Abstract
Neurocysticercosis (NCC), an infection of the brain with the larval stage of the Taenia solium tapeworm, is responsible for an estimated one-third of adult-onset epilepsy cases in regions of the world where it is endemic. Currently, anthelmintic drugs used for treatment of NCC are only partially effective, and there is, therefore, a pressing need for new therapeutic agents. Discovery of new anthelmintics with activity against T. solium has been limited by the lack of suitable sensitive assays that allow high-throughput screening. Using an in vitro culture system with Taenia crassiceps metacestodes, we demonstrate that changes in secretion of parasite-associated alkaline phosphatase (AP) and phosphoglucose isomerase (PGI) can be used to detect and quantify anthelmintic effects of praziquantel (PZQ), a drug with activity against T. solium. We applied two enzyme release assays to screen for anti-T. crassiceps activity in nonconventional antiparasitic drugs and demonstrate that nitazoxanide and artesunate induced release of both AP and PGI in differing time- and dose-related patterns. Furthermore, imatinib, a tyrosine kinase inhibitor previously reported to have parasiticidal activity against Schistosoma mansoni, also induced release of both AP and PGI in a dose-dependent manner, similar in pattern to that observed with the other anthelmintics. We also evaluated release of ATP into cyst supernatants as an indicator of drug effects but did not see any differences between treated and untreated cysts. These data provide the basis for rapid and quantitative screening assays for testing for anthelmintic activity in candidate anticestode agents.
INTRODUCTION
Cysticercosis and echinococcosis are neglected diseases caused by infections of the larval cystic forms of the tapeworms Taenia solium and Echinococcus granulosus (or related tapeworms). Both cause considerable morbidity and mortality in humans worldwide by different pathological mechanisms. Disease caused by E. granulosus is due to large cysts that mostly lodge in the liver or lungs, giving rise to symptoms caused by their large size or to signs and symptoms related to rupture (1). Most disease associated with T. solium cysts is due to signs and symptoms caused by infection of the brain and inflammatory responses to degenerating cysts.
Praziquantel (PZQ) and albendazole were initially developed in the 1970s and 1980s to treat schistosomiasis and intestinal helminths, respectively (2, 3), and later, both were found to be useful in the treatment of neurocysticercosis (NCC) and albendazole was found to be useful against Echinococcus spp. Even though these drugs revolutionized the treatment of both infections, they are only partially effective. Cure rates of parenchymal NCC are about 40 to 50% at the recommended dosing, and complicated disease commonly requires repeated courses of treatment lasting months to years (3). Treatment of E. granulosus with albendazole, although not as well studied, appears to be less effective, requiring months of therapy and being most efficacious in the treatment of small or young cysts (4). Furthermore, liver toxicities sometimes limit the use of albendazole. Therefore, there is a clear need to identify more efficacious and safer drugs.
There is no easy, reliable, or high-throughput method to test for drugs active against metacestodes of the two major cestode pathogens of humans, Echinococcus granulosus and Taenia solium, in vitro. However, there has been considerable progress using in vitro-produced metacestodes of Echinococcus multilocularis, an important but less prevalent human pathogen (5). Under very specialized conditions, metacestodes can be produced in vitro and used for drug testing in vitro using release of cestode larva parasite enzymes as measures of drug damage (6). Nevertheless, there are significant differences in the biology of E. multilocularis and that of T. solium as well as in drugs useful for the treatment of T. solium infections. There are major impediments to screening for anthelmintic activity against T. solium, including an inability to obtain a sufficient number of cysts, the targets of chemotherapy, the variable biology of cysts, imperfect culture conditions, and nonquantitative endpoints for the conventional detection systems. Notably, there are no standard biochemical or morphological endpoints defined that can be used in every system. Release of cestode larva parasite enzymes and antigens has recently been employed as a measure of drug damage to T. solium (7). However, cysts of T. solium are not generally available, since they require special resources and techniques to be obtained for laboratory testing. Therefore, they cannot be easily adapted for screening assays.
The cystic larval form of Taenia crassiceps, a tapeworm of carnivores with an intermediate cystic form in rodents, has been used as a model for cestode infections (8). Since the larval form multiplies in rodents by budding, the cysts are easily available for testing of drug effects. However, to date, effects of anthelmintics on T. crassiceps have been tested only with morphological endpoints, which are nonquantitative and time-consuming. Here, we report the first results from application of a number of biochemical assays for the testing of known or potential cysticidal compounds active against T. crassiceps cysts. These biochemical assays of drug effects may prove useful for screening of new anthelmintic drugs in a rapid and reproducible manner and may expedite the development of new anticestode drugs.
MATERIALS AND METHODS
Parasites and in vitro culture of cysts.
Metacestodes of T. crassiceps (ORF strain), a kind gift from Prema Robinson (Baylor University, Houston, TX), were propagated in vivo by serially passaging cysts with intraperitoneal injection into T and B cell-deficient, T cell receptor (TCR)-α-knockout mice (Jackson strain 2116; Jackson ImmunoResearch, Bar Harbor, ME). The protocol for maintaining T. crassiceps in mice was compliant with the Animal Welfare Act, the Public Health Service Animal Welfare Policy, the policies and procedures of Baylor College of Medicine (Animal Welfare Assurance no. A3823-01), and the National Institute of Allergy and Infectious Diseases Institutional Animal Care and Use Committee (NIAID IACUC). The NIAID IACUC has approved the animal rearing and experimental methods for maintaining parasites in animals. Parasites were harvested by peritoneal lavage, washed 6 times in phosphate-buffered saline (PBS; pH 7.4; Gibco-Invitrogen, Gaithersburg, MD), and maintained at 37°C in 5% CO2 in culture medium consisting of Dulbecco modified Eagle medium (DMEM) supplemented with glucose (4 g/liter), heat-inactivated fetal bovine serum (10%, wt/vol; Thermo Scientific Inc.), and HEPES buffer (10 mM), sodium bicarbonate (1 mM), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) (all purchased from Gibco-Invitrogen, MD). Under these culture conditions, these laboratory-adapted cysts lack scolices and remain viable for weeks but do not multiply.
Drugs.
All drugs were purchased in powder form from commercial sources. Albendazole sulfoxide (ABZSO), the active metabolite of albendazole, was purchased from Toronto Research Chemicals (North York, Ontario, Canada); praziquantel (PZQ) was from Sigma (St. Louis, MO); and imatinib, nitazoxanide, artemisinin, and artesunate were from Santa Cruz Biotechnology (Santa Cruz, CA). Drugs were dissolved in PBS or dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) per manufacturers' recommendations and stored at −80°C. All drugs were diluted in culture medium.
In vitro culture and drug treatment of cysts.
For in vitro drug treatment, 10 T. crassiceps cysts were incubated in 1 ml culture medium per well in 24-well plates. Drugs (ABZSO, PZQ, etc.) were added to achieve concentrations ranging from 1 to 500 ng/ml. The dose ranges of the drugs were based on published studies reporting either in vitro or serum levels of these drugs in humans (6, 7, 9–11). Control wells had medium with drug solvent (DMSO) at the highest concentration employed for each drug assay. Plates were incubated for 4 to 10 days at 37°C in 5% CO2. Every 24 h, the supernatant from each well was harvested and replaced with the same amount of medium containing the same drug and concentration. Each condition was evaluated in triplicate. This allowed calculation of rates of enzyme secretion every 24 h.
Measurement of AP secretion.
Alkaline phosphatase (AP) activity in cyst culture supernatants was measured using the alkaline phosphatase substrate p-nitrophenyl phosphate (PNPP; Sigma, St. Louis, MO) as reported previously (7). Briefly, 30 μl of cyst culture supernatant from each well was added to 170 μl of PNPP substrate, prepared according to manufacturer's instructions, by dissolving one PNPP tablet in 5 ml of sodium carbonate buffer (pH 9.6). Plates were incubated at 37°C for 15 min, and absorbance at 405 nm was determined using an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, Downingtown, PA). Culture medium incubated without parasites and freshly prepared medium were included as controls. Results are compared to those for absorbance with medium alone, using the following formula: % of control = (optical density [OD] of medium with parasite and drug − OD of medium alone)/(OD of medium with untreated parasite − OD of medium alone) × 100.
Measurement of PGI secretion.
Phosphoglucose isomerase (PGI) activity in supernatants was determined according to previously published methods (5) and by using reagents from commercial suppliers (Sigma, St. Louis, MO). Briefly, 95 μl of a glucose-6-phosphate (G6P) dehydrogenase buffer (100 mM Tris-HCl, 0.5 mM NAD, 2 mM EDTA, 1 U glucose-6-phosphate dehydrogenase) was added to 20 μl of supernatant in triplicate wells of 96-well plates. The reaction was initiated with the addition of 5 μl fructose-6-phosphate to a final concentration of 1 mM. NADH formation was measured by reading absorbance at 340 nm at several time points between 0 and 30 min. Culture medium incubated without parasites or freshly prepared medium was included as a control. Results are expressed as corrected OD: corrected OD = (OD test sample 30 min − OD test sample 0 min) − (OD medium blank 30 min − OD medium blank 0 min).
Measurement of ATP.
ATP was measured using a kit, ATPlite (PerkinElmer, Bridgeville, PA) in supernatants that were either used immediately after collection or stored at −20°C until analysis. The substrate vial and buffer solution provided were warmed to room temperature, and buffer was added to substrate (10 ml/vial) and mixed. To measure ATP concentrations, 100 μl of the reconstituted reagent was added to each well containing 100 μl of culture supernatant. The plates were incubated for 2 min with shaking (700 rpm). The plates were then dark adapted for 10 min to reduce plate autophosphorescence, and the luminescence was measured in a luminometer (Victor V multilabel counter; PerkinElmer, Boston, MA). Freshly prepared and/or cultured medium incubated without parasites was used as controls. Results are expressed as percentage of control, determined by the following formula: % of control = {[luminescence units (LU) of test sample − LU of medium without parasites]/(LU of medium with untreated parasite − LU of medium alone)} × 100.
Statistical analysis.
Nonparametric statistical tests, the Mann-Whitney U test (for two-way comparisons) and the Kruskal-Wallis test for multiple conditions, were used for comparison of data between test drugs and conditions. P < 0.05 was the standard criterion for designation of statistical significance for all comparisons.
RESULTS
Selection of AP and PGI release for detection of drug anthelmintic effects on T. crassiceps cysts.
Alterations in secretion as reliable measures of drug activity were first tested using the AP, ATP, and PGI assays with two proven and commonly used drugs with anticestode activity, ABZSO and PZQ. Release of ATP upon exposure to both drugs failed to show significant changes, and therefore, the ATP assay was deemed unsuitable as a screening test (data not shown) and subsequent experiments were performed with the AP and PGI assays.
Effects of conventional anthelmintics on AP and PGI release by T. crassiceps.
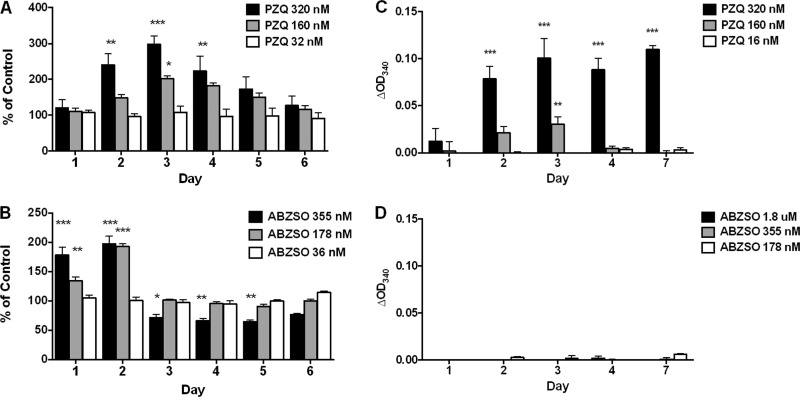
Upon exposure of T. crassiceps cysts to ABZSO, there was a dose-dependent increase in AP release into the supernatant at 178 nM and 355 nM on days 1 and 2 followed by a significant decrease in release to below-baseline levels at 355 nM on days 3, 4, and 5 (Fig. 1). No significant changes were seen at 36 nM. In contrast, for PZQ, there was a dose-dependent increase in release of AP that, though maximal on day 3, was significantly increased over that for untreated cysts on days 2, 3, and 4. Thereafter, values gradually decreased and were similar to day 1 values by day 6. As with ABZSO, no significant changes were noted at 32 nM of PZQ during the course of the experiment. The patterns of release, consisting of an increase followed by a decrease to normal or below-normal levels, were similar between the two drugs (Fig. 1).
Fig 1.
Effects of PZQ and ABZSO on in vitro release/secretion of AP and PGI by T. crassiceps. T. crassiceps metacestodes were cultured in vitro in the indicated concentrations of PZQ (A and C) or ABZSO (B and D) for 6 days. Supernatants collected (and replaced) every 24 h were tested for AP concentrations (A and B) or PGI activity (C and D) (see Materials and Methods). Data are shown as percentages of untreated parasites at the corresponding culture time point (A) or as optical density at 340 nm (OD340) corrected for wells with no drug (medium alone) and are representative of three (repeated) experiments. Molar concentrations of drugs provided in the graph legends correspond to 10 ng/ml, 50 ng/ml, and 100 ng/ml for PZQ and 10 ng/ml, 50 ng/ml, 100 ng/ml, and 500 ng/ml for ABZSO. Significant differences from untreated cysts are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.005).
Unlike AP, PGI is not normally released into supernatant during culture. However, significant changes in PGI release were induced by exposure to PZQ but not following ABZSO exposure. Because OD values were small (albeit reproducible), they are not shown as a percentage of control. There was a statistically significant increase in PGI on and after day 2 at 320 nM and on day 3 at 160 nM, indicating an increase in effect with dose (Fig. 1). Therefore, the T. crassiceps culture system, using changes in AP and PGI drug-induced release, allowed detection and quantification of drug activity for known anticestode agents.
Additive effect/synergy between conventional anthelmintic drugs as revealed by in vitro assays.
To determine if synergy or additive effects could be detected between the two conventional anti-Taenia drugs, ABZSO and PZQ, both ABZSO and PZQ were tested in combination. The combination of 16 nM PZQ and 1.8 μM ABZSO showed additive effects for AP on day 2 (Fig. 2A). Because no PGI secretion was detected using 360 nM albendazole, a higher dose of 1.8 μM was employed along with a suboptimal dose of PZQ. Significant synergistic activity was seen on days 2 and 3 (Fig. 2B), which is similar to synergistic activity previously reported using T. solium cysts (7). Interestingly, in comparison to similar experiments using T. solium cysts in which 180 nM of ABZSO showed significant drug activity, no activity was detected using T. crassiceps even with ABZSO concentrations as high as 9 μM for either AP or PGI (Fig. 2).
Fig 2.
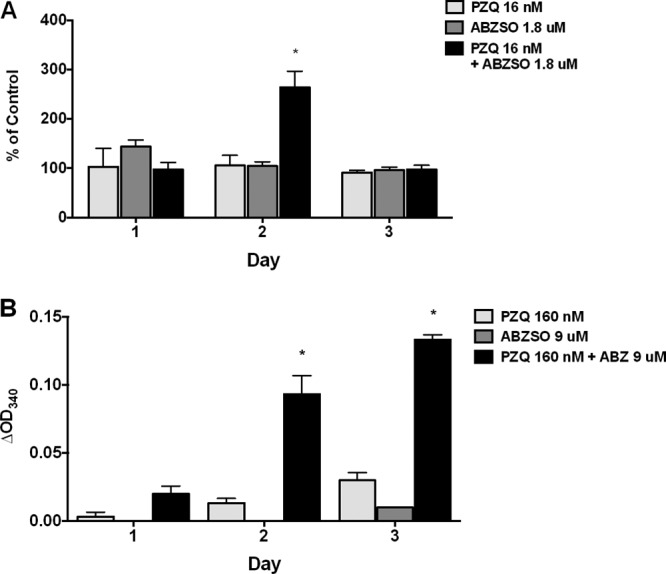
Additive/synergistic effects of PZQ and ABZSO on in vitro release/secretion of AP and PGI by T. crassiceps. T. crassiceps metacestodes were cultured in vitro in the indicated concentrations of PZQ or ABZSO either alone or together, for 3 days. Supernatants collected (and replaced) every 24 h were tested for AP (A) or PGI (B) activity (see Materials and Methods). Data are shown as percentages of corresponding untreated parasites at each time point (for AP) or as OD340 corrected for untreated cysts (for PGI). Molar concentrations provided in graph legends correspond to 5 ng/ml (A) and 50 ng/ml (B) for PZQ and 100 ng/ml (A) and 500 ng/ml (B) for ABZSO. Significant differences from untreated cysts are indicated by asterisks (*, P < 0.005). Data shown are representative of two (repeated) experiments.
Anthelmintic effects of nitazoxanide, artesunate, and imatinib on T. crassiceps demonstrated by AP and PGI assays.
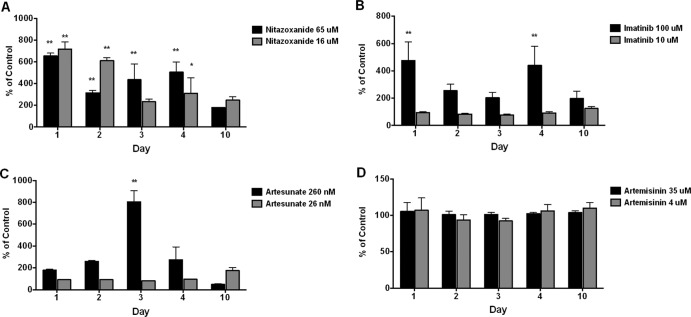
Other commercially available drugs not conventionally used for treatment of cestode infections but with potential anthelmintic activity against Taenia (12–14) were also tested using both the AP and PGI assays. Imatinib, a tyrosine kinase (TK6) inhibitor recently demonstrated to have parasiticidal activity against schistosomes in vitro and in prevention of fibrosis in vivo in a mouse model of schistosomiasis (9, 15), showed a significant increase in release of AP (Fig. 3) and PGI (Fig. 4) at 100 μM but not at 10 μM. AP release was generally increased at all time periods but was significantly increased over controls only at days 1 and 4 (Fig. 3). PGI release was delayed until days 3 to 5, maximal on day 4, and somewhat decreased on day 10 (Fig. 4).
Fig 3.
Effects of nitazoxanide, artemisinin, artesunate, and imatinib on in vitro release/secretion of AP by T. crassiceps. T. crassiceps metacestodes cultured in vitro were exposed to the indicated concentrations of nitazoxanide (A), imatinib (B), artesunate (C), or artemisinin (D), at the indicated concentrations for up to 10 days. Supernatants collected (and replaced) every 24 h were tested for AP activity (see Materials and Methods). Data are shown as percentages of corresponding untreated parasites at each time point. Molar concentrations provided in graph legends correspond to 5 μg/ml and 20 μg/ml for nitazoxanide, 6 μg/ml and 60 μg/ml for imatinib, 10 ng/ml and 100 ng/ml for artesunate, and 1 μg/ml and 10 μg/ml for artemisinin. Significant differences from untreated cysts are indicated by asterisks (*, P < 0.05; **, P < 0.01). Data shown are representative of two replicated experiments.
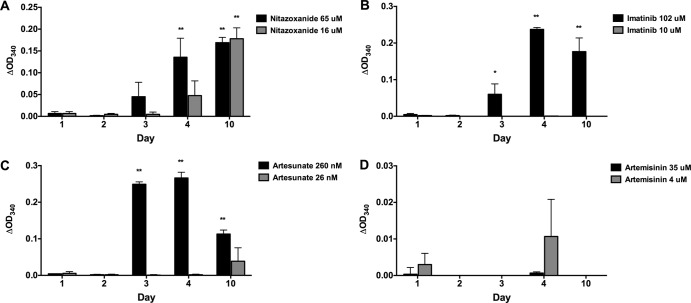
Fig 4.
Effects of nitazoxanide, artemisinin, artesunate, and imatinib on in vitro release/secretion of PGI by T. crassiceps. T. crassiceps metacestodes cultured in vitro were exposed to the indicated concentrations of nitazoxanide (A), imatinib (B), artesunate (C), or artemisinin (D) at the indicated concentrations for up to 10 days. Supernatants collected (and replaced) every 24 h were tested for PGI activity (see Materials and Methods). Representative data from two replicated experiments are shown as OD340 corrected for untreated cysts at the corresponding culture time point. Molar concentrations provided in graph legends correspond to 5 μg/ml and 20 μg/ml for nitazoxanide, 6 μg/ml and 60 μg/ml for imatinib, 10 ng/ml and 100 ng/ml for artesunate, and 1 μg/ml and 10 μg/ml for artemisinin. Significant differences from untreated cysts are indicated by asterisks (*, P < 0.05; **, P < 0.01).
Artesunate was previously shown to have some in vitro activity against E. granulosus and E. multilocularis metacestodes and enhancement of anthelmintic activity of ABZSO in vivo (13). In our experiments, AP release was significantly increased on day 3 with 260 nM of artesunate. Similar to the pattern of PGI release seen with imatinib, there was no release on days 1 and 2 but PGI activity was significantly increased on days 3, 4, and 10 at the same concentration of 260 nM.
Nitazoxanide, a broad-spectrum compound with prior known anticestode activity, showed significantly increased release with both assays (Fig. 3 and 4). AP release with nitazoxanide was significantly higher than that of controls at both concentrations on days 1 to 4 but was greatest on day 1 and lowest on day 10 (Fig. 3). PGI release was initially noted on day 3 at 65 μM and increased to significant levels at 4 and 10 days (Fig. 4). Increased release of PGI at 16 μM was initially noted on day 4 but became significant on day 10. In contrast, no significant changes were seen with either assay (AP or PGI) for artemisinin at 4 μM and 36 μM.
DISCUSSION
The ability to find new and better drugs for treatment of Taenia cestode infections suffers greatly from an inability to develop easy, reliable, low-cost assays. Macroscopic cysts obtained from naturally infected or experimentally infected animals are required (tissue culture cells from cestodes have never been successfully produced). Although the use of T. crassiceps as an experimental model provides, at best, a partial remedy, the ability to utilize T. crassiceps is important because the larval form, which causes disease in animals but rarely in humans, is readily obtained by passage in the peritoneum of mice. The only other system to obtain large number of metacestodes is a recently developed cocultivation method using hepatoma cells and E. multilocularis metacestodes (5, 16). Unfortunately, although T. crassiceps cysts can be obtained relatively easily, the number of cysts that can be harvested is limited by the numbers of mice infected and the number of cysts harvested from these animals. Although a significant or important advance compared to earlier assays, it still requires maintenance of infected mice and a considerable degree of individual manipulation.
The most significant findings in this study are that both conventional and potentially new anticestode drugs altered the pattern and quantity of release of both AP and PGI after exposure to T. crassiceps cysts. Consequently, quantification of both parasite-derived enzymes released into the supernatants can be used to determine drug effects and therefore can be used for drug screening. However, the patterns of release were complex and varied with the drug and the enzyme assayed. Because we measured rates of release (per 24 h), rather than cumulative concentrations of each enzyme over the course of the experiments, varying values over time are not surprising and likely reflect progressive inhibition, damage, or death of the cyst. Importantly, we observed increasing effects that were both dose and time dependent. Earlier studies correlated enzyme release with morphological changes and parasite death (6, 7). Microscopic examination of hematoxylin-eosin-stained parasites from day 3 cultures in the six drugs tested revealed degenerative changes on the surface and tegumentary and subtegumentary layers of T. crassiceps when exposed to PZQ, nitazoxanide, and imatinib (see Fig. S1 in the supplemental material), the three drugs with the greatest effects as determined by AP and PGI release. Artesunate exposure resulted in minimal tegumentary and surface damage compared to that with medium alone, and artemisinin and ABZSO showed no discernible damage to the surface structures and tegument (see Fig. S1).
Use of both AP and PGI as measures of drug effects on cestodes has been mostly limited to E. multilocularis (15), There are limitations in their general use. Both are pathogens of humans, and accidental injection may be infectious. While E. multilocularis can be passaged in mice, E. granulosus is available only from infected animals. As mentioned above, metacestodes can be grown in vitro using a rigorous culture system. More importantly, Echinococcus has a very different biology from that of Taenia spp., and therefore, our results are likely to be more relevant to the latter.
The present study is an extension of a similar study published recently in which T. solium cysts were used (7). The pattern of AP excretion in that study differed dramatically from that seen with T. crassiceps in this study. In contrast to previous patterns of AP release in Echinococcus spp. (6, 7) and T. crassiceps (shown here), release of AP from T. solium is constitutive and increases exponentially over time. Exposure to ABZSO and PZQ led to an inhibition of release rather than an increase in AP activity, as was seen here using T. crassiceps. There are a number of potential reasons for these differences, including continued development and frequent evagination of T. solium after extended culture. Notably, both parasites demonstrated effects of known anticestode drugs, ABZSO and PZQ, over roughly similar concentrations and both were able to show some degree of additive and/or synergistic effects in combination.
In comparing the two enzyme assays, the use of PGI has the advantage of very low levels of release into the supernatants during non-drug-treated culture and superior reproducibility and accuracy. Assays of all the tested drugs with the exception of ABZSO showed a consistent increase of release over time and a clear dose response. The lack of effect of ABZSO on PGI and AP release may be due to a slower effect than that seen with PZQ. Indeed, after 5 days, responses to ABZSO were detected in another system, where T. solium cysts were used (7). In our hands, the ABZSO treatment resulted in low OD values in both assays. The low values precluded the use of OD ratios as a reliable measure of enzyme release. Previous studies, by aggregating the enzyme released over the whole period of culture, avoided this issue and may have thereby allowed detection of ABZSO effects (17, 18), although we believe that the rate of accumulation provides additional useful information about the effects of drugs on the parasite.
The patterns of release of the drugs may be telling. The lack of normal release of PGI indicates that it is in the parasite tissues, and release likely indicates damage. The early increase and rise in release of AP due to ABZSO and PZQ followed by an inhibition or normalization of release are consistent with damage and release of cytosolic AP and then more severe damage that limits normal release or exhaustion of AP. The rise of PGI release fits with severe damage and inability to secrete.
All but one of the drugs tested, imatinib, have been proposed to have anticestode activity in other in vitro or in vivo assays (13, 19–21). Nitazoxanide and artesunate showed effects on the release of both enzymes, which reinforces the utility of these assays for drug screening. The widespread use and availability of these drugs in most regions of endemicity and their relatively low toxicity make them attractive new anticestode drugs. Imatinib, a tyrosine kinase inhibitor, has been previously demonstrated to have activity in vitro against Schistosoma mansoni, which encodes a tyrosine kinase in its genome (9, 15). This drug has also been reported to have activity against the filarial parasite Brugia malayi (S. Bennuru, Laboratory of Parasitic Diseases, NIAID, personal communication). Its activity against T. crassiceps in our experiments suggests the presence of the same class of tyrosine kinases in this parasite. The genome of T. crassiceps remains to be sequenced, but its close biologic and immunological similarity to T. solium recommends a search for the targets of this drug in the latter parasite. Although the use of imatinib for treatment of this infection in developing nations is financially and logistically unfeasible, our experiments suggest that the target enzyme pathways could eventually be exploited with alternative drugs that are more amenable to use in these settings.
In conclusion, we have demonstrated, using an in vitro culture system with T. crassiceps, that AP and PGI serve as sensitive indicators of drug effects on cestode parasites and that these effects can be utilized with ease for screening of drugs for anthelmintic activity. Indeed, in our studies, imatinib and artesunate, drugs that are not traditionally considered to treat cestode infections, showed activities on T. crassiceps cysts that were similar to those seen with the cestocidal drugs ABZSO and PZQ. With some adaptation for high-throughput screening, these assays have promise for the screening and selection of sorely needed new anthelmintics for neglected diseases of considerable public health significance.
Supplementary Material
Footnotes
Published ahead of print 10 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01022-12.
REFERENCES
- 1. Brunetti E, Kern P, Vuitton DA. 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114:1–16 [DOI] [PubMed] [Google Scholar]
- 2. Horton J. 2002. Albendazole: a broad spectrum anthelminthic for treatment of individuals and populations. Curr. Opin. Infect. Dis. 15:599–608 [DOI] [PubMed] [Google Scholar]
- 3. Nash TE, Garcia HH. 2011. Diagnosis and treatment of neurocysticercosis. Nat. Rev. Neurol. 7:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunetti E, Junghanss T. 2009. Update on cystic hydatid disease. Curr. Opin. Infect. Dis. 22:497–502 [DOI] [PubMed] [Google Scholar]
- 5. Hemphill A, Stadelmann B, Scholl S, Muller J, Spiliotis M, Muller N, Gottstein B, Siles-Lucas M. 2010. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 137:569–587 [DOI] [PubMed] [Google Scholar]
- 6. Stadelmann B, Scholl S, Muller J, Hemphill A. 2010. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 65:512–519 [DOI] [PubMed] [Google Scholar]
- 7. Mahanty S, Paredes A, Marzal M, Gonzalez E, Rodriguez S, Dorny P, Guerra-Giraldez C, Garcia HH, Nash T. 2011. Sensitive in vitro system to assess morphological and biochemical effects of praziquantel and albendazole on Taenia solium cysts. Antimicrob. Agents Chemother. 55:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willms K, Zurabian R. 2010. Taenia crassiceps: in vivo and in vitro models. Parasitology 137:335–346 [DOI] [PubMed] [Google Scholar]
- 9. Beckmann S, Grevelding CG. 2010. Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int. J. Parasitol. 40:521–526 [DOI] [PubMed] [Google Scholar]
- 10. Rao RU, Huang Y, Fischer K, Fischer PU, Weil GJ. 2009. Brugia malayi: effects of nitazoxanide and tizoxanide on adult worms and microfilariae of filarial nematodes. Exp. Parasitol. 121:38–45 [DOI] [PubMed] [Google Scholar]
- 11. Stepniewska K, Taylor W, Sirima SB, Ouedraogo EB, Ouedraogo A, Gansane A, Simpson JA, Morgan CC, White NJ, Kiechel JR. 2009. Population pharmacokinetics of artesunate and amodiaquine in African children. Malar. J. 8:200 doi:10.1186/1475-2875-8-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palomares-Alonso F, Piliado JC, Palencia G, Ortiz-Plata A, Jung-Cook H. 2007. Efficacy of nitazoxanide, tizoxanide and tizoxanide/albendazole sulphoxide combination against Taenia crassiceps cysts. J. Antimicrob. Chemother. 59:212–218 [DOI] [PubMed] [Google Scholar]
- 13. Spicher M, Roethlisberger C, Lany C, Stadelmann B, Keiser J, Ortega-Mora LM, Gottstein B, Hemphill A. 2008. In vitro and in vivo treatments of echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrob. Agents Chemother. 52:3447–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winning A, Braslins P, McCarthy JS. 2009. Case report: nitazoxanide for treatment of refractory bony hydatid disease. Am. J. Trop. Med. Hyg. 80:176–178 [PubMed] [Google Scholar]
- 15. El-Agamy DS, Shebl AM, Said SA. 2011. Prevention and treatment of Schistosoma mansoni-induced liver fibrosis in mice. Inflammopharmacology 19:307–316 [DOI] [PubMed] [Google Scholar]
- 16. Jura H, Bader A, Hartmann M, Maschek H, Frosch M. 1996. Hepatic tissue culture model for study of host-parasite interactions in alveolar echinococcosis. Infect. Immun. 64:3484–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palomares F, Palencia G, Ambrosio JR, Ortiz A, Jung-Cook H. 2006. Evaluation of the efficacy of albendazole sulphoxide and praziquantel in combination on Taenia crassiceps cysts: in vitro studies. J. Antimicrob. Chemother. 57:482–488 [DOI] [PubMed] [Google Scholar]
- 18. Palomares F, Palencia G, Perez R, Gonzalez-Esquivel D, Castro N, Cook HJ. 2004. In vitro effects of albendazole sulfoxide and praziquantel against Taenia solium and Taenia crassiceps cysts. Antimicrob. Agents Chemother. 48:2302–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reuter S, Manfras B, Merkle M, Harter G, Kern P. 2006. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrob. Agents Chemother. 50:2966–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stadelmann B, Kuster T, Scholl S, Barna F, Kropf C, Keiser J, Boykin DW, Stephens CE, Hemphill A. 2011. In vitro efficacy of dicationic compounds and mefloquine enantiomers against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 55:4866–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stettler M, Fink R, Walker M, Gottstein B, Geary TG, Rossignol JF, Hemphill A. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.