032 | 5 |
| VO44-58 [unknown] | >8 | 4 | 0.5 | 1 | 0.032 | 5 |
| EMFR-S678P [S678P] (10) | 0.25 | 0.25 | 0.25 | >16 | 0.032 | 5 |
The amino acid substitution refers to the gene cyp51A for azole-resistant strains and the gene fks1p for the echinocandin-resistant strain.
MICs are as reported from the Regional Mycology Laboratory Manchester (RMLM) (2) except for the MICs of AF293, akuBKU80, and EMFR-S678P (this study). ITZ, itraconazole; VCZ, voriconazole; POS, posaconazole.
MEC values as assessed in this study (means of triplicates). Of note, the cutoff for the MIC (>90% growth inhibition) was not reached for caspofungin, FK506, and geldanamycin. CSP, caspofungin; GDA, geldanamycin.
akuBKU80 is the reference (genetic background) strain for EMFR-S678P.
Fig 1.
In vitro antifungal activity of FK506 against the AF293 (wild-type), F16134 (multi-azole-resistant), and EMFR-S678P (echinocandin-resistant) strains. FK506 at the concentration of 0.016 to 0.032 μg/ml is active against all three strains, inducing morphological abnormalities (dense, blunted hyphae with extensive branching) and a substantial hyphal growth defect. Light-microscopy photographs (×10 magnification) after 24 h of growth at 37°C.
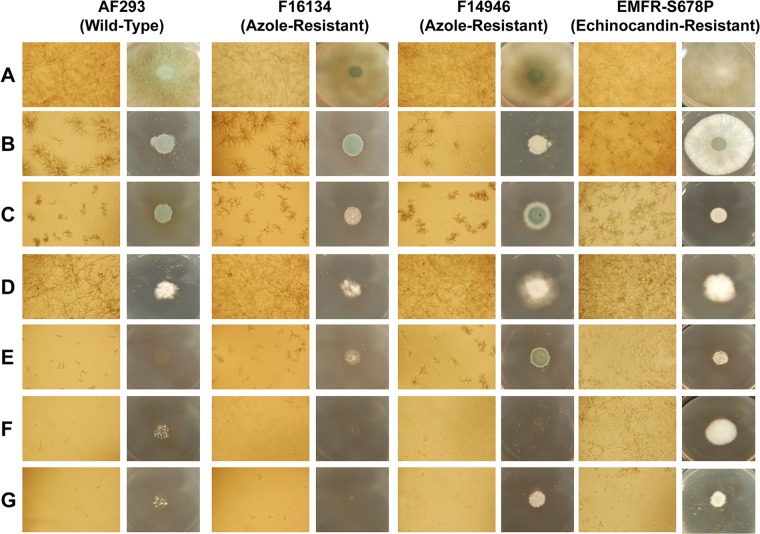
Fig 3.
In vitro antifungal activity of caspofungin (CSP), FK506, and geldanamycin (GDA) alone or in combinations against the AF293 (wild-type) strain and the resistant strains. Caspofungin, FK506, and geldanamycin were tested alone or in combination at the concentrations at which their maximal antifungal activity was reached: 1 μg/ml for caspofungin, 0.1 μg/ml for FK506, and 4 μg/ml for geldanamycin. Light-microscopy photographs (×10 magnification) were taken after conidia were grown at a concentration of 104/ml in liquid RPMI 1640 containing the appropriate drug concentration for 24 h at 37°C (left panels in each strain column). An inoculum of 5,000 conidia was grown on RPMI 1640 agar plates containing the appropriate drug concentration for 72 h at 37°C (right panels in each strain column). Rows are as follows: (A) untreated; (B) caspofungin alone; (C) FK506 alone; (D) geldanamycin alone; (E) caspofungin and FK506; (F) caspofungin and geldanamycin; (G) FK506 and geldanamycin. Each drug used alone has only a fungistatic activity. A near-complete lack of growth is achieved in liquid medium by all three drug combinations against the wild-type AF293. A similar effect is obtained against the azole-resistant strains (F16134 and F14946) by geldanamycin in combination with caspofungin or FK506, while the combination of FK506 and caspofungin has somewhat less activity. The combination of FK506 and geldanamycin has the highest antifungal activity against the echinocandin-resistant strain EMFR-S678P.
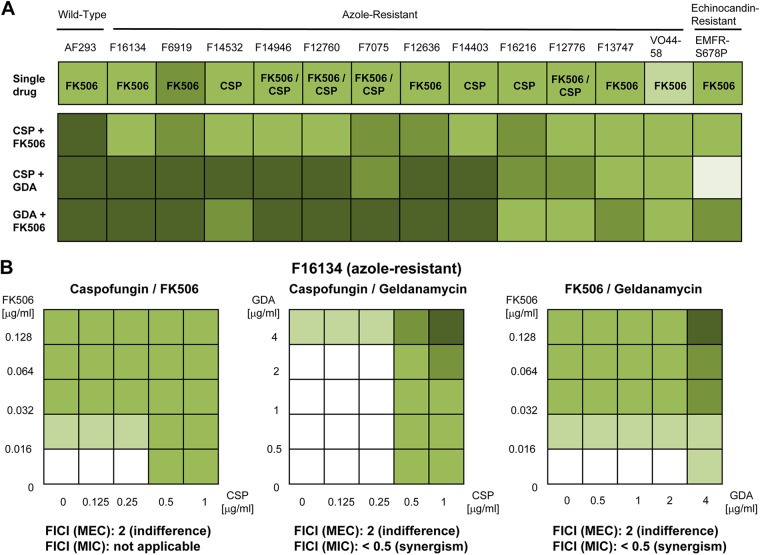
We then tested the effect of various drug combinations against the azole-resistant strains. The addition of FK506 or geldanamycin to voriconazole did not result in any positive interaction. Combining various active antifungal agents (caspofungin with FK506 or geldanamycin and FK506 with geldanamycin) at the concentrations associated with their maximal effect (i.e., 1 μg/ml for caspofungin, 0.1 μg/ml for FK506, and 4 μg/ml for geldanamycin) resulted in various degrees of inhibition according to the drug combination and the strain tested (Fig. 2A). Overall, the combinations of geldanamycin and caspofungin or geldanamycin and FK506 were the most active against the azole-resistant strains, with a near-complete growth inhibition for more than half of the strains (Fig. 2A and 3). The fungicidal activity of geldanamycin in combination with caspofungin or FK506 was confirmed by the viability assay showing ≥97% killing. The combination of FK506 and caspofungin provided a similar benefit against the wild-type strain but did not result in a substantial increase of activity for the azole-resistant strains (less than 90% growth inhibition) compared to the best antifungal activity achieved with one single drug (Fig. 2A and 3). Neither FK506 nor geldanamycin was able to increase the susceptibility to caspofungin of the echinocandin-resistant strain. A substantial increase in activity was achieved with the combination of FK506 and geldanamycin, although residual growth was observed (less than 90% growth inhibition) (Fig. 2A and 3). When these combinations were tested in a checkerboard dilution against the azole-resistant strains, the interaction was defined as indifferent (FICI = 2) for all three drug combinations, as the MEC of each drug was unchanged when used alone and in combination (Fig. 2B). However, considering the cutoff MIC, the interaction of geldanamycin with caspofungin or FK506 was described as synergistic (FICI < 0.5) for the majority of the azole-resistant strains, as an MIC (≥90% growth inhibition) was achieved for the combination and not for any single drug (Fig. 2B).
Fig 2.
Assessment of the drug interactions for the combinations of caspofungin and FK506, caspofungin and geldanamycin, and FK506 and geldanamycin against the AF293 (wild-type) strain and the resistant strains. The antifungal activity of both drugs was assessed visually according to the following criteria: no effect, morphological abnormalities (hyphal blunting and impaired branching) with <25% growth reduction, 25 to 50% growth reduction, 50 to 75% growth reduction, 75 to 90% growth reduction, and >90% growth reduction, as illustrated by the gradient of green colors from white (no activity) to dark green (>90% growth reduction). CSP, caspofungin; GDA, geldanamycin; FICI, fractional inhibitory concentration index; MEC, minimal effective concentration. (A) The greatest antifungal activity achieved with one single drug (FK506, caspofungin, or geldanamycin, as indicated) is shown for each strain and does not exceed 50 to 75% growth reduction (upper panel). When these drugs are combined at the concentrations associated with their maximal effect (caspofungin, 1 μg/ml; FK506, 0.1 μg/ml; geldanamycin, 4 μg/ml), only geldanamycin in combination with caspofungin or FK506 substantially increases activity, with near-complete growth inhibition (>90%) against the majority of the azole-resistant strains. The combination of caspofungin and FK506 is increasingly active against the wild-type strain AF293 but does not provide a substantial benefit against most of the resistant strains. (B) Results of the checkerboard dilution testing of all three drug combinations are shown for the representative multi-azole-resistant strain F16134. The same MEC cutoff is observed for each drug when used alone or in combination (0.5 μg/ml for caspofungin, 0.016 μg/ml for FK506, and 4 μg/ml for geldanamycin). On the basis of this cutoff (MEC), the interaction is described as indifferent for all three combinations (FICI [MEC] = 2). When a higher cutoff is considered (MIC), this level of activity is achieved only with the combination of geldanamycin with either caspofungin or FK506, defining these interactions as synergistic (in this case, the FICI is reported as <0.5, as it cannot be assessed mathematically in the absence of a defined MIC value for the single drugs).
Our results show for the first time that calcineurin inhibitors are effective against a variety of characterized multidrug-resistant clinical and laboratory A. fumigatus strains at FK506 concentrations that are clinically achievable in humans (25). The Hsp90 inhibitor geldanamycin had limited antifungal activity when used alone. However, the combination of geldanamycin with caspofungin or FK506 had the greatest antifungal activity against the azole-resistant strains, achieving a fungicidal activity, while the combination of geldanamycin and FK506 was the most active against the echinocandin-resistant strain. These findings highlight distinct and specific potential roles for targeting the Hsp90-calcineurin axis at different levels. The characterization of these interactions was variable according to the criteria used to define the in vitro antifungal activity of a drug, and the FICI values may not be appropriate to reflect the spectrum of activity of fungistatic antifungal drugs. The fact that synergism was able to be assessed for the requirement of a high cutoff such as that of the MIC, which is more likely to correlate with clinical efficacy, supports the potential benefit of these interactions. The limitation of this in vitro combination method to define drug interactions of antifungal agents has already been highlighted (18, 26), and in vivo studies are warranted to better define the role of these drug combinations for the treatment of invasive aspergillosis.
In conclusion, our results show that targeting the fungal Hsp90-calcineurin pathway may represent an alternative antifungal strategy to combat the increasing antifungal resistance emerging against two critical classes of agents. While the currently available echinocandin or triazole antifungals target the fungal cell wall or membrane, respectively, this novel approach focused on the calcineurin-Hsp90 axis may give rise to a new class of antifungals with a distinct mechanism of action. Both calcineurin and Hsp90 inhibitors have demonstrated their potential for application in humans. Calcineurin inhibitors are the mainstay of immunosuppressive therapy in transplant recipients, and the Hsp90 inhibitors 17-AAG and 17-DMAG have been tested in humans as anticancer therapy with promising results (27). However, their use as antifungal drugs is currently limited by their lack of fungal specificity, resulting in immunosuppressive effects (for calcineurin inhibitors) and low toxic thresholds (for Hsp90 inhibitors). The future of exploiting these important cell signaling pathways lies in the development of novel fungal-specific inhibitors of calcineurin and Hsp90 without their current mammalian cross-reactivity.
ACKNOWLEDGMENTS
F.L. is supported by the Swiss National Science Foundation (PASMP3-142746). W.J.S. is supported by NIH/NIAID (1R56AI077648-01A2). This work was partially funded through an Investigator-Initiated Studies Program from Merck to W.J.S.
Footnotes
Published ahead of print 19 November 2012
REFERENCES
- 1. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 2. Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg. Infect. Dis. 17:1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789–795 [DOI] [PubMed] [Google Scholar]
- 5. Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, Sable CA, Kartsonis NA, Ngai A, Taylor A, Patterson TF, Denning DW, Walsh TJ. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563–1571 [DOI] [PubMed] [Google Scholar]
- 6. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arendrup MC, Garcia-Effron G, Buzina W, Mortensen KL, Reiter N, Lundin C, Jensen HE, Lass-Florl C, Perlin DS, Bruun B. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J. Clin. Microbiol. 47:3323–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardiner RE, Souteropoulos P, Park S, Perlin DS. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 43(Suppl 1):S299–S305 [DOI] [PubMed] [Google Scholar]
- 10. Rocha EM, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:4174–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowen LE. 2008. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 6:187–198 [DOI] [PubMed] [Google Scholar]
- 12. Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 54:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fortwendel JR, Juvvadi PR, Pinchai N, Perfect BZ, Alspaugh JA, Perfect JR, Steinbach WJ. 2009. Differential effects of inhibiting chitin and 1,3-β-d-glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, Steinbach WJ. 2011. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 82:1235–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinbach WJ, Cramer RA, JR, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, JR, Heitman J, Perfect JR. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. 2012. Heat shock protein 90 (Hsp90) is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot. Cell 11:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. 2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51:313–316 [DOI] [PubMed] [Google Scholar]
- 18. Steinbach WJ, Schell WA, Blankenship JR, Onyewu C, Heitman J, Perfect JR. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J. Clin. Microbiol. 47:3142–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Monzon A, Cuenca-Estrella M. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 52:2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi—second edition. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22. Arikan S, Lozano-Chiu M, Paetznick V, Rex JH. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 24. da Silva Ferreira ME, Kress MR, Savoldi M, Goldman MH, Hartl A, Heinekamp T, Brakhage AA, Goldman GH. 2006. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Margreiter R. 2002. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet 359:741–746 [DOI] [PubMed] [Google Scholar]
- 26. Meletiadis J, Verweij PE, TeDorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med. Mycol. 43:133–152 [DOI] [PubMed] [Google Scholar]
- 27. Trepel J, Mollapour M, Giaccone G, Neckers L. 2010. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]