Abstract
Existing antifungal agents are still confronted to activities limited to specific fungal species and to the development of resistance. Several improvements are possible either by tackling and overcoming resistance or exacerbating the activity of existing antifungal agents. In Candida glabrata, azole resistance is almost exclusively mediated by ABC transporters (including C. glabrata CDR1 [CgCDR1] and CgCDR2) via gain-of-function mutations in the transcriptional activator CgPDR1 or by mitochondrial dysfunctions. We also observed that azole resistance was correlating with increasing virulence and fitness of C. glabrata in animal models of infection. This observation motivated the re-exploitation of ABC transporter inhibitors as a possible therapeutic intervention to decrease not only the development of azole resistance but also to interfere with the virulence of C. glabrata. Milbemycins are known ABC transporter inhibitors, and here we used commercially available milbemycin A3/A4 oxim derivatives to verify this effect. As expected, the derivatives were inhibiting C. glabrata efflux with the highest activity for A3 oxim below 1 μg/ml. More surprising was that oxim derivatives had intrinsic fungicidal activity above 3.2 μg/ml, thus highlighting effects additional to the efflux inhibition. Similar values were obtained with C. albicans. Our data show that the fungicidal activity could be related to reactive oxygen species formation in these species. Transcriptional analysis performed both in C. glabrata and C. albicans exposed to A3 oxim highlighted a core of commonly regulated genes involved in stress responses, including genes involved in oxidoreductive processes, protein ubiquitination, and vesicle trafficking, as well as mitogen-activated protein kinases. However, the transcript profiles contained also species-specific signatures. Following these observations, experimental treatments of invasive infections were performed in mice treated with the commercial A3/A4 oxim preparation alone or in combination with fluconazole. Tissue burden analysis revealed that oxims on their own were able to decrease fungal burdens in both Candida species. In azole-resistant isolates, oxims acted synergistically in vivo with fluconazole to reduce fungal burden to levels of azole-susceptible isolates. In conclusion, we show here the potential of milbemycins not only as drug efflux inhibitors but also as effective fungal growth inhibitors in C. glabrata and C. albicans.
INTRODUCTION
Superficial and life-threatening systemic fungal infections have been increasing over the last 2 decades. The majority of systemic fungal infections are caused by Candida, Aspergillus, and Cryptococcus species, but Candida albicans and non-albicans Candida species still account for most of the infections. A few treatment options exist in medical practice, including the use of at least four antifungal chemical classes (azoles, candins, pyrimidine analogues, and polyenes). Emergence of antifungal resistance is a consequence of long-term use of these agents, which is occurring in most immunocompromised patients with HIV or undergoing organ transplants or cancer chemotherapy (1). Clinical criteria can define antifungal resistance, and this has been achieved by the setting of Clinical Break Points (CPB) which indicate a drug concentration for a given fungal pathogen above/under which failure/success of a therapy can be expected (2). For example and according to these criteria, antifungal resistance for azoles is currently the highest for C. glabrata among other Candida spp. and accounts for 10 to 20% of the C. glabrata population (3, 4). This yeast species is ranked as second after C. albicans among bloodstream isolates. Recent studies report in several institutions an epidemiological shift of C. glabrata at the expense of C. albicans, but the reasons behind this phenomenon are still unexplained (4).
Antifungal resistance involves different mechanisms, including principally enhanced drug efflux and target alterations by active-site mutations or overexpression (5). In C. glabrata, one of the prominent resistance mechanisms invokes the participation of multidrug transporters of the ABC transporter family (CgCDR1, CgCDR2, and CgSNQ2) (6–8). We and others have found that these transporters are upregulated in azole-resistant isolates with an MIC higher than 16 μg/ml for fluconazole. The upregulation is associated with mutations, so-called gain-of-function (GOF) mutations, in a transcription factor of the Zn2-Cys6 family, CgPDR1 (1, 9–13). In C. albicans, antifungal resistance is multifactorial and involves the participation of efflux transporters, target mutations, compensatory mutations, and genome rearrangements (5). As in the case of C. glabrata, mutations in the transcription factors TAC1, MRR1, and UPC2 result in the upregulation of target genes participating to the development of azole resistance (14–18). The resistance levels achieved by C. glabrata and C. albicans address the need to overcome and avoid this phenomenon. Several concepts have been proposed in the past and utilize as basic principle the combination of one antifungal with another compound in order to increase antifungal activity (19, 20). Given the importance of ABC-transporters for the development of azole resistance both in C. glabrata and C. albicans, one possible option to tackle resistance could be the use of transporter inhibitors. In cancer research, human P-gp, which are functional homologs of fungal ABC transporters, are important mediators of resistance to many anticancer drugs. A wide range of P-gp-inhibitory compounds identified include natural and synthetic polymers, P-gp substrates (such as FK506), calcium channel modulator (verapamil), calmodulin inhibitors, and quinine analogs (21). Consequently, implementing the concept of combination therapy using an efflux inhibitor in order to increase the activity of fluconazole, a drug still widely used to treat fungal infections, seems an adequate strategy for overcoming resistance development in C. glabrata.
Drug resistance acquisition can also be associated to fitness costs in several microbial systems (22). In C. glabrata, however, we found that azole resistance was on the contrary resulting in enhanced virulence and fitness in animal models. This feature was dependent on the presence of a GOF mutation in CgPDR1 (9). Recently, we found that this effect could be mediated partially by the ABC transporter CgCDR1, which is upregulated to variable levels in azole-resistant isolates in C. glabrata (23).
Since CgCDR1 plays an important role in the development of azole resistance and that it can also contribute to increase virulence and fitness of C. glabrata, we reasoned that ABC transporters inhibitors such as milbemycins could be of potential therapeutic interest. We demonstrate here that specific milbemycin derivatives not only exhibit expected activities as efflux inhibitors but also that they possess intrinsic antifungal and fungicidal activities. Here we show that experimental treatments of C. glabrata infections by combination therapy are feasible and expanded this idea to C. albicans infections. Lastly, we perform transcriptional profiling of both Candida species exposed to milbemycins in order to understand the basis for their unexpected antifungal activity.
MATERIALS AND METHODS
Strains, media, and drugs.
The C. albicans strains used in the present study are listed in Table 1. Yeast strains were grown in liquid YEPD complete medium (1% Bacto peptone [Difco], 0.5% yeast extract [Difco], 2% glucose [Fluka]). To grow the strains on solid media, 2% agar (Difco) was added. Escherichia coli DH5α was used as a host for plasmid construction and propagation. DH5α cells were grown in Luria-Bertani (LB) broth or on LB plates, which were supplemented with ampicillin (0.1 mg/ml) when required. Fluconazole was obtained from Sigma. Milbemycins were obtained from Novartis Animal Health (Basel, Switzerland).
Table 1.
Strains used in this study
| Isolate | Characteristics | Fluconazole MIC (μg/ml)a | Source or reference |
|---|---|---|---|
| SC5314 | Wild type C. albicans isolate | 0.25 | 24 |
| DSY294 | Clinical C. albicans isolate, azole susceptible | 0.5 | 25 |
| DSY296 | Clinical C. albicans isolate, azole resistant | 128 | 25 |
| DSY2321 | Clinical C. albicans isolate, azole susceptible | 0.25 | 26 |
| DSY2323 | Clinical C. albicans isolate, azole resistant | 32 | 26 |
| DSY741 | Clinical C. albicans isolate, azole susceptible | 0.25 | 27 |
| DSY742 | Clinical C. albicans isolate, azole resistant | 16 | 27 |
| DSY562 | Clinical C. glabrata isolate, azole susceptible | 4 | 9 |
| DSY565 | Clinical C. glabrata isolate, azole resistant | 128 | 9 |
| DSY529 | Clinical C. glabrata isolate, azole susceptible | 8 | 6 |
| DSY530 | Clinical C. glabrata isolate, azole resistant | 128 | 6 |
| DSY726 | Clinical C. glabrata isolate, azole susceptible | 4 | 9 |
| DSY727 | Clinical C. glabrata isolate, azole resistant | 128 | 9 |
MICs were measured by the EUCAST protocol (28) as described in Materials and Methods.
Drug susceptibility testing.
Susceptibility assays were performed according to the standard broth microdilution protocols Edef. 7.1 (Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing [AFST-EUCAST]) (28). Briefly, serial 2-fold dilutions of fluconazole in RPMI 1640 broth (with l-glutamine, without bicarbonate and with phenol red as the pH indicator; Sigma), supplemented with 2%, (wt/vol) of d-glucose for Edef. 7.1, were distributed in 50-μl volumes at four times the final desired concentration into the wells of flat-bottom microtiter plates. Fluconazole final concentrations ranged from 128 to 0.25 μg/ml. Cell suspensions were prepared in sterile saline solution from overnight cultures of yeast strains at 35°C in Sabouraud dextrose agar plates. The suspensions were diluted in the test medium and added in 150-μl volumes to the drug solutions in the microtiter plates to yield final inoculum sizes of 0.5 to 2.5 × 105 cells/ml. Drug-free cultures and sterility controls were included in each microtiter plate. The plates were incubated at 35°C, and the MIC values were read after 24 h by measuring the absorbance using a spectrophotometric microdilution plate reader set at 450 nm. Checkerboard tests were performed with a similar procedure but including serial dilutions of milbemycins in a 50-μl volume at four times the final desired concentration for each fluconazole-containing well and with final concentrations ranging from 0.8 to 25.6 μg/ml. Milbemycins were first diluted in dimethyl sulfoxide (DMSO) and diluted accordingly. DMSO concentration in final suspensions was 0.8%. The cell suspensions were added in 100-μl volumes to the drug solutions to obtain final inoculum sizes of 0.5 to 2.5 × 105 cells/ml.
The fractional inhibitory concentration (FIC) index for a drug combination in the checkerboard method is the minimum ΣFIC obtained with the following equation: ΣFIC = FICA + FICB = (CFLC/MICFLC) + (CMil/MICMil), where MICFLC and MICMil are the MICs of fluconazole and the tested milbemycin when tested alone, and CFLC and CMil are the concentrations of fluconazole and the tested milbemycin resulting in at least 50% growth inhibition in a given well in the 96-well microtiter plate. Interactions were categorized according to the method of Te Dorsthorst et al. (29) by the following rules: synergism (FIC, ≤0.5), additivity (FIC, >0.5 to ≤1), and indifference (FIC, >1 to <4).
Efflux of rhodamine 6G.
A whole cells rhodamine 6G (R6G) efflux assay, adapted from a previously developed protocol, was used to measure the drug efflux capacity of C. albicans and C. glabrata isolates (30). Each fungal species required a specific procedure for cell preparation. For C. albicans, cell cultures grown overnight in YEPD were diluted in 5 ml of fresh medium and allowed to grow at 30°C under constant agitation to a density of 2 × 107 cells/ml. Cells were centrifuged, washed in 5 ml of phosphate-buffered saline (PBS; pH 7), and resuspended in 2 ml of PBS. These suspensions were incubated for 1 h at 30°C under constant agitation to energy-deprived cells, and R6G was next added at a concentration of 10 μg/ml. The incubation was continued for 1 h to allow R6G accumulation. After this incubation time, cells were sedimented by centrifugation, washed with PBS at 4°C, and resuspended in a final volume of 300 μl of PBS. For C. glabrata, overnight cultures were diluted to 2 × 107 cells/ml in 50 ml of YEPD and agitated for 4 h at 30°C. Cells were next washed twice with PBS and resuspended in PBS at a 2% (wet weight) concentration. R6G was added to a 10 μM end concentration together with 2 mM deoxyglucose. After 1 h of incubation at 30°C, the cells were washed twice with PBS and resuspended to a concentration of 108 cells/ml.
Fifty-microliter portions of individual suspensions were diluted in 150 μl of PBS and divided into aliquots into a 96-well microtiter plate, which was then placed in a SpectraMax Gemini fluorimeter (Molecular Devices, Sunnyvale, CA, USA) with a temperature control set at 30°C. Baseline emission of fluorescence (excitation wavelength, 344 nm; emission wavelength, 555 nm) was recorded for 5 min as relative fluorescence units (RFU), and d-glucose was next added to each strain at a final concentration of 1% (wt/vol) to initiate R6G efflux. As negative controls, no glucose was added to a series of separate aliquots of each strain. The data points were recorded in duplicate for 60 min in 1-min intervals.
Efflux inhibition with milbemycins was carried out by adding the different compounds to each yeast sample. The milbemycin-yeast cell mixture was incubated for 10 min at room temperature before initiation of efflux experiments.
MitoSOX Red staining.
Intracellular reactive oxygen species (ROS) production was examined using MitoSOX Red (Molecular Probes). MitoSOX Red is a lipid soluble cation that accumulates in the mitochondrial matrix, where it can be oxidized to a fluorescent product by superoxide. Yeast strains from initial concentration of 2 × 106 cells/ml were grown in RPMI medium containing 10 μg of A3Ox/ml. After 1 h of incubation, 500-μl aliquots were washed twice with PBS and incubated in the dark for 20 min in 2.5 μM MitoSOX Red. The cells were washed three times with PBS and resuspended in PBS, and the percentage of cells positively stained with MitoSOX Red was determined by fluorescence microscopy using a Zeiss Axioplan 2 fluorescence microscope. Images were recorded using a Visitron Systems HistoScope Camera and VisiView Imaging Software. Fluorescence of cells was also determined using a FLUOstar OMEGA microplate reader (BMG) with excitation and emission wavelengths of 510 and 580 nm, respectively.
Construction of Candida microarrays.
C. glabrata microarrays were designed according to the Agilent eArray Design guidelines as previously described (23), and custom arrays were manufactured in the 8×15 K format by Agilent Technologies. C. albicans microarrays were also manufactured by Agilent Technologies; however, the array design (design ID 037331) was obtained from Synnott et al. (31). A total of 6,101 genes (including 12 mitochondrial genes) are represented by two sets of probes, both spotted in duplicate. Four copies of each array were printed on a 4×44 K format.
cRNA synthesis, one-color labeling, and array hybridization.
Sample preparation was performed on biological quadruplicate cultures of the C. glabrata strain DSY562 and biological triplicate for the C. albicans strain SC5314. Log-phase cultures at 35°C with agitation in RPMI 1640 medium with 2% d-glucose were treated for 1 h in the same conditions with either 10 μg of milbemycin A3 oxim/ml or the DMSO solvent. Total RNA was extracted after mechanical disruption of the cells with glass beads by a phenol-chloroform-lithium chloride procedure, as previously described (32). The integrity of the input RNA template was determined prior to labeling or amplification using an Agilent RNA 6000 Nano LabChip kit and 2100 BioAnalyzer (Agilent Technologies). Agilent's One-Color Quick Amp labeling kit (Agilent Technologies) was used to generate fluorescent cRNA as previously described (32). Briefly, a spike mix and T7 promoter primers were added to 400 ng of total RNA from each sample. cDNA synthesis was promoted by Moloney murine leukemia virus reverse transcriptase in the presence of deoxynucleoside triphosphates and RNaseOUT. Next, cRNA was produced from this first reaction with T7 RNA polymerase, which simultaneously amplifies target material and incorporates cyanine 3-labeled CTP. The labeled cRNAs were purified with an RNeasy minikit (Qiagen) and quantified using a NanoDrop ND-1000 UV/VIS spectrophotometer. A total of 600 ng of Cy3-labeled cRNAs from each sample were fragmented and hybridized for 17 h at 65°C to specific subarrays of the 8×15 K or 4×44 K format using a gene expression hybridization kit (Agilent Technologies) and a gasket slide.
Microarray data analysis.
Slides were washed and processed according to the Agilent 60-mer Oligo microarray processing protocol and scanned on an Agilent microarray scanner G2565BA (Agilent Technologies). The data were extracted from the images with feature extraction (FE) software (Agilent Technologies). Each slide was processed with spike quality controls to establish the dynamic range of signals which fitted a minimum R2 value of 0.99. The FE software flags outlier features (the percentages ranged from 0.01 to 0.15% of signals overall for cRNA hybridizations) and detects and removes spatial gradients and local backgrounds. The data were normalized using a combined rank consistency filtering with Lowess intensity normalization. The gene expression values obtained with FE software were imported into GeneSpring 11 software (Agilent Technologies) for preprocessing and data analysis. For inter-array comparisons, a linear scaling of the data was performed using the quantile normalization of one-color signal values of noncontrol probes on the microarray. The expression of each gene was normalized by its median expression across all samples. Statistical analyses were performed using unpaired t tests and a corrected P value of 0.05 was chosen as the cutoff for significance. Changes in expression for each gene of at least 2-fold between treated and nontreated samples were considered significant. Microarray data can be retrieved at the Gene Expression Omnibus NCBI site under the accession number GSE40232. Validation of gene expression results from microarray analysis was performed by quantitative real-time PCR (qPCR) as detailed below.
qPCR.
RNA sample preparation was performed as described above for microarray experiments. RNA samples were treated with RNase-free DNase (DNA-free; Ambion) to remove any contaminating genomic DNA and reverse transcribed using random hexamers as the priming method (Transcriptor First-Strand cDNA synthesis kit; Roche). Expression levels of C. glabrata target genes (ERG4, MET3, YPS1, and YPS3) and the C. albicans genes (HXT6, WOR1, and OPT5) were determined by a SYBR green-based quantitative real-time PCR (MesaBlue qPCR kit for SYBR assay; Eurogentec) in a StepOne Plus real-time thermal cycler (Applied Biosystems). Primers used for YPS1 and YPS3 quantification were previously described (23). Primers used for quantification of the remaining genes are listed in Table 2. Relative gene expression in milbemycin A3 oxim-treated samples in comparison to nontreated controls was determined from CgTEF3-normalized expression levels for C. glabrata and ACT1 for C. albicans.
Table 2.
Primers usedin this study
| Primer | Sequence (5′-3′) | Source or reference |
|---|---|---|
| CgMET3-F | GGACGCCATCCAGCTGCCA | This study |
| CgMET3-R | AGCGGCACGCACGGTCAA | This study |
| CgERG4_F | ATTGACCCACTTGAAGA | This study |
| CgERG4_R | GGCACAAGTCATTATTCT | This study |
| CgTEF3-F | CGGTGGTAAGAAGAAGAA | This study |
| CgTEF3-R | AGAAACGTAAGCATCACC | This study |
| CgYPS1a | CCTCCGCCAGATTGTGGCATG | 23 |
| CgYPS1b | CTCGAACAGAGCCGGTTACTA | 23 |
| CgYPS3a | CGACGACCCATCCCCAGGCTC | 23 |
| CgYPS3b | ACTTAGCTCTTCATGGTAACG | 23 |
| CgACT1a | GTGTGAATAACCGCTGCGATACT | 23 |
| CgACT1b | TGCCCACCACTCCTAACTCATAAT | 23 |
| WOR1-qPCR-F | TCCCGACGACGAGTACGACCA | This study |
| WOR1-qPCR-R | CCAGTCGCAAGCAACATTGGACC | This study |
| TNA1-qPCR-F | TGCTGCCTCGGGAGCCATTG | This study |
| TNA1-qPCR-R | AGAATGGCGCAACCAGCAGTT | This study |
| OPT5-qPCR-F | TCTTGGGTTTGGGCTTTCTGGA | This study |
| OPT5-qPCR-R | TGCTTTCACTTCAGGCGACA | This study |
| HXT6-qPCR-F | GGGCCTTGTGTTTGCTTGGTGG | This study |
| HXT6-qPCR-R | TGCAGGGTCCTCTGGAGAAA | This study |
| ACT1-RT-F | GTTCCCAGGTATTGCTGAAC | 33 |
| ACT1-RT-R | CAATGGATGGACCAGATTCG | 33 |
Animal experiments.
Female BALB/c mice (20 to 25 g) were purchased from Harlan Italy S.r.l (San Pietro al Natisone, Udine, Italy) and inbred in-house. The mice were housed in filter-top cages with free access to food and water. To establish C. glabrata infection, mice were injected into their lateral vein with saline suspensions of the C. glabrata strains (each in a volume of 200 μl).
Groups of 10 mice were established for each yeast strain. For tissue burden experiments, immunocompetent mice were inoculated with 4 × 107 CFU of each C. glabrata strains and 7 × 104 CFU of each C. albicans strains. After 7 days, mice were sacrificed by use of CO2 inhalation, and target organs (spleen and kidney) were excised aseptically, weighted individually, and homogenized in sterile saline by using a Stomacher 80 device (Pbi International, Milan, Italy) for 120 s at high speed. Organ homogenates were diluted and plated onto YPD. Colonies were counted after 2 days of incubation at 30°C, and the numbers of CFU g of organ−1 were calculated. CFU counts were analyzed with nonparametric Wilcoxon rank-sum tests. A P value of <0.05 was considered to be significant.
The milbemycin injectable solution was prepared by adding 1 volume of PEG 400 to 2 volumes of a milbemycin DMSO solution (0.2 mg/ml) in order to obtain a 0.1-mg/ml solution with DMSO/PEG 400 ratio of 2:1. The DMSO/PEG solution was injected in a 100-μl volume intraperitoneally each day. Fluconazole was injected intraperitoneally at dosages of 100 mg/kg/day dosage for C. glabrata and of 3 mg/kg/day for C. albicans.
RESULTS
Inhibition profiles with milbemycins.
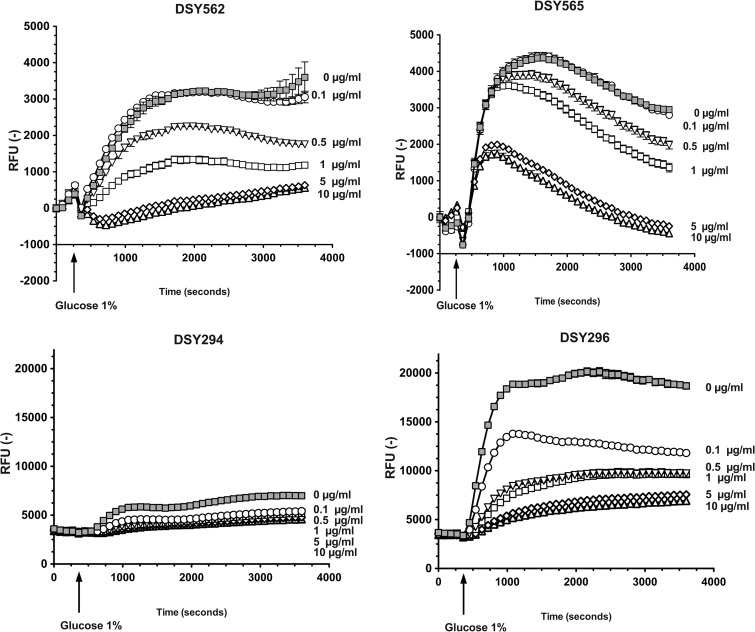
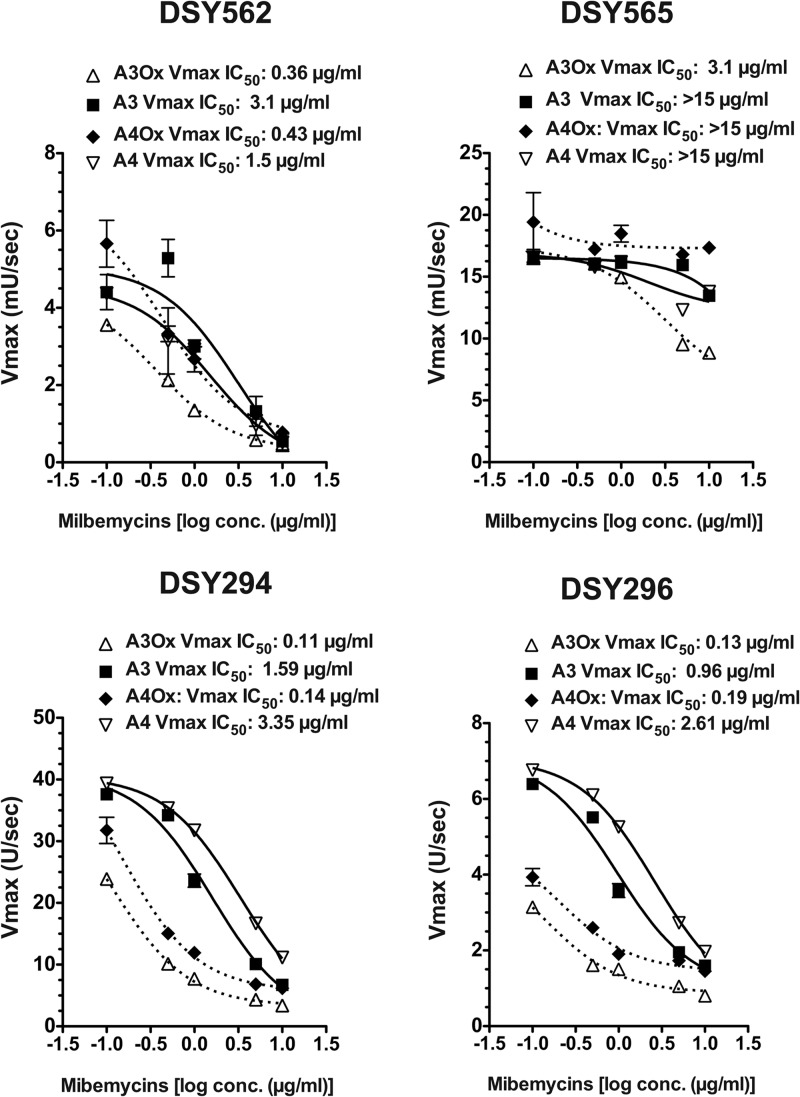
Several milbemycin derivatives are known to exert an inhibition of drug efflux in fungi by targeting the class of ABC transporters (21). Here, we tested the properties of two different milbemycin derivatives (milbemycin A3 and A4), as well as their oxim derivatives which are widely used in veterinary practice as antiparasitic drugs (34). We used an efflux assay based on fluorophore rhodamine 6G (R6G)-loaded cells and a real-time recording of effluxed compound. Milbemycins A3 and A4 (referred here as A3 and A4) as well as their oxim derivatives (referred here as A3Ox and A4Ox) were used at different concentrations to estimate their inhibition capacities. As observed in Fig. 1A and B, R6G efflux in the azole-susceptible isolate DSY562 and the azole-resistant isolate DSY565, respectively, could be stimulated by glucose addition but was gradually decreased with increasing concentrations of A3Ox, this derivative taken as an illustrative example. One can observe that A3Ox was more effective at inhibiting R6G efflux in DSY562 compared to DSY565. This difference reflects that DSY565 contains higher CgCDR1 levels compared to DSY562 and that higher A3Ox concentrations are needed to cause a decrease in effluxed R6G in DSY565. Additional experiments with a mutant lacking CgCDR1 (DSY1041) confirmed that the recorded signals were due to R6G efflux (data not shown). To estimate inhibition kinetics of the four different milbemycins in DSY562 and DSY565, Vmax of R6G efflux calculated from RFU values over time were plotted against inhibitor concentrations (Fig. 2). The obtained plots were used to deduce inhibitor concentrations decreasing Vmax values by 50% (IC50). The obtained IC50s with DSY562 showed that oxims derivatives were more potent than their parent compounds at inhibiting R6G efflux. A3Ox appeared as the most effective substance (Vmax IC50 = 0.36 μg/ml). IC50s obtained with DSY565 were higher than for DSY562. This could be expected since CgCDR1 is overexpressed in this isolate. However, A3Ox (Vmax IC50 = 3.1 μg/ml) was also the most potent inhibitor of R6G efflux for this isolate, although this value is 10-fold higher than in DSY562.
Fig 1.
Rhodamine 6G efflux inhibition in C. glabrata and C. albicans. R6G efflux was recorded over 1 h at regular intervals. Experiments were carried out in triplicates, and mean data (± the standard deviation [SD]) points are shown. Concentrations of A3Ox are shown next to each curve.
Fig 2.
Inhibition characteristics of milbemycins and their oxim derivatives in C. glabrata and C. albicans. IC50s were calculated using GraphPad Prism software.
The same procedures were used to characterize the properties of milbemycins with the azole-susceptible C. albicans isolate DSY294 and its azole-resistant parent DSY296. The azole-resistant isolate of this clinical pair upregulates the ABC transporters CDR1 and CDR2, which is one of the mechanisms contributing to azole resistance (25, 26). As observed in Fig. 1, milbemycins could decrease R6G efflux in a concentration-dependent manner. Vmax IC50s revealed that oxim derivatives possessed the highest inhibition capacity compared to parent compounds (Fig. 2), which was similar to that observed in C. glabrata.
Taken together, our results demonstrate that milbemycins A3 and A4 and their oxim derivatives were able to inhibit drug efflux in C. glabrata and C. albicans and that oxim derivatives exhibited the highest inhibitory capacity.
Combination of milbemycins with fluconazole in vitro.
Given that A3, A4, and their oxim derivatives can inhibit drug efflux, we expected that fluconazole MICs could be reduced in their presence. We therefore undertook checkerboard assays with fluconazole and each milbemycin compound to estimate fractional inhibitory concentration (FIC) indexes between both drugs. As shown in Fig. 3, we first observed that A3Ox had an antifungal effect on both C. glabrata DSY562 and DSY565 (MIC = 6.4 μg/ml, Table 3). This effect was less pronounced for the parent derivative A3 (MIC = 25.6 μg/ml, Table 3). A4Ox and A4 were less active than A3 derivatives, but again the A4Ox was the most active compound (MIC = 25.6 μg/ml, Table 3). FIC indexes were calculated for each drug combination (Table 4) and synergistic and additive effects were made visible in the checkerboard (Fig. 3: white area: synergistic interactions; red area: additive interactions). The 3-color heat map shown in Fig. 3 illustrates drug combinations resulting in cutoff 50% growth reduction (blue color) compared to drug-free growth (green color) and those with absence of growth (black color). Inspection of the data shows that milbemycin-fluconazole interactions were almost exclusively additive, with the exception of a synergistic combination with A3Ox in DSY562 (Fig. 3 and Table 4). We found the same intrinsic antifungal activity A3Ox and derivatives in two other pairs of clinical isolates (DSY529/DSY539 and DSY727/DSY728) that were described elsewhere (see Tables 1 and 3). In addition, A3Ox-fluconazole interaction patterns in these isolates were similar than those observed in DSY562/DSY565 (Table 4). Therefore, it is likely that milbemycins act by the same mechanisms in all clinical C. glabrata isolates.
Fig 3.
Three-color heat maps of checkerboard MIC tests of A3Ox and A3 with C. glabrata. Each box corresponds to relative growth (as compared to drug-free growth) resulting from a specific combination between milbemycins and fluconazole. White-delimited zones correspond to synergistic interactions (FIC index ≤ 0.5). Red-delimited zones correspond to additive interactions (FIC index > 0.5 and ≤ 1). Positive interactions (synergistic or additive) can be visualized when relative growth is ≤50% relative to control (blue to black colored wells). MICs were performed according to EUCAST protocols.
Table 3.
MICs of C.glabrata and C. albicans against fluconazole and milbemycins
| Isolate | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| Fluconazole | A3Ox | A4Ox | A3 | A4 | |
| C. glabrata | |||||
| DSY562 | 4 | 6.4 | 25.6 | >25.6 | >25.6 |
| DSY565 | 128 | 6.4 | 25.6 | 25.6 | >25.6 |
| DSY529 | 8 | 6.4 | 25.6 | >25.6 | >25.6 |
| DSY530 | 128 | 6.4 | 25.6 | 25.6 | >25.6 |
| DSY726 | 4 | 6.4 | 25.6 | 25.6 | >25.6 |
| DSY727 | 128 | 6.4 | 25.6 | >25.6 | >25.6 |
| C. albicans | |||||
| DSY294 | 0.5 | 6.4 | 6.4 | 25.6 | 25.6 |
| DSY296 | 128 | 6.4 | 3.2 | >25.6 | 12.8 |
| DSY2321 | 0.25 | 6.4 | 6.4 | 25.6 | 25.6 |
| DSY2323 | 32 | 6.4 | 3.2 | >25.6 | >25.6 |
| DSY741 | 0.25 | 6.4 | 6.4 | 25.6 | 25.6 |
| DSY742 | 16 | 6.4 | 3.2 | >25.6 | >25.6 |
| SC5314 | 0.25 | 6.4 | 6.4 | 25.6 | 25.6 |
Table 4.
FIC indexes of combination of fluconazole with milbemycins
| Isolate | Milbemycin derivativea |
|||||||
|---|---|---|---|---|---|---|---|---|
| A3Ox |
A4Ox |
A3 |
A4 |
|||||
| Index | Drug effect | Index | Drug effect | Index | Drug effect | Index | Drug effect | |
| C. glabrata | ||||||||
| DSY562 | 0.4 | Synergism | 0.8 | Additivity | 1 | Additivity | 1 | Additivity |
| DSY565 | 0.6 | Additivity | 0.4 | Synergism | 0.8 | Additivity | 0.6 | Additivity |
| DSY529 | 0.5 | Synergism | – | – | – | – | – | – |
| DSY530 | 0.6 | Additivity | – | – | – | – | – | – |
| DSY726 | 0.4 | Synergism | – | – | – | – | – | – |
| DSY727 | 0.6 | Additivity | – | – | – | – | – | – |
| C. albicans | ||||||||
| DSY294 | 0.4 | Synergism | 0.4 | Synergism | 0.3 | Synergism | 0.3 | Synergism |
| DSY296 | 0.1 | Synergism | 0.2 | Synergism | 0.6 | Additivity | 0.2 | Synergism |
| DSY2321 | 0.6 | Additivity | – | – | – | – | – | – |
| DSY2323 | 0.1 | Synergism | – | – | – | – | – | – |
| DSY741 | 0.6 | Additivity | – | – | – | – | – | – |
| DSY742 | 1.1 | Indifference | – | – | – | – | – | – |
Index, FIC indexes were calculated as described in Materials and Methods. Drug effect, interpretation corresponded to the following definitions: synergism, FIC index ≤ 0.5; additivity, FIC index >0.5 and ≤1; and indifference, FIC index >1 and <4. –, Not tested.
Milbemycin-fluconazole interactions were tested with the C. albicans strains DSY294 and DSY296 (Table 3 and 4). With C. albicans, both the A3Ox and A4Ox derivative exhibited higher activity than their parent compounds, which is slightly different from C. glabrata, in which only the A3Ox derivative was highly active. The A3Ox and A4Ox MICs in C. albicans had the same value as measured in C. glabrata (3.2–6.4 μg/ml). On the opposite to C. glabrata, the combination of milbemycins with fluconazole resulted mostly in synergistic interactions (Table 4). A3Ox and other derivatives had the same range of activity in other pairs of C. albicans clinical strains including DSY2321/DSY2323 and DSY741/DSY742 (see Tables 1 and 3). Interestingly, the A3Ox-fluconazole interaction was synergistic for DSY2323, while indifferent for DSY742 (Table 4). The likely explanation behind this opposite behavior is that, even if both isolates are azole-resistant, one (DSY2323) has acquired resistance by ABC transporter upregulation (26) while the other (DSY742) by MDR1 upregulation (27). Since A3Ox targets ABC transporters, it was expected that fluconazole potentiation could only be revealed in strains overexpressing this type of multidrug transporter.
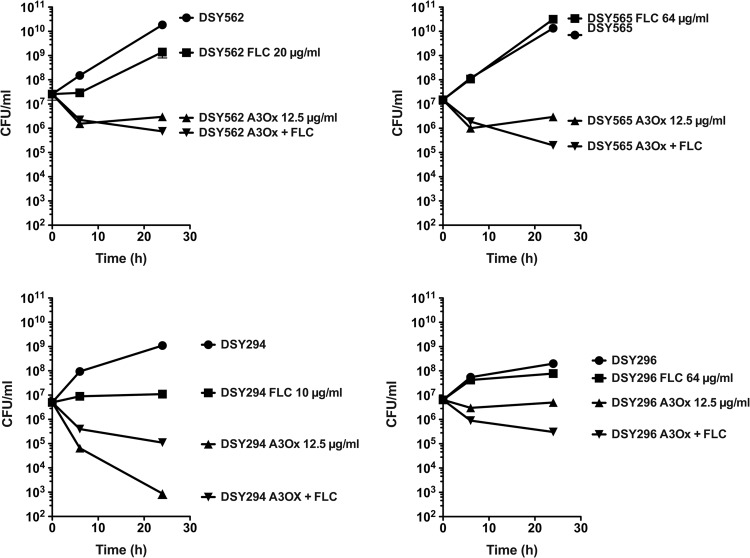
Milbemycin oxims are fungicidal.
Since A3Ox exhibits antifungal activity, we addressed whether this compound could decrease survival upon incubation of cells at the supra-MIC of 10 μg/ml. In C. glabrata, A3Ox exhibited a clear fungicidal effect: after 24 h of incubation survival of azole-susceptible and azole-resistant cells decreased by 95% compared to the initial inoculum (Fig. 4). Combining fluconazole with A3Ox slightly increased the fungicidal effect of milbemycin. A3Ox had a strong fungicidal effect on the C. albicans DSY294 isolate compared to C. glabrata and fluconazole increased this effect substantially. In the azole-resistant C. albicans isolate DSY296, A3Ox exhibited still a fungicidal effect (78% decrease in survival compared to inoculum), however it could be still increased by fluconazole, even though this azole had almost no inhibitory effect when used as single drug at the subinhibitory concentration of 64 μg/ml. In conclusion, our data show that A3Ox has fungicidal effect, that the extent of this effect is species-dependent and that fluconazole-A3Ox combinations enhance milbemycin fungicidal effect.
Fig 4.
Time-kill curves of A3Ox in C. glabrata and C. albicans. Each value is the mean of two separate experiments.
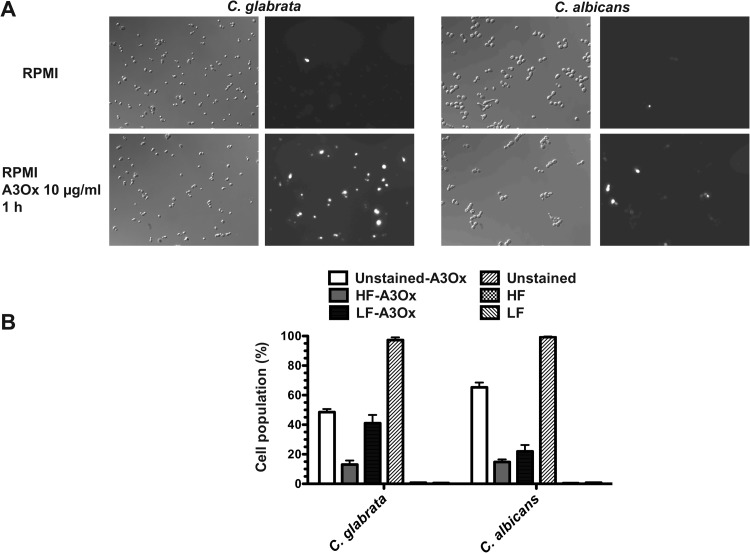
Given the fungicidal properties of A3Ox, we tested the formation of reactive oxygen species (ROS) during A3Ox exposure using MitoSOX Red, a specific indicator of ROS in mitochondria. As described by Batova et al. (35), the fluorescence intensity of this dye can also distinguish between dead cells (bright fluorescence) and actively ROS-producing cells (low fluorescence). Figure 5A illustrates that ROS could be detected both in C. glabrata and C. albicans. Quantification of fluorescence signals with MitoSOX Red (Fig. 5B) indicated that A3Ox treatments in both yeast species induced cell death and ROS production. Thus, it is likely that the fungicidal effect of A3Ox involves at some point the formation of ROS, which then could promote the killing of C. glabrata and C. albicans.
Fig 5.
ROS production following A3Ox treatment. (A) Microscopy pictures of C. glabrata and C. albicans under phase contrast and under fluorescence. The cells were incubated with RPMI or RPMI containing 10 μg of A3Ox/ml at 35°C under constant shaking. Next, the cells were stained with MitoSOX Red (2.5 μg/ml) for 20 min at room temperature, centrifuged, and washed two times with PBS and observed under microscopy. Cells with high fluorescence (HF) are dead cells, while those with low fluorescence (LF) produce ROS. (B) Quantification of MitoSOX Red staining. Approximately 200 cells were counted for each experimental condition (untreated and A3Ox treated).
Experimental treatment of invasive candidiasis with milbemycins.
Milbemycin oxims (A3Ox and A4Ox) are commercially available. The single substances in the mixture are usually contained in a 2.5:7.5 proportion. We used this commercial preparation since pure substances were available in limited quantities only. Given the efflux-inhibiting capacity of A3Ox and A4Ox, as well as the fungicidal properties of the A3Ox derivative, we tested their potential therapeutic effect in experimental infections in single treatment or in combination with fluconazole. The properties of milbemycin oxims could also have an impact on the virulence of C. glabrata, since ABC transporters participate to this phenotype, as described above-mentioned. Animal experiments were carried out with immunocompetent animals and with two different milbemycin regimens, i.e., 0.5 and 2.5 mg/kg/day. Fluconazole was used at a dosage of 100 mg/kg/day. Quantification of milbemycin serum levels at day 7 ranged from 5 to 10 μg/ml (see Fig. S1 in the supplemental material).
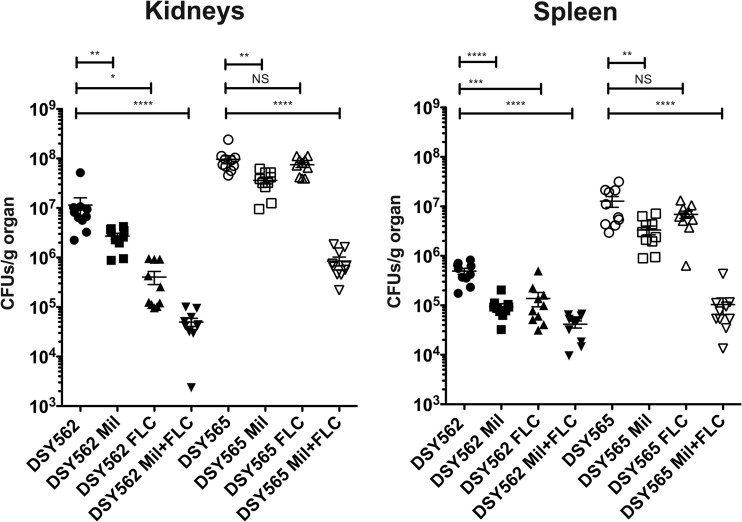
Figure 6 shows results of tissue burdens obtained after 7 days experiments in spleen and kidneys with a 2.5-mg/kg/day milbemycin regimen. Treatment at 0.5 mg/kg/day had similar effects (see Fig. S2 in the supplemental material). Single drug treatments had the following effects on tissue burden (including spleen and kidneys) in infected mice: (i) azole treatment significantly decreased CFU counts only in mice infected with the azole-susceptible isolate DSY562 and (ii) milbemycin treatment decreased CFU counts in mice infected with the two fungal isolates. Combined treatments increased both the milbemycin and fluconazole antifungal effect in animals infected with both strain types. Interestingly, milbemycin and fluconazole treatment reduced tissue burdens of animals infected with the azole-resistant isolate DSY565 to levels comparable to the azole-susceptible isolate DSY562, thus highlighting the power of drug combinations in this animal model.
Fig 6.
Fungal tissue burdens of mice infected with C. glabrata DSY562 and DSY565 and treated with fluconazole (FLC) and commercial milbemycins (Mil: A3Ox/A4Ox mix, 2.5:7.5) or their combination. The regimens for milbemycin and FLC were 2.5 and 100 mg/kg/day, respectively. Each data point corresponds to one animal, and the origin of tissue (kidney or spleen) is shown for each diagram. Geometric means are indicated by horizontal bars, and asterisks indicate statistically significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). NS, no significance (P > 0.05). CFU counts were analyzed with nonparametric Wilcoxon rank-sum tests.
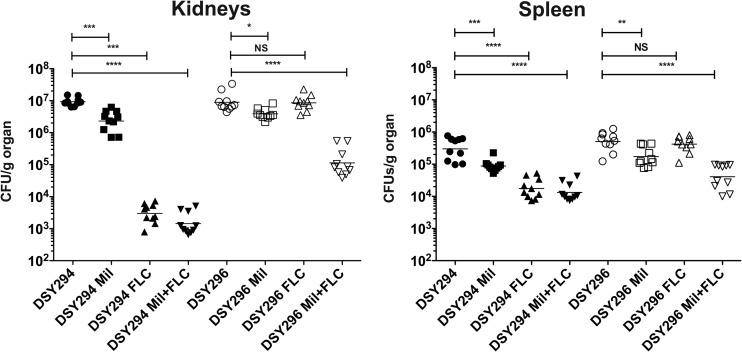
Similar experiments were carried out with C. albicans infections in mice. Both 0.5- and 2.5-mg/kg/day milbemycin regimens were applied; however, only the 2.5-mg/kg/day results are shown in Fig. 7 (the 0.5-mg/kg/day results are shown in Fig. S3 in the supplemental material). Fluconazole was used at a dosage of 3 mg/kg/day. As in the case of C. glabrata, single drug regimen reduced CFU counts in selected organs, with the exception of fluconazole treatment of the azole-resistant isolate DSY296, which could be expected. Combining both drugs resulted in a sharp decrease of CFU counts of the azole-resistant isolate (Fig. 7). This combination was however as efficient as the single azole regimen for the azole-susceptible isolate DSY294.
Fig 7.
Fungal tissue burdens of mice infected with C. albicans DSY294 and DSY296 and treated with fluconazole (FLC) and commercial milbemycins (Mil: A3Ox/A4Ox mix, 2.5:7.5) or their combination. The regimens for milbemycin and FLC were 2.5 and 100 mg/kg/day, respectively. Each data point corresponds to one animal, and the origin of tissue (kidney or spleen) is shown for each diagram. Other figure details are explained in Fig. 6.
Microarray analysis of milbemycin exposure in C. albicans and C. glabrata.
In order to better understand the effect of milbemycin on C. albicans and C. glabrata, we subjected both yeast species (azole-susceptible isolates) to a 1-h treatment at supra-MICs (10 μg/ml) to clearly observe their effects and analyzed their transcriptome by microarrays. The conventional laboratory C. albicans isolate SC5314 was chosen in these experiments to enable transcriptomic comparisons with experimental data from other laboratories. SC5314 also exhibited the same A3Ox MIC values than DSY294 (Table 3, 6.4 μg/ml). A 2-fold variation in any gene as a result of A3Ox exposure was used as regulation threshold. Microarray data were validated by qPCR with a few genes in C. albicans and C. glabrata (see Fig. S4 in the supplemental material).
C. glabrata.
A3Ox treatment resulted in a 2-fold gene expression variation for 887 genes (421 up- and 466 downregulated genes, respectively). GO term analysis of upregulated genes revealed enrichment of genes involved in oxido-reductive processes and sulfur amino acid biosynthesis (see File S1 in the supplemental material). This observation is consistent with ROS production induced by A3Ox. This was also confirmed by the upregulation of cytochrome c oxidase subunits (COX13, COX5B, and COX7), which participate to ROS production from electron transport activities (36). Strikingly, among the most upregulated genes were those encoding heat shock proteins (SSA3, HSP104, HSP42, and HSP30) and BNT2 encoding for a v-SNARE binding protein that facilitates specific protein retrieval from a late endosome to the Golgi. Together with the expression of the above-mentioned chaperones, BNT2 has been shown to be upregulated in stressed cells in S. cerevisiae to promote the sorting of misfolded proteins (37). In relation with this observation, several genes involved in protein ubiquitination (UBI4, UBP7, UBP9, UBX3, and UBX7) were upregulated by A3Ox treatment. Thus, this expression profile confirms that A3Ox imposes a stress in C. glabrata, which itself perturbs protein processing. As a matter of fact, several genes implicated in protein targeting to the vacuole (VPS genes) were upregulated by A3Ox treatment, including VPS8, VPS70, VPS38, VPS27, and VPS21. VPS genes are part of ESCRT system and are involved in the degradation of ubiquitylated proteins via the formation of multivesicular bodies (38).
We also observed that several genes of the calcineurin pathway were upregulated by A3Ox treatment, including CCH1, MID2 CMK2, CRZ1, and CMP2. The calcineurin pathway has been shown to be critical for responding to chemical stress in several yeast species and thus upregulation of this system might represent a mechanism by which C. glabrata protects itself against A3Ox toxicity (5, 39, 40). Interestingly, A3Ox treatment seems to upregulate several peroxisomal enzymes (PEX13, PEX18, and PEX6) and among them those involved in the glyoxylate cycle (ÌCL1 and MLS1).
C. albicans.
A3Ox treatment resulted in a 2-fold gene expression variation for 1,809 genes (1,438 up- and 371 downregulated genes, respectively), thus indicating large perturbations of this substance in the C. albicans transcriptome. GO term analysis of upregulated genes showed enrichment of genes involved in proteolytic and ubiquitination processes and involving proteasome assembly (see File S2 in the supplemental material). Thus, A3Ox treatment had a similar outcome as in C. glabrata reflecting a stress situation and enhancement of protein degradation. GO terms analysis of upregulated genes revealed enrichments in genes involved mitochondrial electron transport (NAD6, NAD1, NAD2, NAD3, NAD4, NAD5, and NAD4). The fact that the mitochondrial electron transport chain is stimulated upon A3Ox treatment and that cytochrome c oxidase subunits COX2 and COX3B, as well as the mitochondrial F1F0 ATP synthase subunits ATP6, ATP8, and ATP9, were upregulated may be related to a stimulus of mitochondrial respiration and consequent ROS production in C. albicans as described above measured. Consistent with these observations, orf19.148, orf19.639.1, and orf19.2438 (encoding for mitochondrial large ribosomal subunits MRPL31, MRPL44, and MRPS12, respectively) were upregulated in A3Ox-treated cells. We observed that upregulated genes were also enriched in oxidoreductive functions, especially those related to peroxide detoxification (URE2, CCP1, GTT12, orf19.4436, PRX1, DOT5, orf19.584, TTR1, CAT1, TSA1, and GPX1), probably reflecting the enhanced mitochondrial activity upon A3Ox treatment. GO terms analysis of upregulated genes also revealed enrichments in genes involved in cell wall biogenesis, including, for example, DIT1, PHR3, PHR2, CSK1, CHS2, CDA2, and ROM2. Several genes encoding GPI-anchored proteins (PGA11, PGA14, PGA15, PGA17, PGA19, PGA22, PGA23, PGA25, PGA27, PGA28, PGA30, PGA31, PGA39, PGA41, PGA42, PGA44, PGA46, PGA49, PGA58, PGA60, and PGA63) were also upregulated by A3Ox treatment. We noticed that MKC1 encoding for a mitogen-activated protein (MAP) kinase involved in cell wall integrity was upregulated by A3Ox, thus raising the possibility that A3Ox is sensed by the cell wall integrity pathway, the activation of which results in upregulation of several cell wall genes, including those above listed (41). Interestingly, several genes encoding heat shock and chaperone proteins (ASR1, HSP104, HSP30, HSP31, and HSP70) were upregulated upon A3Ox treatment and thus highlight that C. albicans is under stress as in the case of C. glabrata.
Intriguingly, we noticed a strong upregulation of WOR1, a master regulator of white-opaque switching in C. albicans (42) upon A3Ox treatment. White and opaque cells show dramatic differences in terms of cell shape, gene expression profile, drug permeability, and virulence (42–46). It is known that ectopic expression of this master regulator in the background of C. albicans isolates homozygous for the mating type locus (MTLa or MTLα) results in a massive conversion of white cells to the opaque state (47). We found that 50 of 214 genes upregulated in the opaque phase as published by Tsong et al. (46) were also upregulated by A3Ox. Normally, WOR1 is repressed in the strain SC5314 (which was used here in microarray experiments) heterozygote for the mating locus but this repression can be released by inhibition of the a1/α2 homeobox transcriptional complex. Thus, A3Ox was capable to release this complex directly or indirectly and thus favored the upregulation of WOR1 and its targets. We verified that A3Ox could induce white-opaque switching using a A3Ox disk assay onto Lee's medium. Opaque cells could be observed around the disk zone, thus confirming that A3Ox could stimulate white-opaque switching (see Fig. 4S in the supplemental material). The stimulation of the opaque state upon exposure to toxic environments has been documented in the past (48). This stimulation is the consequence of growth inhibition in the background of cells homozygous for the mating locus (48).
Other genes regulated by A3Ox treatment were reminiscent of stress conditions consequent of exposure to exogenous toxic compounds: we noticed upregulation of genes involved in DNA repair (RAD genes), upregulation of MCA1, a caspase-like gene, and also genes involved in drug resistance such as the transcription factor MRR1. The upregulation of MRR1 is accompanied by upregulation of some of its target genes (35 out of 84 target genes), among which MDR1, a multidrug transporter of the major facilitator family (49). MRR1 upregulation by A3Ox is consistent with a response of C. albicans to metabolic inhibitors.
DISCUSSION
In this work, we demonstrated that milbemycins are effective agents to treat C. albicans and C. glabrata infections in combination with fluconazole in animal models and even reverse azole resistance to levels of wild-type isolates. We showed that milbemycins A3 and A4 and especially their oxim derivatives have potent drug efflux inhibition activities. Several studies already established that this family of substances inhibit fungal ABC transporters (20, 21, 50); however, it is the first time, to our knowledge, that this is demonstrated for oxim derivatives. These substances are active components of commercial antiparasitic drugs and thus are easy to obtain in large quantities.
One of the most remarkable properties of milbemycin oxims was their antifungal and fungicidal activities both in C. albicans and C. glabrata, which to our knowledge has not been reported yet for other efflux inhibitors. This activity seems to be mediated by the oxim prosthetic group, since A4 and A3 were not exhibiting the same activity. Furthermore, A3Ox was the most potent compound in both Candida species. Thus, both the difference in chemical structure between A3 and A4 (Fig. 8, one ethyl and methyl group at position 25, respectively) and the addition of an oxim group (Fig. 8, at position 5) (51) conferred a strong antifungal activity. Milbemycin oxims carry two activities, one including the inhibition of drug efflux at low concentration (efflux inhibition was detectable starting from 0.1 μg/ml) and the other including antifungal activity at higher drug concentrations (3.2 to 6.4 μg/ml). The antifungal activity is independent of the presence or absence of ABC transporters, since mutants lacking major ABC transporters involved in azole resistance (CgCdr1/CgCdr2, Cdr1, and Cdr2) exhibit similar A3Ox MIC values to wild-type isolates (data not shown). Interestingly, efflux inhibition is more potent against drug-resistant C. albicans than C. glabrata, as reflected by the respective inhibition of R6G efflux (Vmax IC50s are >20-fold higher for C. glabrata, Fig. 2). This is possibly due to structural differences of ABC transporters between the two species which may result in different inhibitor affinities. The intrinsic antifungal activity of A3Ox, on the other hand, shows no difference between the two types of isolates, therefore highlighting that it is mediated by an ABC-transporter-independent mechanism of action.
Fig 8.

Chemical structures of milbemycins A3/A4 (left) and oxim derivatives (right).
With the idea to better understand how the fungicidal effect of A3Ox was exerted on C. albicans and C. glabrata, we performed whole-genome transcript profiling with the same drug concentration and experiment duration. The results obtained revealed, as expected, several features common to drug stress that include the mobilization of oxidoreductive processes, of chaperoning of misfolded proteins and of protein degradation pathways. The upregulation of the caspase-like gene MCA1 in C. albicans could be related to the fungicidal effect of A3Ox. It has been shown that a striking caspase activity pattern can occur in C. albicans during oxidative stress-induced programmed cell death (PCD) (52). Together with the ROS-producing activity of A3Ox, MCA1 upregulation suggests that A3Ox has the capacity to induce PCD in C. albicans, although it can be expected that cell necrosis would be an important readout of A3Ox exposure.
One surprising observation was the apparent divergent transcriptional response of both Candida species upon A3Ox exposure. We found that 204 gene orthologs between C. glabrata (of 887 genes) and C. albicans (of 1,809 genes) were commonly regulated by A3Ox, which reflects a common response mechanism, but also demonstrated quite different effects of this drug on the transcriptome of the two yeast species (see File S3 in the supplemental material). The cluster analysis of these 204 regulated genes showed that 62 were regulated in the opposite way between the two yeast species (Fig. 9). The remaining genes that were commonly up- and downregulated could constitute a common core of genes responding to A3Ox in a species-independent way. The GO term analysis of the 144 coregulated genes revealed enrichments of genes involved in oxidoreductive processes and in oxidative stress response, which is consistent with a stress situation (see File S3 in the supplemental material). From the annotated genes involved in oxidoreductive processes, 75% belonged to genes commonly upregulated by A3Ox between both species (Fig. 9). Oxidative stress is a signature common in response to xenobiotics in yeast (53–55), and our transcript profile analyses therefore underline a similar effect for A3Ox. In addition to this oxidative stress response, the presence of chaperone genes (HSP gene family), of genes involved in protein ubiquitination process, and of genes involved in ESCRT vesicle trafficking was reminiscent of the response signature involving protein damage (55). Interestingly, MAP kinases from both C. albicans (MKC1) and C. glabrata (SLT2) were also upregulated upon A3Ox treatment. Besides the role of these kinases in cell wall integrity as described above, it has been suggested that at least MKC1 participates to oxidative stress response (56), which is again in agreement with the idea that A3Ox stress responses involve oxidative processes.
Fig 9.
Cluster analysis of coregulated and ortholog genes between C. albicans and C. glabrata. Gene orthologues were obtained from data available at Candida Genome database (CGD) and were extracted from data available on Files S1 and S3 in the supplemental material. Genes were clustered with the CLUSTER 3.0 software using centroid linkage preset. The data were viewed with TreeView (version 1.1.6r2). Gene symbols contain the C. albicans with the corresponding C. glabrata ortholog and the attached gene name. Three subclusters are shown. (A) Genes upregulated in C. albicans and C. glabrata; (B) genes downregulated in C. albicans and down-/upregulated in C. glabrata; (C) genes upregulated in C. albicans and downregulated in C. glabrata. “Fold-Ca” and “Fold-Cg” indicate the fold change expression in the presence of A3Ox compared to untreated controls. Arrows indicate genes assigned by GO term analysis to oxidoreductive processes (see File S3 in the supplemental material for details). The colored scale corresponds to the fold changes in gene expression ranging from <3.5-fold to >3.5-fold.
At this stage, it is not possible to predict direct cellular targets of this substance in addition to efflux transporters in the tested Candida isolates. A few studies comparing the effect of the same drug on different yeast species using microarrays are available. These studies have investigated the effect of antifungal drugs on fungal metabolism and generally could identify common response patterns between the different investigated species (1). Here, we identified a limited overlap of gene expression between C. glabrata and C. albicans, thus suggesting species-specific response signatures. This could be due to the evolutionary distance between both yeast species, which determines how a specific pathogen behaves in contact with exogenous substances. However, even if species are more closely related, drug response can be still different as illustrated by transcriptome comparisons between C. glabrata and S. cerevisiae in the presence of benomyl (57). The same conclusion was drawn for the response of diverse yeast species to azoles (58).
Testing efflux inhibitors in animal models has been achieved in a few occasions. For example, Hayama et al. (59) have used the d-octapeptide derivative RC21v3, a Cdr1 inhibitor, in the treatment of murine oral candidiasis caused by azole-susceptible and azole-resistant C. albicans clinical isolates. RC21v3 potentiated the therapeutic efficacy of fluconazole for mice infected with either strain. Sorensen et al. (60) reported that milbemycin α9 could potentiate fluconazole in an experimental systemic pyelonephritis with C. albicans. We showed here that milbemycin oxims could potentiate fluconazole efficacy both in C. albicans and C. glabrata using a systemic model of infection, and thus we expand the concept of drug combination to both of these important fungal pathogens. We have used different milbemycin regimens ranging from 0.5 to 2.5 mg/kg/day in mice without signs of toxicity. These dosages correspond to those given in animal care (61). A higher dosage (5 mg/kg/day) exhibited slight toxicity effects in treated mice without increasing fluconazole efficacy (data not shown). The serum levels obtained after 7-day treatments reached concentrations near to the MIC measured in C. glabrata and C. albicans. This could explain why milbemycin oxims on their own could decrease fungal burden in tissues infected with C. glabrata and C. albicans. On the other hand, we have shown in previous studies that the ABC transporter CgCDR1 could participate to C. glabrata virulence (23). Thus, inhibition of this transporter by milbemycins could also impact on C. glabrata virulence and therefore could contribute to the decrease of tissue burden upon inhibitor treatment.
Whether or not milbemycin oxims with fluconazole can be used in human is an open question. The primary targets of the substance are glutamate sensitive chloride channels in neurons and myocytes of invertebrates, leading to hyperpolarization of these cells and blocking of signal transfer. These glutamate channels are specific for invertebrates and are not expressed in mammalian hosts (62). Some milbemycin derivatives have been used in humans already. For example, Cotreau et al. (63) reported the use of moxidectin to treat onchocerciasis (river blindness). Onchocerciasis is a parasitic disease caused by the helminth Onchocerca volvulus and is transmitted to humans through the bite of a black fly of the genus Simulium. This study showed that such substances have low toxicity in humans when administered in the 3- to 36-mg range. Our preliminary data show that moxidectin acts synergistically with fluconazole in C. glabrata and C. albicans as in the case of milbemycin A3Ox. Therefore, the use of milbemycin for treating Candida infections is potentially possible in humans. Candida infections, and especially C. glabrata infections, are rising in diverse countries and, together with azole resistance levels reached nowadays by C. glabrata, alternative therapeutic approaches should be proposed in the future (4).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Françoise Ischer for technical assistance and Alix Coste for stimulating discussion. We also thank Sandra Schorderet-Weber and Jacques Bouvier (Novartis Animal Health) for discussions and help for providing milbemycin derivatives.
This study was partially supported by a grant from the Swiss Research National Foundation 31003A_127378 and from a Swiss-Indo Collaborative Research grant ISJRP 122917.
Footnotes
Published ahead of print 3 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02040-12.
REFERENCES
- 1. Sanglard D, Coste A, Ferrari S. 2009. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 9:1029–1050 [DOI] [PubMed] [Google Scholar]
- 2. Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 125:S3–S13 [DOI] [PubMed] [Google Scholar]
- 3. Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. using Clinical and Laboratory Standards Institute broth microdilution methods, 2010–2012. J. Clin. Microbiol. 50:2846–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lockhart SR, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility of Candida bloodstream isolates from population-based surveillance in two US cities: 2008–2011. J. Clin. Microbiol. 50:3435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75:213–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanglard D, Ischer F, Bille J. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torelli R, Posteraro B, Ferrari S, La Sorda M, Fadda G, Sanglard D, Sanguinetti M. 2008. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata. Mol. Microbiol. 68:186–201 [DOI] [PubMed] [Google Scholar]
- 9. Ferrari S, Ischer F, Calabrese D, Posteraro B, Sanguinetti M, Fadda G, Rohde B, Bauser C, Bader O, Sanglard D. 2009. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog. 5:e1000268 doi:10.1371/journal.ppat.1000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsai H, Krol A, Sarti K, Bennett J. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob. Agents Chemother. 50:1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermitsky J-P, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. 2006. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol. Microbiol. 61:704–722 [DOI] [PubMed] [Google Scholar]
- 12. Tsai H, Sammons L, Zhang X, Suffis S, Su Q, Myers T, Marr K, Bennett J. 2010. Microarray and molecular analyses of the azole resistance mechanism in Candida glabrata oropharyngeal isolates. Antimicrob. Agents Chemother. 54:3308–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caudle KE, Barker KS, Wiederhold NP, Xu L, Homayouni R, Rogers PD. 2011. Genomewide expression profile analysis of the Candida glabrata Pdr1 regulon. Eukaryot. Cell 10:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coste AT, Crittin J, Bauser C, Rohde B, Sanglard D. 2009. Functional analysis of cis- and trans-acting elements of the Candida albicans CDR2 promoter with a novel promoter reporter system. Eukaryot. Cell 8:1250–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunkel N, Blass J, Rogers P, Morschhauser J. 2008. Mutations in the multidrug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heilmann C, Schneider S, Barker K, Rogers P, Morschhauser J. 2010. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 54:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoot SJ, Smith AR, Brown RP, White TC. 2011. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 55:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whiteway M. 2012. Seeking antifungal drug synergies. Microbe 7:234–237 [Google Scholar]
- 20. Niimi K, Harding D, Parshot R, King A, Lun D, Decottignies A, Niimi M, Lin S, Cannon R, Goffeau A, Monk B. 2004. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a d-octapeptide derivative. Antimicrob. Agents Chemother. 48:1256–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. 2009. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22:291–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson JB. 2005. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat. Rev. Microbiol. 3:547–556 [DOI] [PubMed] [Google Scholar]
- 23. Ferrari S, Sanguinetti M, Torelli R, Posteraro B, Sanglard D. 2011. Contribution of CgPDR1-regulated genes in enhanced virulence of azole-resistant Candida glabrata. PLoS One 6:e17589 doi:10.1371/journal.pone.0017589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 25. Maccallum DM, Coste A, Ischer F, Jacobsen MD, Odds FC, Sanglard D. 2010. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob. Agents Chemother. 54:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coste A, Selmecki A, Forche A, Diogo D, Bougnoux M-E, D'enfert C, Berman J, Sanglard D. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 6:1889–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh A, Prasad R. 2011. Comparative lipidomics of azole sensitive and resistant clinical isolates of Candida albicans reveals unexpected diversity in molecular lipid imprints. PLoS One 6:e19266 doi:10.1371/journal.pone.0039812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 29. Te Dorsthorst DTA, Verweij PE, Meletiadis J, Bergervoet M, Punt NC, Meis JFGM, Mouton JW. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maesaki S, Marichal P, Vanden Bossche H, Sanglard D, Kohno S. 1999. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 44:27–31 [DOI] [PubMed] [Google Scholar]
- 31. Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG, Butler G. 2010. Regulation of the hypoxic response in Candida albicans. Eukaryot. Cell 9:1734–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrari S, Sanguinetti M, De Bernardis F, Torelli R, Posteraro B, Vandeputte P, Sanglard D. 2011. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob. Agents Chemother. 55:1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vandeputte P, Pradervand S, Ischer F, Coste AT, Ferrari S, Harshman K, Sanglard D. 2012. Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans. Eukaryot. Cell 11:916–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dryden M, Payne P. 2005. Preventing parasites in cats. Vet. Ther. 6:260–267 [PubMed] [Google Scholar]
- 35. Batova M, Klobucnikova V, Oblasova Z, Gregan J, Zahradnik P, Hapala I, Subik J, Schüller C. 2010. Chemogenomic and transcriptome analysis identifies mode of action of the chemosensitizing agent CTBT (7-chlorotetrazolo[5,1-c]benzo[1,2,4]triazine). BMC Genomics 11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bender T, Leidhold C, Ruppert T, Franken S, Voos W. 2010. The role of protein quality control in mitochondrial protein homeostasis under oxidative stress. Proteomics 10:1426–1443 [DOI] [PubMed] [Google Scholar]
- 37. Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. 2012. Molecular chaperones and stress-inducible protein sorting factors coordinate the spatio-temporal distribution of protein aggregates. Mol. Biol. Cell 23:3041–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fabrizio P, Hoon S, Shamalnasab M, Galbani A, Wei M, Giaever G, Nislow C, Longo VD. 2010. Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in life span regulation. PLoS Genet. 6:e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FG, Bungay AA, Bigol UG, Nicolas MG, Abraham SN, Thompson DA, Regev A, Heitman J. 2012. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 (Bethesda) 2:675–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959–976 [DOI] [PubMed] [Google Scholar]
- 41. Monge RA, Roman E, Nombela C, Pla J. 2006. The MAP kinase signal transduction network in Candida albicans. Microbiology 152:905–912 [DOI] [PubMed] [Google Scholar]
- 42. Zordan RE, Galgoczy DJ, Johnson AD. 2006. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. U. S. A. 103:12807–12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lohse MB, Johnson AD. 2009. White-opaque switching in Candida albicans. Curr. Opin. Microbiol. 12:650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alby K., Jr 2009. To switch or not to switch?: Phenotypic switching is sensitive to multiple inputs in a pathogenic fungus. Commun. Integr. Biol. 2:509–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lohse MB, Johnson AD. 2008. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One 3:e1473 doi:10.1371/journal.pone.0001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsong AE, Miller MG, Raisner RM, Johnson AD. 2003. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115:389–399 [DOI] [PubMed] [Google Scholar]
- 47. Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. 2006. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 103:12813–12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alby K, Bennett RJ. 2009. Stress-induced phenotypic switching in Candida albicans. Mol. Biol. Cell 20:3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morschhauser J, Barker K, Liu T, Bla B, Homayouni R, Rogers P. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3:e164 doi:10.1371/journal.ppat.0030164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holmes A, Lin Y, Niimi K, Lamping E, Keniya M, Niimi M, Tanabe K, Monk B, Cannon R. 2008. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 52:3851–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shoop WL, Mrozik H, Fisher MH. 1995. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 59:139–156 [DOI] [PubMed] [Google Scholar]
- 52. Cao Y, Huang S, Dai B, Zhu Z, Lu H, Dong L, Cao Y, Wang Y, Gao P, Chai Y, Jiang Y. 2009. Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress-induced death and change in energy metabolism. Fungal Genet. Biol. 46:183–189 [DOI] [PubMed] [Google Scholar]
- 53. Santos PM, Simões T, Sá-Correia I. 2009. Insights into yeast adaptive response to the agricultural fungicide mancozeb: a toxicoproteomics approach. Proteomics 9:657–670 [DOI] [PubMed] [Google Scholar]
- 54. Dias PJ, Teixeira MC, Telo JP, Sá-Correia I. 2010. Insights into the mechanisms of toxicity and tolerance to the agricultural fungicide mancozeb in yeast, as suggested by a chemogenomic approach. OMICS 14:211–227 [DOI] [PubMed] [Google Scholar]
- 55. dos Santos SC, Teixeira MC, Cabrito TR, Sá-Correia I. 2012. Yeast toxicogenomics: genome-wide responses to chemical stresses with impact in environmental health, pharmacology, and biotechnology. Front. Genet. 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Dios CH, Roman E, Monge RA, Pla J. 2010. The role of MAPK signal transduction pathways in the response to oxidative stress in the fungal pathogen Candida albicans: implications in virulence. Curr. Protein Pept. Sci. 11:693–703 [DOI] [PubMed] [Google Scholar]
- 57. Lelandais G, Tanty V, Geneix C, Etchebest C, Jacq C, Devaux F. 2008. Genome adaptation to chemical stress: clues from comparative transcriptomics in Saccharomyces cerevisiae and Candida glabrata. Genome Biol. 9:R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuo D, Tan K, Zinman G, Ravasi T, Bar-Joseph Z, Ideker T. 2010. Evolutionary divergence in the fungal response to fluconazole revealed by soft clustering. Genome Biol. 11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hayama K, Ishibashi H, Ishijima SA, Niimi K, Tansho S, Ono Y, Monk BC, Holmes AR, Harding DR, Cannon RD, Abe S. 2012. A d-octapeptide drug efflux pump inhibitor acts synergistically with azoles in a murine oral candidiasis infection model. FEMS Microbiol. Lett. 328:130–137 [DOI] [PubMed] [Google Scholar]
- 60. Sorensen K, Corcoran E, Huie K, Chen S, Tembe V, Griffith D, Lomovskaya O, Watkins W, Dudley NM. 2000. In vivo potentiation of fluconazole by the fungal efflux pump inhibitor MC-510,011 in a mouse model of pyelonephritis due to fluconazole-susceptible and -resistant Candida albicans, abstr 1502, p 209. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Ontario, Canada [Google Scholar]
- 61. Tranquilli WJ, Paul AJ, Todd KS. 1991. Assessment of toxicosis induced by high-dose administration of milbemycin oxime in collies. Am. J. Vet. Res. 52:1170–1172 [PubMed] [Google Scholar]
- 62. Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. 1994. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371:707–711 [DOI] [PubMed] [Google Scholar]
- 63. Cotreau MM. 2003. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J. Clin. Pharmacol. 43:1108–1115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.