Abstract
The opportunistic yeast pathogen Candida glabrata is recognized for its ability to acquire resistance during prolonged treatment with azole antifungals (J. E. Bennett, K. Izumikawa, and K. A. Marr. Antimicrob. Agents Chemother. 48:1773–1777, 2004). Resistance to azoles is largely mediated by the transcription factor PDR1, resulting in the upregulation of ATP-binding cassette (ABC) transporter proteins and drug efflux. Studies in the related yeast Saccharomyces cerevisiae have shown that Pdr1p forms a heterodimer with another transcription factor, Stb5p. In C. glabrata, the open reading frame (ORF) designated CAGL0I02552g has 38.8% amino acid identity with STB5 (YHR178w) and shares an N-terminal Zn2Cys6 binuclear cluster domain and a fungus-specific transcriptional factor domain, prompting us to test for homologous function and a possible role in azole resistance. Complementation of a Δyhr178w (Δstb5) mutant with CAGL0I02552g resolved the increased sensitivity to cold, hydrogen peroxide, and caffeine of the mutant, for which reason we designated CAGl0I02552g CgSTB5. Overexpression of CgSTB5 in C. glabrata repressed azole resistance, whereas deletion of CgSTB5 caused a modest increase in resistance. Expression analysis found that CgSTB5 shares many transcriptional targets with CgPDR1 but, unlike the latter, is a negative regulator of pleiotropic drug resistance, including the ABC transporter genes CDR1, PDH1, and YOR1.
INTRODUCTION
The haploid yeast Candida glabrata is closely related to Saccharomyces cerevisiae. C. glabrata is the second-most-common yeast species known to cause fungemia (2, 11). Antifungals used to treat C. glabrata infections include amphotericin B, echinocandins, and azoles. Although azoles, particularly fluconazole, are often used to treat candidemia, C. glabrata is intrinsically more resistant than most other Candida species and develops further resistance during prolonged azole therapy.
Drug efflux, resulting from the increased expression of ATP-binding cassette (ABC) transporter proteins, is the predominant mechanism by which C. glabrata mediates resistance to a wide range of azoles and other antifungal compounds. Several ABC transporters, including Cdr1p, Pdh1p, Yor1p, and Snq2p, contribute to xenobiotic drug efflux. The transcription factor CgPdr1p is the principal regulator of ABC transporter gene expression and has been found to be a key component of Pleiotropic Drug Resistance (PDR) (1, 3–5).
In the related yeast Saccharomyces cerevisiae, Pdr1p has been shown to form a heterodimer with another transcription factor, S. cerevisiae Sin3 Binding Protein 5 (ScStb5p) (6). Studies in S. cerevisiae found that ScStb5p is a Zn2Cys6 transcription factor (7, 8) which regulates genes involved in the oxidative stress response by increasing the supply of NADPH through the pentose phosphate pathway (9). Deletion of ScSTB5 resulted in a growth defect and sensitivity to cold (20°C), caffeine, hydrogen peroxide, diamide, benomyl, calcofluor, methyl methane sulfonate, acetaldehyde, and cycloheximide (7, 9, 10). In addition, a ΔScstb5 mutant has been reported to require uracil and methionine for growth (12). Although Akache and Turcotte reported that susceptibility to ketoconazole was not affected in a ΔScstb5 mutant (13), we postulated a role of STB5 in azole resistance in C. glabrata because they and their colleagues also reported that Pdr1p and Stb5p dimerize and directly bind the promoter of PDR5 in S. cerevisiae (6).
Here, we report that the open reading frame (ORF) CAGL0I02552g is a homologue of ScSTB5 (YHR178w). We observed the effect that CgSTB5 has on C. glabrata azole susceptibility by gene deletion and overexpression. Furthermore, using microarray hybridization for a genome-wide survey of transcript levels, we studied the effects of CgSTB5 deletion and overexpression. We conclude that CgSTB5 can complement some but not all of the defects in the Δyhr178w strain and is a transcriptional repressor of several genes implicated in azole resistance in C. glabrata.
MATERIALS AND METHODS
Strains and culture conditions.
Saccharomyces cerevisiae strains were obtained from Open Biosystems (Huntville, AL) (Table 1). Plasmids were maintained in Escherichia coli TOP 10 (Invitrogen, Carlsbad, CA) (Table 2). Host cells were grown in LB with 50 μg/ml ampicillin or 50 μg/ml kanamycin, depending on the plasmids.
Table 1.
List of Saccharomyces cerevisiae and Candida glabrata strains
| Strain | Genotype | Source or reference |
|---|---|---|
| S. cerevisiae | ||
| BY4741 | MATa his3-Δ1 leu2-Δ0 met15-Δ0 ura3-Δ0 | ATCC 4040002 |
| Δyhr178w | MATa his3-Δ1 leu2-Δ0 met15Δ0 ura3-Δ0 STB5::kanMX | ATCC 4002872 |
| Δyhr178wSTB5 | Δyhr178w expressing CgSTB5 in pH392 vector | This study |
| Δyhr178wP | Δyhr178w with empty vector pH392 | This study |
| C. glabrata | ||
| NCCLS84 (Cg84) | Wild-type strain 84 | ATCC 90030 |
| 84u | NCCLS84 ura3 mutant | 3 |
| Δcgstb5 | 84u cgstb5Δ::ScURA3 | This study |
| CgSTB5OE | Overexpression of CgSTB5 in 84u with pGRB2.2 | This study |
| 84uP | 84u transformed with pGRB2.2 vector | This study |
| Δpdr1 | 84u pdr1Δ::ScURA3 | This study |
| Δpdr1Δstb5 | Δpdr1 stb5Δ::ScURA3 | This study |
| Δpdr1STB5 | Δpdr1 overexpressing CgSTB5 in pGRB2.2 | This study |
| Δpdr1P | Δpdr1 transformed with pGRB2.2 | This study |
| CgPDR1OE | Overexpression of PDR1 in 84u with pCgCgPDR1 | This study |
Table 2.
List of plasmids used in current study
| Plasmid | Source or reference |
|---|---|
| STB5-Topo | This study |
| pH392 | Kind gift of Herman Edskes |
| pH392-CgSTB5 | This study |
| pGRB2.2 | Kind gift of Brendan Cormack |
| pGRB2.2-CgSTB5 | This study |
| pCgACU | Kind gift of K. Kitada (14) |
| pCgPDR1 | This study |
C. glabrata and S. cerevisiae strains were cultured either on yeast extract-peptone-dextrose (YPD) medium containing 1% Bacto yeast extract (Difco Laboratories, Detroit, MI), 2% Bacto peptone (Difco Laboratories), and 2% glucose or on minimum (MIN) medium containing 0.67% yeast nitrogen base without amino acids (Difco Laboratories, Franklin Lakes, NJ) and 2% glucose. Cells were shaken overnight at 30°C and washed in distilled water three times, and cell density was determined by the optical density at 600 nm (OD600) and used as described below.
Drug sensitivity assays.
MICs of fluconazole and voriconazole (Etest; AB Biodisk, Solna, Sweden) were determined by plating 1 × 106 cells on MIN agar plates and reading the zone of inhibition at the paper strip after incubation at 30°C for 2 days. The CLSI microdilution method M27-A3 was also used for susceptibility testing with the following modifications: MIN broth, 30°C incubation for 48 h, and an 80% growth inhibition (15). MIN agar and broth provided better growth than RPMI 1640 of the slow-growing stb5 deletant and preserved the plasmid in the STB5-overexpressing strains.
Caffeine and cold sensitivity assays of Saccharomyces cerevisiae strains.
As described by Akache et al. (7), cells were shaken at 30°C overnight in YPD media and diluted in fresh YPD media to a concentration of 1 × 105 cells per 5 μl. Four 1:10 serial dilutions were made, and 5 μl of cells were spotted onto YPD agar plates, with or without the presence of 0.15% caffeine (Sigma-Aldrich). Plates were incubated at either 30°C for 2 days or 20°C for 4 days to assess their cold sensitivity.
Oxidative stress sensitivity assays of Candida glabrata strains.
Cells were grown in YPD media overnight and resuspended at a concentration of 1 × 105 cells per 5 μl. As described by Larochelle et al. (9), a series of four 1:10 serial dilutions were made using deionized water, and 5-μl spots were placed on YPD agar plates or YPD agar plates with 10 mM hydrogen peroxide. Plates were incubated at 30°C for 2 days.
Cloning of CgSTB5.
The ORF of CAGL0I02252g was obtained by PCR using the genomic DNA of C. glabrata strain 84 as a template and primers CgSTB5S and CgSTB5AS, containing flanking BamHI and EcoRI restriction sites (Table 3). For sequencing, PCR-amplified CgSTB5 was ligated into the pCR-BluntII-Topo vector (Invitrogen). The amino acid sequence of our strain was identical to that in GenBank (accession no. CR380955.2), encoding a 1,005-amino-acid protein with an N-terminal Zn2Cys6 motif (C-X2-C-X6-C-X6-C-X2-C-X6-C) at amino acids 32 to 59, a conserved fungus-specific transcription factor domain (PF04082) at amino acids 408 to 657, and a polyglutamine sequence (Q3-I-Q4-I-Q5) at amino acids 242 to 259.
Table 3.
Primers and probes used in this study (grouped by application)
| Primer | Sequence (5′–3′) |
|---|---|
| Plasmid construction primers | |
| CgSTB5S | GCATGGATCCATGAGTGGCCCAGATAAGGG |
| CgSTB5AS | GCATGAATTCTTATCTAAAGCGATCCTCCCA |
| Deletion cassette primers | |
| CgSTB5DS1 | TCATATGTCATCATAGCCTACTTGACTAGGAGTTATAACAGATATATTAATAGAGTAGGATTTACAAGGATTTCACTATGTCGAAAGCTACATATAAGGA |
| CgSTB5DAS2 | CACAATTATTGACAAGTTTCAGTTTCATAATCCGTCTGCATCTTCATTGATATCATCCTGGGGCTTACTTCTTATCTTATTAGTTTTGCTGGCCGCATCT |
| CgSTB5DS3 | CAAGCTAGTGATCAGATAAAACTGGGGAGTTTAATCAACAAGAGTTTTTAGCCCTTTCTTAGTCCCAATCCAACTGAATCATATGTCATCATAGCCTACT |
| CgSTB5DAS4 | TCTTGTGCTATACGGCAGAAACATAAATAGATTAAGATAAACTATTAGCACTAATCGGAATAAAGGGAACTGAATAGCACAATTATTGACAAGTTTCAGT |
| CgPDR1D1 | TGGAATTAGTGTTTTATTCTGCCTTTTTTTTTAGAATATATTGGTAAAGTCATTCTTTAGCTACGTTATTGAGAGAATATGTCGAAAGCTACATATAAGG |
| CgPDR1D2 | GCAACATAACCACTAACAAATGATTTTTCAGGTTAAATATAAAATTATACAGGCTATGCACACTGTCTAAATTAATAGCATTAGTTTTGCTGGCCGCATC |
| CgPDR1D3 | TTATTACTTAAACAATTTTTAAGTAACACATTCAAACTTCCATTACTTCGTACCCCATATCGTATTGCCATTGTGATATGGAATTAGTGTTTTATTCTGC |
| CgPDR1D4 | ATTAAACTACAATTTTTTAATGAGAGATATTGTAGTGTTATCGCTAATTTGAGGTAGTCTAAGTCTCATGTAAAATTATGCAACATAACCACTAACAAAT |
| qRT-PCR primers | |
| CgACT1F | TTGGACTCTGGTGACGGTGTTA |
| CgACT1R | AAAATAGCGTGTGGCAAAGAGAA |
| CgCDR1F | AGATGTGTTGGTTCTGTCTCAAAGAC |
| CgCDR1R | CCGGAATACATTGACAAACCAAG |
| CgPDH1F | AATGGATGTTAGAAGTAGTTGGAGCAG |
| CgPDH1R | TGTTCGGAATTTCTCCACACCT |
| CgYOR1F | CGCTGGGAAGGCCAAGA |
| CgYOR1R | CTCCCCGGACGTCAGAATAG |
| CgPDR1F | AACGATTATTCAATTGCAACAACG |
| CgPDR1R | CCTCACAATAAGGAAAGTCTGCG |
| qRT- PCR probes | |
| CgACT1 | CCACGTTGTTCCAATTTACGCCGG |
| CgCDR1 | TTATCTGCTGCGATGGTTCCTGCTTCC |
| CgPDH1 | CAGGCTCACATGCAAACCAAGACTACCAT |
| CgYOR1 | CTCGCCGGTGCAGGATTACGATCTAGA |
| CgPDR1 | TCGAATATTATGCACCATCATGTCTGTGTTTAGCT |
Construction of the Δcgstb5 and Δpdr1 mutants.
Gene deletions were performed as previously described (4). All primer sequences are presented in Table 3. Primer pair CgSTB5DS1 and CgSTB5DAS2 and primer pair CgPDR1D1 and CgPDR1D2 were designed to create deletion cassettes for CAGL0I02552g and CgPDR1, respectively. An additional round of PCR using primer pair CgSTB5DS3 and CgSTB5DAS4 and primer pair CgPDR1D3 and CgPDR1D4 extended the 5′ and 3′ ends of the deletion cassettes. The S. cerevisiae URA3 gene in the deletion cassettes was used to replace the CAGL0I02552g and CgPDR1 ORFs. Transformants were selected on MIN media, and deletion of the target genes was confirmed by Southern blot analysis (data not shown). To create the Δpdr1 Δstb5 mutant, we plated the Δpdr1 strain on 0.1% 5-fluoroorotic acid (Lancaster, Pelham, NH) in MIN medium and selected for uracil auxotrophy for recycling of the URA3 selection marker, followed by STB5 gene deletion as described above.
Plasmid construction.
CgSTB5 in the STB5-Topo plasmid was digested with BamHI and EcoRI (both New England BioLabs) and ligated (Rapid DNA Ligation kit; Roche Diagnostics, Germany) into pGRB2.2 (pGRB2.2-STB5) for overexpression under the control of a PGK1 promoter and transformed into the C. glabrata 84u strain (CgSTB5OE).
For expression of CgSTB5 in S. cerevisiae, the STB5-Topo plasmid was digested with EcoRI, and the CgSTB5 fragment was gel purified. The purified fragment was blunt ended with T4 DNA polymerase, digested with BamHI, and ligated into the pH392 plasmid digested with BamHI and PvuII (New England BioLabs) to create pH392-CgSTB5. This plasmid was used to transform the Δyhr178w strain using uracil prototrophy as a selection marker. The pCgPDR1 plasmid containing the CgPDR1 from strain 38a in pCgACU with the S. cerevisiae ADH1 promoter was used to overexpress CgPDR1 in C. glabrata (3). The pCgPDR1 was transformed into 84u in this study to create CgPDR1OE for the microarray study.
Growth curve.
Yeast cells were grown overnight in MIN media and diluted in MIN media to a final OD600 of 0.3. Cells were then placed in a 30°C shaker, and cell concentrations were measured by OD600 over a 6-h period.
qRT-PCR.
The same sample of total RNA used for cDNA reverse transcription, fluorescent labeling, and microarray hybridization was also DNase treated to remove the minute contamination of genomic DNA. Then, the DNase-treated RNA was reverse transcribed with a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). The parallel amplification between CgACT1 and the gene of interest was confirmed for each probe and primer set. Quantitative real-time PCR (qRT-PCR) was used to determine the expression level of CgACT1, CgPDR1, CgCDR1, CgPDH1, CgSNQ2, CgYOR1, GND1, PGI1, and ZWF1 in C. glabrata. The sequences of TaqMan probes and forward and reverse primers are listed in Table 3. CgACT1 was used as an internal control for normalization. The threshold cycle (2−ΔΔCT) method was used for calculating the differences in gene expression.
Whole-genome mRNA expression analysis by microarray.
Microarray analysis was used to compare the transcription profiles of strains in which CgSTB5 had been deleted or overexpressed in a plasmid with a PGK1 promoter. For comparison, the strain CgPDR1OE, in which CgPDR1 was overexpressed, was also studied. Total RNA was isolated from log-phase cultures of C. glabrata grown in MIN media by using TRIzol (Invitrogen, Carlsbad, CA) and an RNeasy MinElute cleanup kit (Qiagen, Valencia, CA). Ten micrograms of total RNA from strain Cg84 and either the Δcgstb5 or the CgSTB5OE mutant was reverse transcribed to cDNA to incorporate the fluorescent Cy3-dUTP and Cy5-dUTP (GE Health Care, Piscataway, NJ), respectively. The expression arrays used for analysis of CgPDR1 overexpression were as previously reported (16). This array used 70-mer oligonucleotides spotted on glass arrays by the NIAID Microarray Research Facility (Gene Expression Omnibus [GEO] accession no. GPL8174; see http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL8174). Microarray images were analyzed using GenPix software. For analysis of the CgSTB5 effect, SurePrint custom arrays (Agilent Technologies, Santa Clara, CA) (GEO accession no. GPL10325; see http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL10325) were used. Agilent Feature Extraction software was used for image analysis. Both sets of arrays used cDNA prepared from Cg84 for the reference channel in two-color hybridization experiments. Analysis of the in-house arrays used 5 microarrays, and analysis of the Agilent arrays used 4 microarrays prepared from 2 to 3 separated RNA preparations, with 1 prepared using reciprocal labeling. Our microarray analysis utilized the mAdb (microArray Database) system provided by the National Institute of Allergy and Infectious Diseases and the Center for Information Technology at the National Institutes of Health, Bethesda, MD (http://madb.niaid.nih.gov/). We applied the Significance Analysis of Microarrays (SAM) method with a delta of 1.0 and a false-discovery-rate cutoff of 0.1285 for the Δcgstb5 mutant versus the Cg84 strain and a delta of 0.7 and a false-discovery-rate cutoff of 0.0320 for the CgPDR1OE versus Cg84 arrays. After SAM, data were further filtered by including only differences detected in three or more arrays per group with an expression ratio greater than 2.0 or less than 0.5. After the data were filtered, a total of 68 genes remained in the Δcgstb5-versus-Cg84 comparison, and a total of 273 genes remained in the CgPDR1OE-versus-Cg84 comparison. The 34 genes differentially expressed in both microarrays are presented in Table 4, together with microarray results for those same genes in a Δpdr1 Δcgstb5 mutant.
Table 4.
C. glabrata genes upregulated ≥2-fold in response to both STB5 deletion (cgstb5Δ) in 84 host and PDR1 overexpression (PDR1OE)a
| Group | C. glabrata designation | S. cerevisiae homologue | Description | Fold expression upregulation |
||
|---|---|---|---|---|---|---|
| Δcgstb5 | CgPDR1OE | Δpdr1 Δcgstb5 | ||||
| Small-molecule transport | CAGL0B04455g | AVT4 | Export of large neutral amino acids from vacuoles | 2.21 | 8.99 | 3.06 |
| CAGL0H06017g | FLR1 | Multidrug transporter of the major facilitator superfamily | 2.28 | 4.3 | 5.85 | |
| CAGL0B04433g | FUR4 | Uracil and cation symporter activity | 2.51 | 2.74 | 1.37 | |
| CAGL0M01760g | PDR5 | ABC transporter involved in azole/multidrug resistance | 8.75 | 13.07 | 1.89 | |
| CAGL0B02475g | PHO84 | High-affinity inorganic phosphate transporter and low-affinity manganese transporter | 4.48 | 8.34 | 3.23 | |
| CAGL0K00715g | RTA1 | Involved in 7-aminocholesterol resistance | 2.75 | 3.93 | 0.89 | |
| CAGL0L13354g | THI7 | Putative nicotinamide transporter | 4.2 | 5.5 | 40.35 | |
| CAGL0C03289g | YBT1 | ABC transporter involved in bile acid transport | 2.98 | 5.11 | 1.67 | |
| CAGL0G00242g | YOR1 | ABC transporter involved in oligomycin/multidrug efflux | 7.04 | 2.74 | 0.58 | |
| Transcription | CAGL0K04257g | RME1 | Zinc finger protein involved in control of meiosis | 4.32 | 8.38 | 4.20 |
| CAGL0L05786g | YPR013c | Uncharacterized; potential zinc finger | 4.39 | 3.35 | 2.61 | |
| CAGL0M01870g | YPR013c | Uncharacterized; potential zinc finger | 3.74 | 2.23 | 0.77 | |
| DNA damage response | CAGL0M02035g | REV3 | DNA repair and translesion synthesis | 3.85 | 3.38 | 1.63 |
| CAGL0M09713g | YIM1 | Implicated in DNA damage response | 8.24 | 9.89 | 1.81 | |
| Biosynthesis | CAGL0H09064g | FUR1 | Putative uracil phosphoribosyltransferase | 2.57 | 4.78 | 1.68 |
| CAGL0M12881g | URA1 | Dihydroorotate dehydrogenase | 2.52 | 3.04 | 2.43 | |
| CAGL0L05676g | URA2 | Bifunctional carbamoylphosphate synthetase | 4.08 | 3.21 | 1.97 | |
| Cell wall | CAGL0I10362g | FLO5 | Cell wall adhesin | 17.12 | 15.35 | 1.68 |
| CAGL0B00616g | SPS22 | Implicated in organization of the beta-glucan layer of the spore wall | 3.5 | 10.72 | 1.21 | |
| Cell organization | CAGL0E01265g | NUM1 | Protein required for nuclear migration | 3.3 | 2.74 | 1.52 |
| Mitochondria | CAGL0M12232g | AIM1 | Involved in mitochondrial function or organization | 4.15 | 2.76 | 1.13 |
| CAGL0I09394g | FMP21 | Uncharacterized | 2.78 | 3.76 | 1.70 | |
| CAGL0D01496g | ISA2 | Protein required for maturation of mitochondrial and cytosolic Fe/S proteins | 2.16 | 4.24 | 1.03 | |
| CAGL0M12947g | YIL077c (PUP1) | Mitochondrial protein of unknown function | 16.17 | 22.93 | 1.89 | |
| CAGL0J04004g | YOR228c | Uncharacterized protein of the mitochondrial outer membrane | 4.48 | 3.1 | 0.94 | |
| Other metabolism | CAGL0K05775g | ACN9 | Carbon utilization and regulation of gluconeogenesis | 2.61 | 2.52 | 1.23 |
| CAGL0M07271g | GRX5 | Disulfide oxidoreductase activity and response to oxidative stress | 3.41 | 2.17 | 0.85 | |
| CAGL0E01705g | MDH2 | l-malate dehydrogenase | 2.16 | 3.37 | 1.20 | |
| CAGL0J00847g | SDH1 | Flavoprotein subunit of succinate dehydrogenase | 5.29 | 4.1 | 1.64 | |
| CAGL0E03850g | SDH2 | Iron-sulfur subunit of sucinate dehydrogenase | 4.63 | 2.52 | 1.21 | |
| CAGL0F05863g | SDH4 | Membrane anchor subunit of succinate dehydrogenase | 2.89 | 2.48 | 1.25 | |
| CAGL0M14091g | No similarity | Putative quinone reductase/NADPH dehydrogenase | 5.45 | 13.68 | 1.39 | |
| Uncharacterized | CAGL0L05434g | NCA3 | Uncharacterized | 2.46 | 13.28 | 2.14 |
| CAGL0M12210g | YAL044W-A | Uncharacterized | 3.37 | 2.33 | 1.11 | |
Δpdr1Δstb5 was added for comparison.
Motif sequence analysis.
The 5′ sequences of all 34 open reading frames presented in Table 4 were searched for regulatory protein binding motifs using the MEME Suite Motif-based sequence analysis tool (T. L. Bailey and C. Elkan, presented at the Second International Conference on Intelligent Systems for Molecular Biology, Menlo Park, CA, 1994). We selected the 1,500 bp immediately upstream of the transcription start site for each gene and searched for motifs with widths between 6 and 10 bp that were distributed any number of times per sequence.
CgSTB5 complementation.
The Saccharomyces cerevisiae stb5 Δyhr178w mutant strain was transformed with pH392-CgSTB5 using a Yeast Easy Transformation kit (Zymogen) and the transformant, S. cerevisiae Δyhr178wSTB5, assessed for susceptibility to fluconazole, voriconazole, hydrogen peroxide, caffeine, and cold temperature. Similarly, 84u was transformed with pGRB2.2-CgSTB5 to create strain CgSTB5OE. Putative transformants were screened by PCR using the primer set CgSTB5S and CgSTB5AS with the following parameters: 95°C for 2 min; 35 cycles of 95°C for 30 s, 53°C for 30 s, and 72°C for 2 min; and 72°C for 10 min.
RESULTS
Complementation of the Δyhr178w strain with CgSTB5.
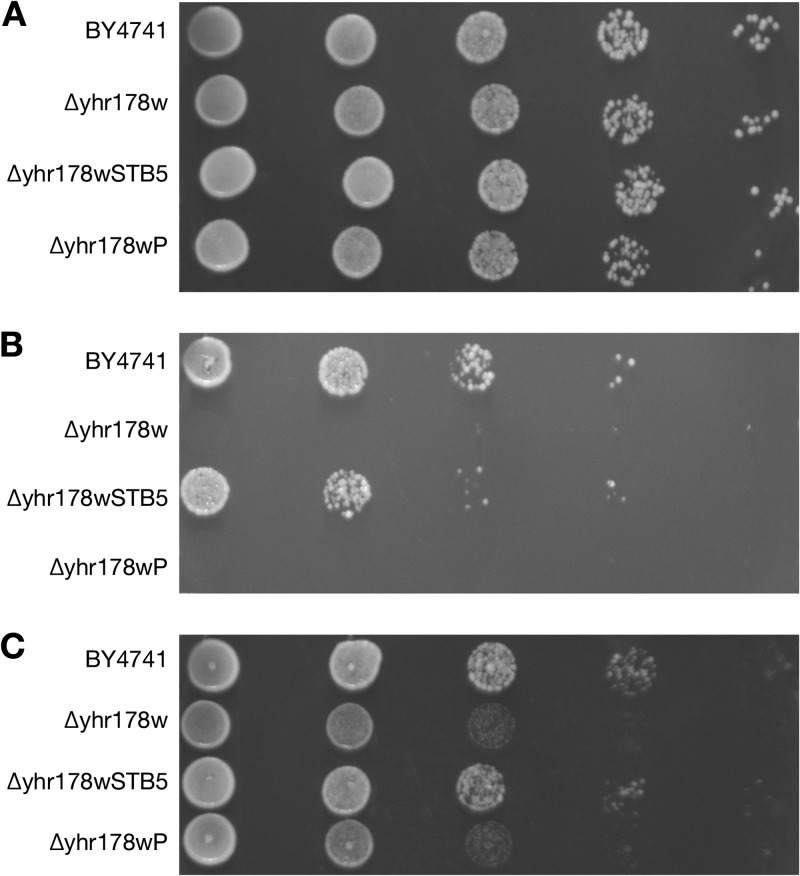
The susceptibilities of S. cerevisiae cells to cold and caffeine were determined by spotting S. cerevisiae cells on YPD agar. Overexpression of CAGL0I02552g in the Δyhr178w strain restored its wild-type sensitivity to caffeine (Fig. 1B), cold (Fig. 1C), and hydrogen peroxide (Fig. 2B) but not cycloheximide (data not shown). Both the parent and mutant were methionine and uracil auxotrophs, negating the value of testing these auxotrophies. Other phenotypes of the Δyhr178w strain were not assessed for complementation by CgSTB5.
Fig 1.
Complementation with CgSTB5 abrogates the sensitivities to caffeine and cold in the Δyhr178w mutant. (A) Growth on YPD agar plates at 30°C. (B) Growth in the presence of 0.15% caffeine. (C) Growth on YPD agar plates at 20°C. For strains, see Table 1. Cells were diluted to 1 × 105 cells per 5 μl, followed by four 1:10 dilutions, and 5 μl of diluted cell culture was spotted on YPD or YPD with 0.15% caffeine agar plates and incubated at 30°C or 20°C for 48 h.
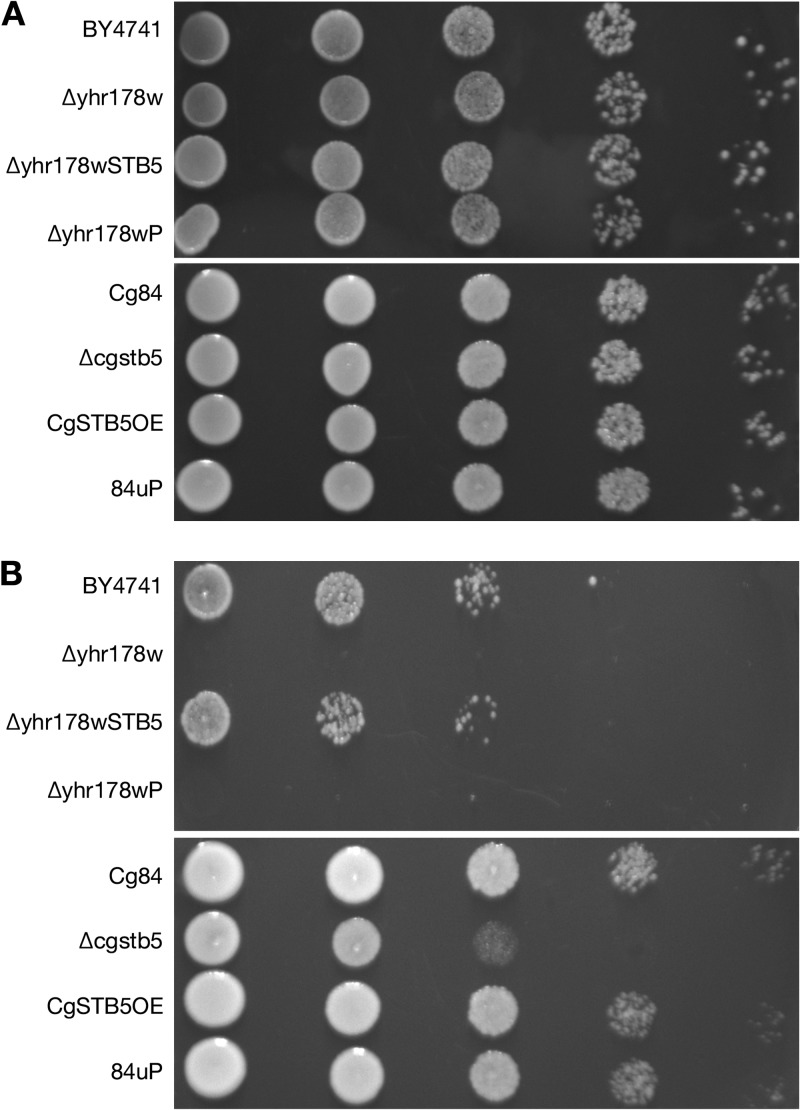
Fig 2.
S. cerevisiae and C. glabrata stb5 mutants are sensitive to hydrogen peroxide. Overexpression of CgSTB5 in 84u had no effect. (A) Growth on YPD agar plates. (B) Growth on YPD agar plates with hydrogen peroxide. Strains were diluted to 1 × 105 cells per 5 μl, followed by four 1:10 dilutions, and 5 μl of diluted cell culture was spotted on medium. S. cerevisiae strains were plated on YPD with 5 mM hydrogen peroxide, and C. glabrata strains were plated on YPD with 10 mM hydrogen peroxide. All plates were incubated at 30°C for 48 h.
Phenotype of the Candida glabrata STB5 null mutant.
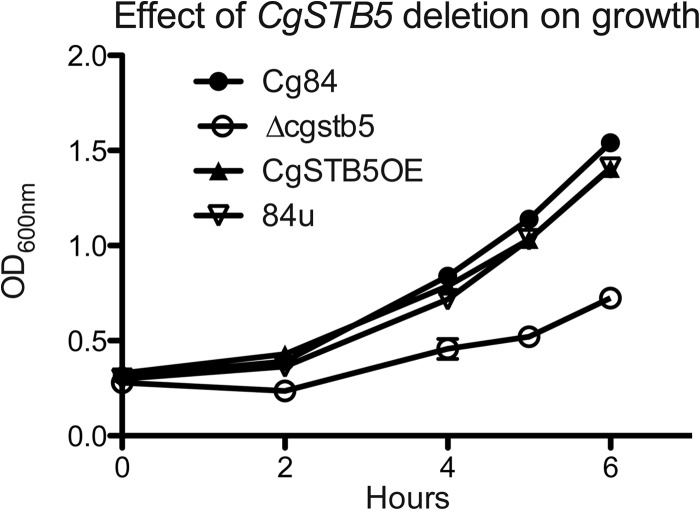
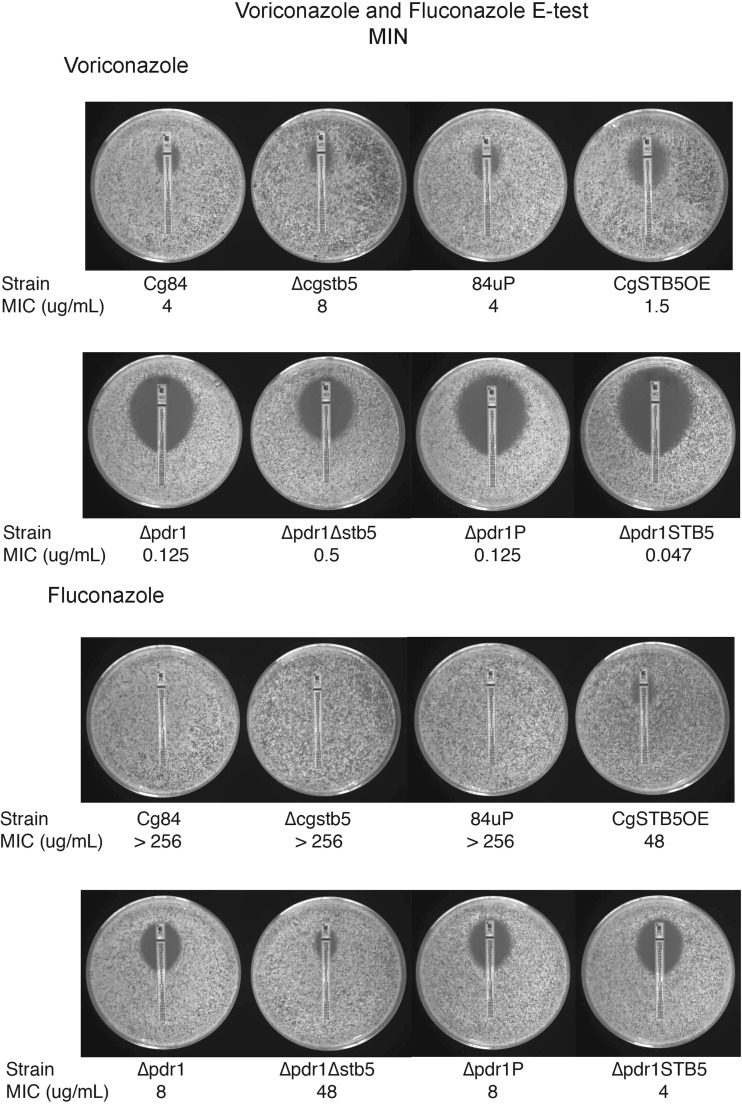
Deletion of CAGL0I02552g (CgSTB5) resulted in a mutant (C. glabrata Δcgstb5) that grew poorly on MIN agar plates with 2 mM hydrogen peroxide (Fig. 2B) and grew more slowly in MIN media at 30°C than the wild-type strain (Fig. 3). Overexpression of CgSTB5 in 84u had no effect on hydrogen peroxide susceptibility (Fig. 2B). Unlike the phenotypes reported for the Δyhr178w mutant, the Δcgstb5 mutant did not exhibit altered susceptibility to cycloheximide or caffeine and grew at 20°C. The ΔCgstb5 mutant consistently showed slightly decreased susceptibility to voriconazole by Etest (Fig. 4), though this could not be confirmed by the tube dilution MIC, which was 8 μg/ml in the host and the deletant. This difference also could not be detected with fluconazole (Fig. 4), but the high MIC seen with the host strain limited the value of this observation. Deletion of CgSTB5 decreased susceptibility to both azoles in the more susceptible Δpdr1 background (Fig. 4). Overexpression of CgSTB5 in the 84u and Δpdr1 backgrounds increased their azole susceptibilities. Microdilution susceptibility testing confirmed the effect of overexpression, though the MICs determined were different from those determined in the Etest. Microdilution testing found a 2-fold increase in fluconazole susceptibility in the CgSTB5-overexpressing strains. In the 84 strain, overexpression decreased the fluconazole MIC from 128 to 64 μg/ml. In the Δpdr1 background, CgSTB5 overexpression decreased the fluconazole MIC from 16 to 8 μg/ml.
Fig 3.
The deletion of CgSTB5 causes a growth defect. Deletion of CgSTB5 resulted in poor growth in minimal media. The doubling times for the Cg84, CgSTB5OE, Δcgstb5, and 84uP strains were determined to be 2.3, 2.5, 3.3, and 2.2 h, respectively.
Fig 4.
Decreased azole susceptibility of the Δcgstb5 mutant. The deletion of STB5 resulted in a decrease in susceptibility, and overexpression caused an increase in susceptibility to voriconazole and fluconazole. This finding was also reproducible in a pdr1 mutant.
CgSTB5 functions as a transcriptional repressor of ATP-binding cassette (ABC) transporter genes.
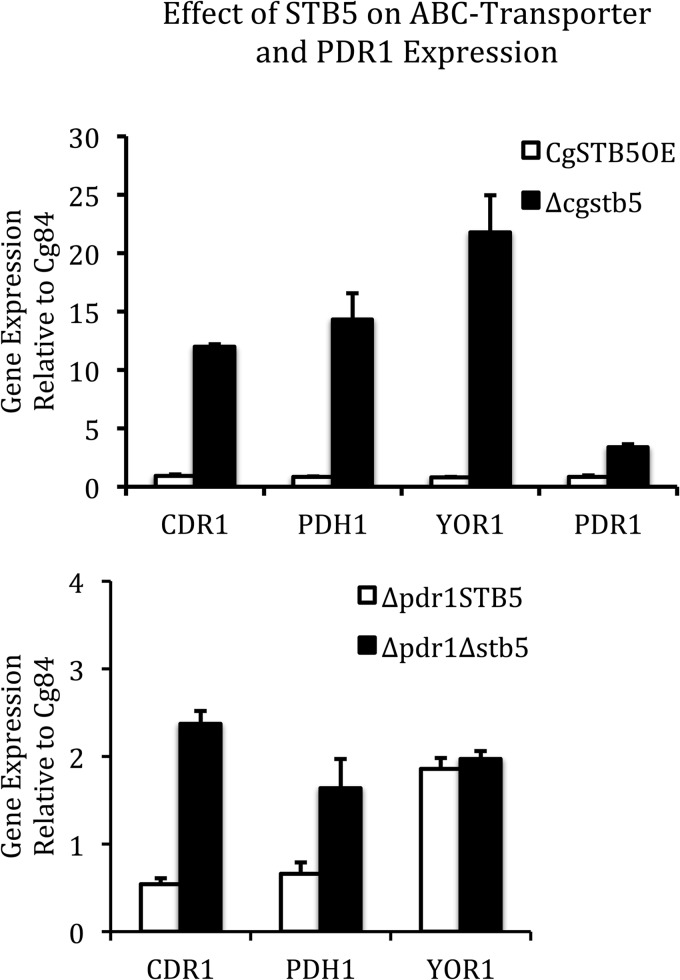
Whole-genome DNA microarray analysis was performed to determine the impact that the CgSTB5 deletion has on mRNA expression levels. Complete microarray results are available at http://www.ncbi.nlm.nih.gov/geo/ (acquisition no. GSE37071). In the Δcgstb5 strain, 68 genes were upregulated at least 2-fold relative to Cg84. Of the 68 genes upregulated by deletion of CgSTB5, 34 were found to be upregulated in the PDR1-overexpressing strain, indicating the presence of an overlapping regulon but with opposite transcriptional effects. These 34 genes are listed in Table 4 and grouped by annotated function. For comparison, expression analysis of PDR1 overexpressed in 84u is also shown. Of the genes upregulated in the Δcgstb5 mutant, nine genes, including the multidrug transporters PDR5 (CgCDR1), FLR1, and YOR1, have roles in small-molecule transport. Additionally, the 7-aminocholesterol-resistance gene RTA1 and the bile acid ABC transporter YBT1 were both upregulated by either PDR1 overexpression or STB5 deletion. Other proteins and genes listed in Table 4 that have been previously implicated in azole resistance include the transcription factor YIM1, the mitochondrial membrane protein encoded by YIL077c (PUP1), the l-malate dehydrogenase MDH2, and the open reading frame CAGL0M14091g, which encodes a putative quinone reductase/NADPH dehydrogenase (16, 17). Expression analysis by microarray found that deletion of CgSTB5 in a Δpdr1 host had much less of an impact on the same genes (Table 4). The microarray experiments, done in the absence of oxidative stress, did not allow us to assess the relationship between CgSTB5 and genes in the pentose phosphate pathway which respond to oxidative stress, such as GND1, TKL1, ZWF1, PGI1, and TAL1 (9). We did, however, address the impact of CgSTB5 deletion on the expression of GND1, PGI1, and ZWF1 under conditions of oxidative stress using qRT-PCR. We exposed early-log-phase Cg84 and Δcgstb5 cell cultures to 25 mM hydrogen peroxide for 15 and 30 min before isolating RNA. There was no significant difference between the levels of Cg84 and Δcgstb5 expression of GND1, PGI1, and ZWF1 at either time point (data not shown). The upregulation of ABC transporters CDR1, YOR1, and PDH1, as well as PDR1, in the Δcgstb5 mutant was confirmed by qRT-PCR in strain 84 (upper panel, Fig. 5). As seen in the lower panel of Fig. 5, two transporters were also upregulated in the Δpdr1 host, though at a lower magnitude (Fig. 5). Overexpression of CgSTB5 did not result in a substantial decrease in transporter expression, though it did cause increased azole susceptibility. Unlike the results seen with S. cerevisiae (13), the membrane transporter SNQ2 was not affected by deletion of CgSTB5 (data not shown) The CDR1, YOR1, PDH1, and PDR1 genes, as well as YIL077c, YIM1, and CAGL0M14091g, are thought to be targets of CgPDR1. Microarray analysis of CgSTB5OE, in which CgSTB5 was overexpressed 7-fold, was not informative. Only 1 of 26 genes downregulated 2-fold by overexpression of CgSTB5 was upregulated 2-fold in the deletant. None of the 25 genes that were upregulated 2-fold by overexpression were downregulated in the deletant. This lack of congruity between overexpression and deletion was interpreted to mean that the suppressive effect of CgSTB5 could be exposed by deletion but could be not increased by overexpression.
Fig 5.
Real-time PCR verification of microarray analysis. The deletion of CgSTB5 resulted in the upregulation of CgCDR1, CgPDH1, and CgYOR1, all members of the ABC transporter family of proteins, as well as CgPDR1, the transcriptional regulator of ABC transporters (upper panel). The same effect was seen in the Δpdr1 background but at a lower magnitude (lower panel). Overexpression of CgSTB5 did not significantly decrease transcription of these transporters despite increasing azole susceptibility. The standard error of the mean is shown for each gene.
DISCUSSION
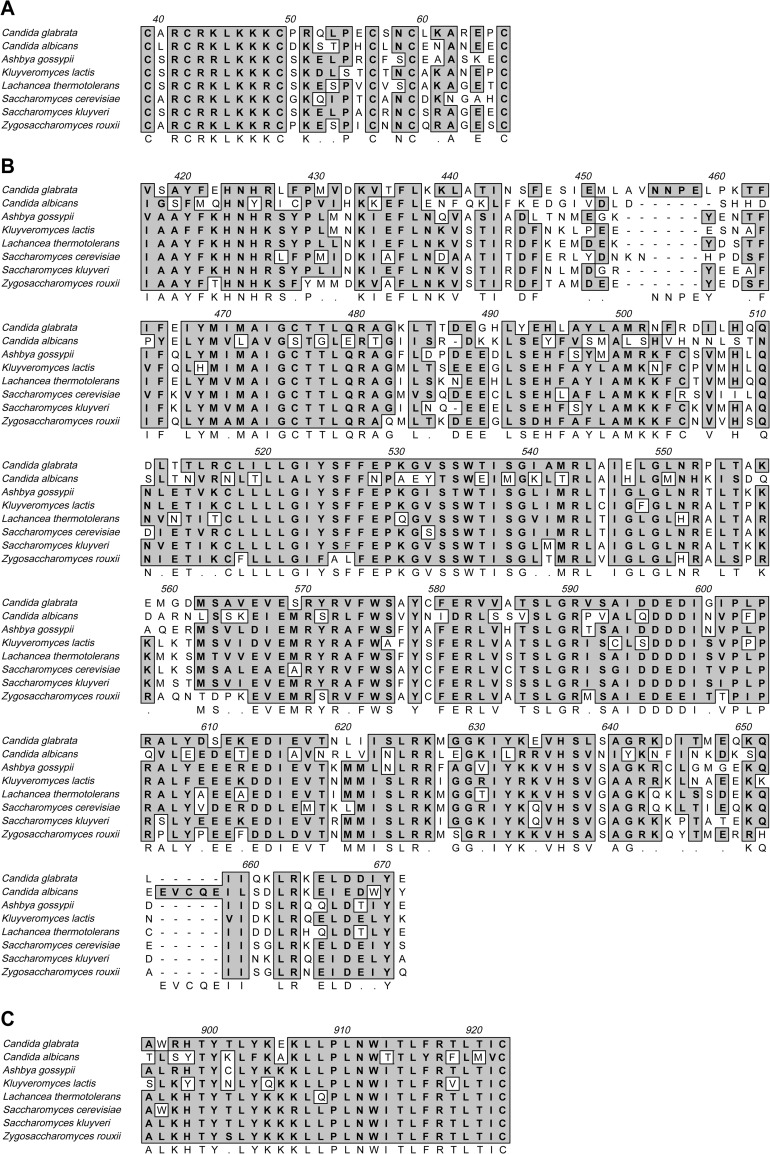
A C. glabrata ORF, CAGL0I02552g, codes for a protein conserved among hemiascomycete binuclear zinc cluster proteins, indicating a common ancestry and likely an important function in these yeasts (Fig. 6). The N-terminal GAL4-like Zn2Cys6 binuclear cluster DNA-binding domain (Fig. 6A) is highly conserved (18), as are the fungal transcription factor domain at amino acids 417 to 671 (Fig. 6B) and the C-terminal 200 amino acid residues (Fig. 6C). In addition, synteny is preserved between all eight species with FMO1 adjacent to the 3′ end, except for in S. cerevisiae, which has one intervening ORF. An NADPH dehydrogenase is located 5′ to STB5 in 4 of the 8 species. Insufficient information is available to evaluate phenotypic similarities among the 8 genes annotated as STB5. CgSTB5 is closely related to STB5 in S. cerevisiae in that they share 50.0% amino acid similarity and 38.8% identity. The ability of CgSTB5 to complement the Δyhr178w (Δstb5) strain in tolerance to cold, caffeine, and hydrogen peroxide indicates sufficient functional similarity to consider the C. glabrata gene a homologue of STB5. Although it is not shown in Fig. 6, Candida albicans has a gene, FCR1, with phenotypic but not genotypic resemblance to CgSTB5. FCR1 is a Zn2Cys6 transcription factor with only 7.8% amino acid identity to CgSTB5, shares no obvious synteny with it, and has no fungus-specific transcriptional factor. Similar to CgSTB5 and unlike the other genes in Fig. 6, FCR1 has a short polyglutamine repeat (Q4-L-Q5). FCR1 is a negative regulator of drug resistance, decreasing resistance to fluconazole, fluphenazine, cycloheximide, and brefeldin (19). FCR1 decreases the expression of the CDR1 ABC transporter and decreases the efflux of rhodamine 6G from the yeast cell (20). FCR1 and CgSTB5 are negative regulators of the major azole transporter, CDR1 in C. albicans and CgCDR1 in C. glabrata. The function of these genes is somewhat analogous to those of the negative regulator of drug resistance in S. cerevisiae, RDR1 (cycloheximide) (21), and the transporter HXT11, the loss of which confers 4-NQO, cycloheximide, and sulfometuron methyl resistance (22).
Fig 6.
Alignment of encoded amino acids in CgSTB5 homologues in 7 other yeasts. Numbers shown in the alignment are not identical to their order in CgSTB5p, which are as follows: (A) 32 to 59; (B) 408 to 657; (C) 881 to 908.
As stated previously, Pdr1p has been found to form a heterodimer with Stb5p in S. cerevisiae. Our transcriptional analysis indicated a shared regulon between the homologues of these two genes, i.e., CgPDR1 and CgSTB5, in C. glabrata. Many of the genes upregulated by overexpression of CgPDR1 were upregulated by deletion of CgSTB5 (Table 4). Pearson's correlation analysis showed that the CgPDR1 overexpression and CgSTB5 deletion microarray data were correlated, with a P value of <0.0001. The microarray result was confirmed by qRT-PCR, showing the effect of CgSTB5 deletion and overexpression on the major ABC transporter targets of PDR1: CDR1, PDH1, YOR1, and PDR1 (Fig. 5). The transporters were also upregulated in the Δpdr1 host (Fig. 5). A MEME search to find common elements in the genes coregulated by CgSTB5 and CgPDR1 5′ to the initiation ATG found only the pleiotropic drug response element (PDRE) of CgPDR1 (TCCACGGA/G) in 7 genes and no sequences similar to those reported for STB5 in S. cerevisiae [CGGN(c/g)TA] (13, 23). Whether the negative regulation by CgSTB5 is due to altered promoter occupancy of some transcriptional targets of CgPdr1p is unknown but is suggested by the coregulation and probable overlapping of motifs.
We searched the microarrays of eight paired azole-susceptible and -resistant isolates of C. glabrata that we have previously published but found none with a 2-fold difference in CgSTB5 expression (16). It is possible that the decrease in fitness of the Cgstb5 mutant (Fig. 1) decreases the likelihood that azole pressure would select for this mutation in the oropharynx.
ACKNOWLEDGMENTS
We thank Herman Edskes and Brendan Cormack for generously providing plasmids and Kathleen Meyer for assistance with GEO submission.
Financial support was provided by the NIAID Division of Intramural Research.
Footnotes
Published ahead of print 10 December 2012
REFERENCES
- 1. Bennett JE, Izumikawa K, Marr KA. 2004. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 48:1773–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai HF, Krol AA, Sarti KE, Bennett JE. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob. Agents Chemother. 50:1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. 2006. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol. Microbiol. 61:704–722 [DOI] [PubMed] [Google Scholar]
- 5. Vermitsky JP, Edlind TD. 2004. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents Chemother. 48:3773–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akache B, MacPherson S, Sylvain MA, Turcotte B. 2004. Complex interplay among regulators of drug resistance genes in Saccharomyces cerevisiae. J. Biol. Chem. 279:27855–27860 [DOI] [PubMed] [Google Scholar]
- 7. Akache B, Wu K, Turcotte B. 2001. Phenotypic analysis of genes encoding yeast zinc cluster proteins. Nucleic Acids Res. 29:2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasten MM, Stillman DJ. 1997. Identification of the Saccharomyces cerevisiae genes STB1-STB5 encoding Sin3p binding proteins. Mol. Gen. Genet. 256:376–386 [DOI] [PubMed] [Google Scholar]
- 9. Larochelle M, Drouin S, Robert F, Turcotte B. 2006. Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol. Cell. Biol. 26:6690–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsufuji Y, Nakagawa T, Fujimura S, Tani SA, Nakagawa J. 2010. Transcription factor Stb5p is essential for acetaldehyde tolerance in Saccharomyces cerevisiae. J. Basic Microbiol. 50:494–498 [DOI] [PubMed] [Google Scholar]
- 11. Pfaller MA, Jones RN, Doern GV, Sader HS, Messer SA, Houston A, Coffman S, Hollis RJ. 2000. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob. Agents Chemother. 44:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cadière A, Galeote V, Dequin S. 2010. The Saccharomyces cerevisiae zinc factor protein Stb5p is required as a basal regulator of the pentose phosphate pathway. FEMS Yeast Res. 10:819–827 [DOI] [PubMed] [Google Scholar]
- 13. Akache B, Turcotte B. 2002. New regulators of drug sensitivity in the family of yeast zinc cluster proteins. J. Biol. Chem. 277:21254–21260 [DOI] [PubMed] [Google Scholar]
- 14. Kitada K, Yamaguchi R, Arisawa M. 1996. Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene 175:105–108 [DOI] [PubMed] [Google Scholar]
- 15. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16. Tsai HF, Sammons LR, Zhang X, Suffis SD, Su Q, Myers TG, Marr KA, Bennett JE. 2010. Microarray and molecular analyses of the azole resistance mechanism in Candida glabrata oropharyngeal isolates. Antimicrob. Agents Chemother. 54:3308–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caudle KE, Barker KS, Wiederhold NP, Xu L, Homayouni R, Rogers PD. 2011. Genomewide expression profile analysis of the Candida glabrata Pdr1 regulon. Eukaryot. Cell 10:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacPherson S, Larochelle M, Turcotte B. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70:583–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talibi D, Raymond M. 1999. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J. Bacteriol. 181:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen H, An MM, Wang de J, Xu Z, Zhang JD, Gao PH, Cao YY, Cao YB, Jiang YY. 2007. Fcr1p inhibits development of fluconazole resistance in Candida albicans by abolishing CDR1 induction. Biol. Pharm. Bull. 30:68–73 [DOI] [PubMed] [Google Scholar]
- 21. Hellauer K, Akache B, MacPherson S, Sirard E, Turcotte B. 2002. Zinc cluster protein Rdr1p is a transcriptional repressor of the PDR5 gene encoding a multidrug transporter. J. Biol. Chem. 277:17671–17676 [DOI] [PubMed] [Google Scholar]
- 22. Nourani A, Wesolowski-Louvel M, Delaveau T, Jacq C, Delahodde A. 1997. Multiple-drug-resistance phenomenon in the yeast Saccharomyces cerevisiae: involvement of two hexose transporters. Mol. Cell. Biol. 17:5453–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hector RE, Bowman MJ, Skory CD, Cotta MA. 2009. The Saccharomyces cerevisiae YMR315W gene encodes an NADP(H)-specific oxidoreductase regulated by the transcription factor Stb5p in response to NADPH limitation. N. Biotechnol. 26:171–180 [DOI] [PubMed] [Google Scholar]