Abstract
Antibiotic treatment, including vancomycin, for Clostridium difficile infection (CDI) has been associated with recurrence of disease in up to 25% of infected persons. This study investigated the effects of vancomycin on the clinical outcomes, intestinal histopathology, and anaerobic community during and after treatment in a murine model of CDI. C57BL/6 mice were challenged with C. difficile strain VPI 10463 after pretreatment with an antibiotic cocktail. Twenty-four hours after infection, mice were treated daily with vancomycin, nitazoxanide, fidaxomicin, or metronidazaole for 5 days. Mice were monitored for either 6 or 12 days postinfection. Clinical, diarrhea, and histopathology scores were measured. Cecal contents or stool samples were assayed for clostridial or Bacteroides DNA and C. difficile toxins A and B. Vancomycin treatment of infected mice was associated with improved clinical, diarrhea, and histopathology scores and survival during treatment. However, after discontinuation of the drug, clinical scores and histopathology were worse in treated mice than in untreated infected controls. At the end of the study, 62% of the vancomycin-treated mice succumbed to recurrence, with an overall mortality rate equivalent to that of the untreated infected control group. Fidaxomicin-treated mice had outcomes similar to those of vancomycin-treated mice. C. difficile predominated over Bacteroides in cecal contents of vancomycin-treated mice, similar to findings for untreated infected mice. Decreasing the duration of vancomycin treatment from 5 days to 1 day decreased recurrence and deaths. In conclusion, vancomycin improved clinical scores and histopathology acutely but was associated with poor outcome posttreatment in C. difficile-infected mice. Decreasing vancomycin exposure may decrease relapse and improve survival in CDI.
INTRODUCTION
Clostridium difficile infection (CDI) is a significant cause of antibiotic-associated nosocomial diarrhea. While discontinuation of the offending antibiotic is the ideal strategy to control the disease, in most cases, treatment with antimicrobial agents active against C. difficile is deemed necessary because of the severity of the gastrointestinal disease or the presence other active infections. Unfortunately, antimicrobial treatment for a first episode of CDI is associated with up to 25% recurrence of the disease (1). After one incidence of recurrence, rates increase to up to 60% (2). A recurrence rate of up to almost 50% has been noted with metronidazole use (3). Even in the carrier state, it had been shown that treatment with either metronidazole or vancomycin of individuals with C.difficile is associated with the reisolation of the organism in the stool 2 months later, with recurrence not necessarily coming from the original strain (4).
The recent epidemic saw the increasing failure of metronidazole to cure CDI (3, 5, 6). Vancomycin is now the drug of choice for severe disease (1, 7). Fidaxomicin, a drug newly approved by the FDA, the European Medicines Agency, and Canada Health, is at least as effective as vancomycin in treating acute infection but has been shown to have less recurrence (8). However, in infections due to NAP1/BI/027, the rates of recurrence in subjects treated with fidaxomicin and vancomycin were similar: 24.4% and 23.6%, respectively. Treatment of recurrent disease is problematic. For the first recurrence, a repetition of the regimen for the initial episode of CDI is recommended. Although there are no solid data for efficacy, prolonged and tapering or pulsed doses of vancomycin are the recommended strategy to treat a second recurrence (1). Even less evidence is available for alternative therapies for succeeding recurrences. Other antibiotics such as nitazoxanide and rifaximin, among others, had been considered (9, 10).
Alteration of the indigenous intestinal flora is critical to susceptibility to CDI and its recurrence. Antibiotic treatment may further disrupt the already abnormal flora and thereby enhance the growth of any leftover C. difficile organisms or of a newly acquired strain once antibiotics are discontinued. Using a murine model of CDI, we compared the effects of vancomycin and alternative antibiotics—fidaxomicin, metronidazole, and nitazoxanide—in treated mice versus controls with respect to clinical disease, relapse, mortality, intestinal histopathology, and fecal clostridial, toxin, and Bacteroides levels.
MATERIALS AND METHODS
Murine model of C. difficile infection and treatment.
The infection model is a modification of the published protocol of Chen et al. (11). This protocol has been approved by the Center for Comparative Medicine at University of Virginia. C57BL/6 mice, male, 8 weeks old, were used. From 6 to 4 days prior to infection, mice were given an antibiotic cocktail containing vancomycin (0.0045 mg/g), colistin (0.0042 mg/g), gentamicin (0.0035 mg/g), and metronidazole (0.0215 mg/g) in drinking water. One day prior to infection, clindamycin (32 mg/kg of body weight) was injected subcutaneously. The mice were divided into the following groups: control uninfected, control infected, infected and treated with vancomycin (20 mg/kg), and infected and treated with comparator drugs—nitazoxanide, fidaxomicin, and metronidazole (all drugs given at 20 mg/kg/day). Food and water were allowed ad libitum. Although each mouse or treatment group was housed in a separate cage, all mice were housed in the same pod of the vivarium. Infection was performed with VPI 10463 (ATCC) as an inoculum of 104 or 105 administered by oral gavage. This strain produces both C. difficile toxins A (TcdA) and B (TcdB). One day postinfection, treated mice were given either vancomycin or nitazoxanide at 20 mg/kg each by oral gavage daily for 5 days and monitored for either 1 or 2 weeks postinfection. One set of experiments was performed in which infected mice were treated with vancomycin (50 mg/kg) daily for 1, 2, 3, or 5 days and were observed for 21 days postinfection or with vancomycin (20 mg/kg) daily for either 5 or 10 days and monitoring for 15 days postinfection. In a separate experiment, mice given a preinfection antibiotic regimen described above were treated with either vancomycin, fidaxomicin, or metronidazole at 20 mg/kg/day for 5 days and infected another 5 days later. Except when indicated, all comparator drugs were administered using the same dosage (20 mg/kg/day for 5 days) to equally compare efficacies, outcomes, and effects on selected gut floras between treatment groups as previously described (12). From another study, a group of control mice was given vancomycin but was not infected. A clinical scoring system was developed on the basis of weight loss, diarrhea, activity level, and appearance of eyes and hair (each parameter scored from 0 to 3, where 0 is normal and 3 is the worst; maximum score of 20) (12). Stool specimens were collected daily. Diarrhea was scored as follows: 1 for soft or color change (yellow), 2 for wet tail or mucoid, and 3 for liquid or no stool (ileus). Mice judged moribund by the clinical score (score of >14) at any day and all surviving mice at the end of the experiment were sacrificed, and intestinal tissues and cecal contents were collected as described below. A separate set of experiments was performed for harvesting cecal contents for clostridial bacterial and toxin burdens at days 3, 6, 9, and 12 to 13 postinfection to follow changes at different time points of the study.
Histopathology.
Upon euthanasia, cecal and colonic tissues were fixed in 10% zinc formalin overnight and then placed in 10% ethanol before being sent for paraffin embedding and hematoxylin and eosin (H&E) staining at the University of Virginia Histology Research Core. Histopathologic scoring was performed coded (C.A.W. and M.S.R.). H&E-stained tissues were scored for mucosal disruption, mucosal hypertrophy, inflammation, vascular congestion and exudates, and submucosal edema (each parameter was graded from 0 to 3, with 0 as normal and 3 worst; maximum score of 15) as we previously described in detail (13).
Quantification of C. difficile and Bacteroides.
Genomic DNA was extracted from the stool or cecal samples using a modified protocol for the QIAamp DNA stool minikit (Qiagen). Stool lysis buffer (400 μl) was added to a frozen stool sample-diluent (from enzyme-linked immunosorbent assay [ELISA]) mixture. Samples were then vortexed for 15 s and incubated at 82.5°C for 5 min. The samples were vortexed for another 15 s and then centrifuged at 14,000 rpm for 2 min. The supernatant was added to a clean 1.5-ml microcentrifuge tube. Thirty microliters of proteinase K was added to the wall of each tube, followed by 400 μl of lysis buffer. Samples were vortexed for 15 s and then incubated at 70°C for 10 min. After incubation, 400 μl of 100% ethanol was added to each sample and then mixed by vortexing. A total of 650 μl of the sample was then added to a spin column provided in the kit and centrifuged at 14,000 rpm for 1 min. The remaining volume of each sample was then added to the spin column and centrifuged under the same conditions. The samples were washed first with 500 μl of AW1 buffer and centrifuged at 14,000 rpm for 1 min and then with 500 μl of AW2 buffer and centrifuged at 14,000 rpm for 3 min. The DNA was eluted by adding 200 μl of elution buffer to the spin column and then centrifuging at 14,000 rpm for 1 min. All quantitative PCRs (qPCRs) were performed on the Bio-Rad iCycler using primers to detect the toxin B (tcdB) gene (forward, GGAGAGTCATCCAACTTATATG; reverse, CCACCAATTTCTTTTAATGCAG) or gene sequence common to Bacteroides spp. (forward, GGARCATGTGGTTTAATTCGATGAT; reverse, AGCTGACGACAACCATGCAG) (14). Mixtures for qPCRs, in a final volume of 20 μl, consisted of 10 μl of QuantiFast SYBR green Supermix (Qiagen), 1 μl each of forward and reverse primers, 4 μl of sterile water, and 4 μl of sample DNA. PCR assays were performed in duplicate under the following cycling conditions: 3 min at 95°C, 10 s at 95°C, and 30 s at 57°C (59°C for Bacteroides) for 40 repeats and a melt curve between 65°C and 95°C of 5 s at 0.5°C intervals. For both C. difficile and Bacteroides spp., standard curves were generated from known concentrations of bacteria grown under anaerobic conditions. Bacterial counts were expressed as counts or colonies per mg of mouse stool assayed.
C. difficile toxin assay.
C. difficile toxins A and B were detected using a modified protocol for the Tech Lab Toxin A/B II ELISA kit. Each stool sample was weighed and the amount of diluent per sample was normalized to provide the same stool mass-to-diluent ratio for each sample. The diluent-sample mixtures were homogenized by grinding and vortexing, and 1:10, 1:100, and 1:1,000 serial dilutions were made of the sample. A total of 150 μl of the 1:1,000 dilution of each sample was added to a precoated well provided in the kit. A negative control consisted of 150 μl of diluent, and a positive control consisted of 135 μl of diluent plus 3 drops of the positive control toxin A-B mixture provided in the kit. One drop of conjugate was added to each well, and the plate was incubated at 37°C for 50 min. Each well was washed three times with 150 μl of a 1× dilution of the wash buffer provided in the kit. Two drops of substrate were added to each well. After 10 min, 1 drop of stop solution was added to each well. The plate was allowed to sit for 2 min before being read in an ELISA reader.
Statistical analysis.
Results were expressed as means ± standard errors of the means (SEM), as generated by GraphPad Prism, version 5.0 (GraphPad Software, San Diego, CA). The differences between experimental groups were compared using analysis of variance (ANOVA) with Bonferroni's multiple-comparison test. Two-way ANOVA was used to compare experimental groups across time periods. If needed, differences between 2 groups were analyzed using unpaired Student's t test. Mortality rates between treatment groups were analyzed with the log rank (Mantel-Cox) test or the log rank test for trend. Statistical significance was set at a P value of <0.05.
RESULTS
Effects of vancomycin on acute CDI (1st week postinfection).
Mice infected with VPI 10463 showed clinical symptoms 2 days after inoculation with the bacteria by gastric gavage. Clinical disease scores, weight loss, and diarrhea, among other parameters, were noted to increase at day 2 (Fig. 1B). Vancomycin treatment of infected mice was associated with improved mean clinical score versus that for infected controls (1.7 ± 0.3 versus 5.7 ± 0.9; P < 0.01), while no difference was seen in nitazoxanide-treated mice (5 ± 1 versus 5.7 ± 0.9) in the first week after infection. Likewise, weights in infected mice were minimally affected by vancomycin treatment. Infected mice which started treatment with vancomycin 1 day after inoculation remained relatively well, almost similar to uninfected control mice, during treatment. In comparison, the infected group treated with nitazoxanide had deaths between days 2 and 6, similar to the infected control group. Untreated infected mice had an overall survival rate of 38% in this study, and vancomycin prevented 100% of these deaths during the acute infection period (Fig. 1A). Nitazoxanide was able to prevent deaths in infected mice by 60% only.
Fig 1.

Effect of vancomycin on Clostridium difficile-infected mice during acute infection and posttreatment. C57BL/6 mice were inoculated with VPI 10463 at 104 to 105 by oral gavage on day 0. The results were data pooled from 94 mice from 3 1-week-long and 2 2-week-long experiments: 20 uninfected mice, 31 infected mice, 26 mice infected and treated with vancomycin, and 17 mice infected and treated with nitazoxanide. (A) Survival curve. P < 0.0001 for uninfected versus infected mice, P = 0.0064 for infected mice versus mice infected and treated with vancomycin, and P = NS for infected mice versus mice infected and treated with nitazoxanide by the log rank (Mantel-Cox) test. (B) Mean diarrhea scores. *, P < 0.05 to 0.001 for uninfected versus infected mice; #, P < 0.05 to 0.001 for infected mice versus mice infected and treated with vancomycin; and ##, P < 0.05 for uninfected mice versus mice infected and treated with vancomycin; statistical significance was determined by two-way ANOVA with Bonferroni's correction.
Effects of vancomycin treatment after acute CDI (2nd week postinfection).
Infected mice which did not receive antimicrobial treatment but survived acute infection (first week postinfection) were able to recover almost completely during the second week of the study (Fig. 1). No additional deaths occurred during the post-acute infection period. Likewise, infected mice treated with nitazoxanide continued to recover, and no additional deaths were noted. Interestingly, vancomycin-treated mice started exhibiting signs of infection a few days after the last dose of the drug was given. The mean clinical scores in the second week postinfection were worse for vancomycin-treated mice than for surviving untreated infected mice (6.2 ± 0.2 versus 2.6; P < 0.001). Clinical scores worsened, weight loss was observed, and deaths (62%) occurred by day 9 and onwards in the vancomycin-treated group.
Of note, in a separate study, uninfected mice treated with a 5-day course of vancomycin alone did not show weight loss or an increase in disease score (see Fig. S1 in the supplemental material).
Histopathologic changes during and after acute CDI.
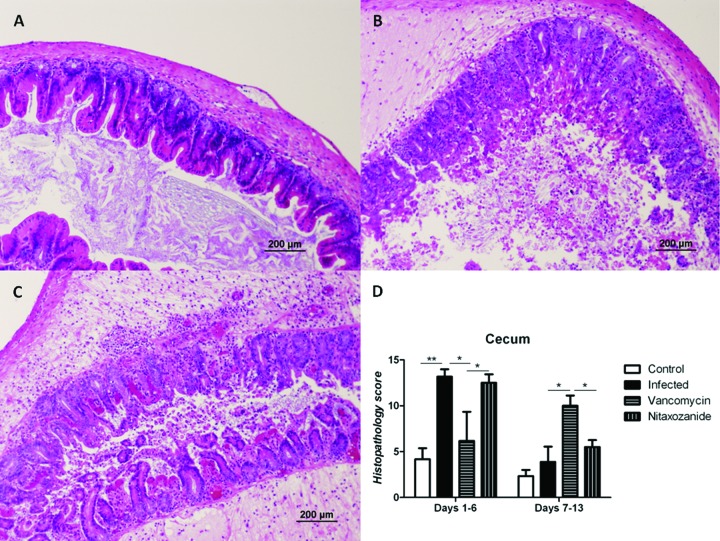
To investigate further the underlying changes that infection and antibiotic treatment cause at the site of infection, we pooled the histopathologic scores of cecal and colonic tissues from moribund mice during the acute infection period of the study (1st week postinoculation) and compared these with the scores of tissues collected after acute infection (2nd week postinoculation). In one experiment, intestinal tissues from 3 mice each from the uninfected control and vancomycin treatment groups were collected at day 3 (peak of infection) for comparison with the others at the 1st week. As expected, in the first week, untreated infected mice had the worst histopathology (12.5 ± 0.7), followed by nitazoxanide-treated mice (11.53 ± 0.9). Only minimal intestinal tissue changes were seen in vancomycin-treated infected mice (4.5 ± 1.6 versus 2.2 ± 1 in uninfected control mice; P value, not significant [NS]) at the peak of infection. By the second week of observation, infected control mice had near-normal intestinal tissue morphology, similar to that of uninfected control mice (2.7 ± 0.9 versus 1.6 ± 0.26, respectively). In contrast, vancomycin-treated mice had significantly worse histopathology scores than infected control mice (9.3 ± 0.9 versus 2.7 ± 0.9, respectively; P < 0.001) in this period. Compared to the elevated score (11.53 ± 0.9) in the first week, nitazoxanide-treated mice showed improved histopathology by the 2nd week, with scores (4.2 ± 0.6) now not significantly different from those of the uninfected controls (1.6 ± 0.26). Figure 2 shows representative H&E-stained sections and histopathologic scores of cecal tissues from the different treatment groups.
Fig 2.
Intestinal histopathology. Shown are representative H&E-stained cecal tissues from infected mice treated or not with vancomycin for 5 days. (A) Uninfected control; (B) infected cecum at day 3; (C) vancomycin treated infection at day 9 (relapse); (D) histopathology scores of cecal tissues of uninfected mice (n = 12), infected mice (n = 16), mice infected and treated with vancomycin (n = 15), and mice infected treated with nitazoxanide (n = 16) moribund (at any day) or sacrificed (at the end of the experiment). *, P < 0.01, and **, P < 0.001, as determined by two-way ANOVA with Bonferroni's correction.
Fecal burden of clostridial infection and toxin posttreatment.
To determine whether the delayed histopathologic changes and increased mortality were associated with increased clostridial burden, we evaluated the presence of the C. difficile gene tcdB in cecal specimens from mice treated or not with vancomycin at the peak of infection (day 3), post-vancomycin treatment (day 6), at relapse (day 9), and at the end of the experiment (day 12). C. difficile DNA (tcdB) and toxin levels in the vancomycin-treated group were low, almost similar to those in the uninfected group on day 3 (Fig. 3A and B). At day 6 (1 day after antibiotic was discontinued), increased clostridial burden was noted in the treatment group, which persisted on days 9 and 12. Similarly, toxin levels were lower on day 3 but increased progressively at the succeeding time points. Consistent with their initial low bacterial and toxin levels, vancomycin-treated mice sacrificed at day 3 had reduced histopathology scores compared with those of infected controls (Fig. 3C). Of note, all mice (including uninfected controls) underwent preinfection antibiotic cocktail treatment. Uninfected mice, even without overt disease, were observed to shed tcdB and/or TcdB.
Fig 3.

Clostridial and toxin burdens in cecal contents at peak of infection, post-vancomycin treatment, and at relapse. Mice were infected with VPI 10463 (105) and treated (vanc; n = 9) or not (infected control [IC]; n = 9) with vancomycin. The uninfected group (control) had 9 mice also. Cecal contents and tissues were collected at days 3 (peak of infection), 6 (postvancomycin), 9 (relapse), and 12 (end of experiment). (A) Cecal tcdB shedding; (B) TcdA and TcdB levels in cecal contents; (C) histopathology scores.
Effects of duration of vancomycin treatment on clinical disease and histopathology.
To evaluate if the survival from CDI correlated with the length of exposure to vancomycin, various durations (1, 2, 3, and 5 days) of treatment were tested in the mouse model. In this study, we used 50 mg, instead of 20 mg, of vancomycin to indirectly assess the effect of a higher dose of the drug on CDI. Vancomycin at 50 mg, given for 5 days, had an overall mortality rate of 100%, compared to 75% in infected controls (Fig. 4). As the duration of treatment was progressively decreased from 5 days to 1 day, survival from CDI increased. As observed in weights, clinical scores, and diarrhea scores, the advantage of vancomycin was consistently evident only during treatment of acute infection; weights decreased and scores worsened few days after discontinuation of vancomycin. The least weight loss and best clinical outcomes were noted in the group that was treated with the vancomycin for 1 day only. Combined histopathologic scores (regardless of day of tissue harvest) showed a trend of decreased intestinal injury in 1- and 2-day versus 3- and 5-day courses, although these differences were not statistically significant (Fig. 4B).
Fig 4.

Effect of duration of vancomycin treatment on disease and survival in mice with CDI. For panels A and B, mice were infected with VPI 10463 (105) and treated or not with vancomycin (50 mg) given for 1, 2, 3, or 5 days. Each treatment group, including uninfected and infected controls, had 8 mice each. For panel C, infected mice were treated with vancomycin (20 mg) given for either 5 days or 10 days. Each treatment group had 6 mice. (A) Survival curve. P = 0.026 by log rank test for trend; P = 0.004 for infected versus uninfected mice and P = 0.092 for infected controls versus mice treated with vancomycin for 1 day by log rank (Mantel-Cox) test. (B) Histopathology scores of colonic and cecal tissues at time of sacrifice and/or at the end of the observation period. (C) Survival curve. P = 0.3790 by log rank test for trend.
Next, we compared the outcomes in infected mice treated with vancomycin (20 mg/kg/day) given for 5 and 10 days. Consistent with what is described above, relapse from the 5-day treatment course occurred 4 days later (Fig. 4C). Interestingly, the 10-day treatment course resulted to earlier relapse—3 days after discontinuation of the antibiotic—and a higher mortality rate. In both treatment groups, weights were stable and the clinical score was <1 until recurrence of disease.
Outcome of infection in mice treated with or exposed to vancomycin, fidaxomicin, or metronidazole.
We compared infected mice treated with vancomycin to those treated with either fidaxomicin or metronidazole, with all antibiotics given at 20 mg/kg/day for 5 days. Compared to untreated infected mice and similar to mice treated with vancomycin, mice that received fidaxomicin did not have weight loss or diarrhea while on antibiotics (see Fig. S2A and B in the supplemental material), but almost all eventually succumbed to the disease a few days after treatment (Fig. 5A). Metronidazole-treated mice had diarrhea and weight loss during treatment, although to a lesser degree than infected controls. During treatment, one mouse from the metronidazole-treated group, versus none from other treated groups, died of infection. However, while most of the infected mice treated with vancomycin (67%) or fidaxomicin (83%) relapsed, none of the metronidazole-treated or untreated infected controls had a recurrence of the disease. Bacterial, toxin shedding, and histopathology scores are shown in Fig. 5B and C.
Fig 5.

Effect of vancomycin, fidaxomicin, or metronidazole on Clostridium difficile-infected mice during acute infection and posttreatment. C57BL/6 mice were inoculated with VPI 10463 at 105 by oral gavage on day 0. Anti-C. difficile drugs (20 mg/kg/day) were given from days 1 to 5. Each group had 6 mice. (A) Survival curve. P = 0.42 by log rank test for trend. (B) Fecal TcdA and TcdB levels. OD, optical density. (C) Histopathology scores from sacrificed moribund mice (at any day) and surviving mice (at the end of the study). P = 0.36 by one-way ANOVA.
To determine the effects of the anti-C. difficile drugs on uninfected mice and their predisposition to a later infection, uninfected mice were exposed to vancomycin, fidaxomicin, or metronidazole prior to infection. Except for mild weight loss (<10%) in mice treated with fidaxomicin and metronidazole, the overall disease scores in all treatment and untreated control groups were similar prior to infection (see Fig. S2C and D in the supplemental material). Upon infection, mice previously exposed to the antibiotics developed disease, whereas none of the untreated controls did. All antibiotic-treated groups were equally susceptible to death from infection (Fig. 6A).
Fig 6.
Effect of preexposure to vancomycin, fidaxomicin, or metronidazole on C. difficile-infected mice. All mice were pretreated with an antibiotic cocktail from day −6 to day −1 (described in the text), then given anti-C. difficile drugs (20 mg/kg/day) or not (untreated control) from days 1 to 5, and then infected with VPI 10463 at day 10. Each treatment group had 6 mice. (A) Survival curve. P = 0.11 by log rank test for trend. (B) Fecal TcdA and TcdB levels by ELISA. (C) Fecal Bacteroides levels by PCR. (D) Histopathology scores. P = 0.03 by one-way ANOVA.
Effects of anti-C. difficile treatment on cecal clostridial burden and anaerobes.
Given that antibiotic treatment during CDI was associated with relapses and that exposure to anti-C. difficile drugs predisposed to a later infection, we then investigated whether cecal clostridial burden and toxin levels or the anaerobic bacterial community would also be altered by the treatment. Bacteroides spp. are the most common anaerobes in the gut and were therefore chosen to represent the anaerobic flora other than C. difficile (15). The amounts of clostridial DNA and toxin from the cecal contents of infected mice that received vancomycin were similar to those in untreated infected mice (see Fig. S3 in the supplemental material). All groups had uniformly low levels of Bacteroides (102 to 104/mg of stool). Except for uninfected controls, all infected mice had overgrowth of C. difficile in their cecal contents as measured by the C. difficile/Bacteroides ratio. Antibiotic treatment of uninfected mice variably affected the anaerobic community in the treated group (Fig. 6C). Untreated controls, although showing an alteration in gut Bacteroides spp. similar to those in treated groups, when infected with the same amount of C. difficile did not develop disease (see Fig. S2C and D in the supplemental material) and had normal histology at the end of the observation period (Fig. 6D).
DISCUSSION
Vancomycin is the current drug of choice for severe CDI (1). In this report, we have demonstrated that while vancomycin was effective in controlling acute infection, the antibiotic-treated infected mice were highly susceptible to severe disease of a later onset, resulting in overall mortality rates at least similar to those of untreated infected controls. In the mouse infection model, vancomycin was not able to completely eradicate C. difficile bacterial and toxin shedding, thus perpetuating clostridial predominance over the commensal anaerobes in the intestinal lumen. Moreover, we confirmed that fidaxomicin-treated mice had outcomes similar to those of vancomycin-infected mice.
In humans, vancomycin had been shown to be more effective than metronidazole in treating severe disease (7). Other newer antibiotics also claim efficacy against CDI (8–10, 16–18). However, recurrence of CDI after an initial successful treatment with antibiotics occurs in 24%, and the rate increases to 65% in those patients with a history of a prior recurrence (19). In our animal study, both vancomycin and fidaxomicin were able to control disease during treatment but were uniformly ineffective in preventing recurrent disease. Similar observations with vancomycin had been reported by others (11, 20). Mechanisms underlying recurrent disease remain unclear. Inability to eradicate spores may lead to a later recrudescence when the anti-C. difficile agents are stopped. While highly active against vegetative bacteria in vitro (21), vancomycin was shown to have no antispore activity (22), and although fidaxomicin was reported to inhibit sporulation (23), its activity against preexisting spores is unproven. Second, inadequate suppression of toxin production may also contribute to recurrence of disease. Clinical studies have shown that individuals that had a robust anti-C. difficile toxin A (TcdA) or B (TcdB) response were less susceptible to symptomatic infection and experienced fewer reinfections (24, 25). The use of monoclonal antibodies against TcdA and TcdB, after standard antibiotic treatment, was shown to be effective in reducing recurrences (26). The toxin binder tolevamer, although inferior to comparator drugs in treating acute infection, was also associated with significantly less recurrence (27). Failure to completely suppress toxin production in situ (as fecal levels of TcdA and TcdB were actually depressed in treated mice) by either vancomycin or fidaxomicin may partially explain recrudescence of the disease.
Inadvertent inhibition and delay of recovery of gut anaerobes and other beneficial floras because of further antibiotic use may also increase susceptibility to relapse or reinfection. In contrast to antibiotic-treated mice, untreated infected mice that survived acute infection recovered completely, suggesting eventual intestinal mucosal recovery in the absence of antibiotics if acute infection is overcome. These observations suggest that anti-C. difficile treatment may only delay recovery of the intestinal flora, which is essential in preventing the overgrowth of the pathogen. One study has evaluated the effects of vancomycin in the intestinal microbiotas of 10 individuals pretreated with cefuroxime (28). In these subjects, vancomycin treatment caused decreases in Enterococcus and anaerobes, including Bifidobacterium and Bacteroides species. Enterococcus with decreased susceptibility or resistant to vancomycin and other vancomycin-resistant strains of Pedicoccus, Lactobacillus, and various Gram-negative organisms emerged. Interestingly, although not as effective in treating acute infection, nitazoxanide and metronidazole were associated with relapse to a lesser degree. We have previously reported that nitazoxanide and its novel derivative amixicile selectively inhibit pyruvate-ferredoxin oxidoreductase in anaerobic bacteria, possibly sparing beneficial bacteria such as Lactobacillus spp., Bifidobacterium, and Enterobacteriaceae that lack this drug target (12). On the other hand, metronidazole has a very broad spectrum of anaerobic coverage. However, its activity against certain beneficial floras may be species and dose dependent. In this study, we used the same dosage (20 mg/kg/day) for all comparator drugs as previously published (12). Although found to be effective against infection, this dose of metronidazole may be lower than those of the other drugs relative to what is given in human disease and probably, did not have the same effect in the gut flora, resulting in less recurrence. The newly FDA-approved drug fidaxomicin was reported to cause less disruption of the gut flora in infected patients than vancomycin (29, 30). It apparently spared Bacteroides species and affected less of clostridial clusters XIVa and IV and Bifidobacterium during treatment for CDI. It has been shown to be at least noninferior to vancomycin in treating CDI and was associated with significantly lower rates of recurrence, but only in non-NAP1 strains (8). However, similar to what was observed in clinical trials in individuals infected with NAP1, we demonstrated that the rates and severity of recurrence in mice treated with fidaxomicin resembled those of vancomycin-treated mice. Thus, it appears that while the gut microbiome may be essential, antibiotic-induced alteration in several other factors, such as host and bacterial factors, are probably also significantly involved in the development of recurrent CDI. Moreover, other non-Bacteroides members of the gut flora, that are susceptible to vancomycin or fidaxomicin, may play a more critical role in controlling infection. That decreasing the duration of treatment with vancomycin reduced recurrence and improved survival in treated mice confirms that further exposure to antibiotics, even agents active against C. difficile, is detrimental to this antibiotic-induced disease.
At present, the use of drugs active against C. difficile is critical in controlling moderate to severe infection. In our study, we demonstrated that vancomycin, while very effective in treating acute CDI in the mouse model, is associated with persistent predominance of toxigenic C. difficile over Bacteroides in the gut and recurrence of severe disease posttreatment. Decreasing the dose and duration of vancomycin treatment actually improved overall survival and prevented recurrences in infected mice. These findings suggest that limited antibiotic exposure may be sufficient to control the disease and may actually improve outcomes. Investigating the optimal duration of antimicrobial treatment for CDI and employing adjunctive therapies that limit the use of antibiotics would be ideal.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID U01 grants AI075520 and AI075526.
Footnotes
Published ahead of print 12 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00877-12.
REFERENCES
- 1. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 31:431–455 doi:10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 2. Surawicz CM, Alexander J. 2011. Treatment of refractory and recurrent Clostridium difficile infection. Nat. Rev. Gastroenterol. Hepatol. 8:330–339 doi:10.1038/nrgastro.2011.59 [DOI] [PubMed] [Google Scholar]
- 3. Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591–1597 doi:10.1086/430315 [DOI] [PubMed] [Google Scholar]
- 4. Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, Gerding DN. 1992. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann. Intern. Med. 117:297–302 [DOI] [PubMed] [Google Scholar]
- 5. Lagrotteria D, Holmes S, Smieja M, Smaill F, Lee C. 2006. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 43:547–552 doi:10.1086/506354 [DOI] [PubMed] [Google Scholar]
- 6. Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586–1590 doi:10.1086/430311 [DOI] [PubMed] [Google Scholar]
- 7. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302–307 [DOI] [PubMed] [Google Scholar]
- 8. Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:422–431 doi:10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 9. Garey KW, Jiang ZD, Bellard A, Dupont HL. 2008. Rifaximin in treatment of recurrent Clostridium difficile-associated diarrhea: an uncontrolled pilot study. J. Clin. Gastroenterol. doi:10.1097/MCG.0b013e31814a4e97 [DOI] [PubMed] [Google Scholar]
- 10. Musher DM, Logan N, Mehendiratta V, Melgarejo NA, Garud S, Hamill RJ. 2007. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J. Antimicrob. Chemother. 59:705–710 doi:10.1093/jac/dkl553 [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 doi:10.1053/j.gastro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Warren CA, OEvan Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. 2012. Amixicile, a novel inhibitor of pyruvate:ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob. Agents Chemother. 56:4103–4111 doi:10.1128/AAC.00360-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pawlowski SW, Calabrese G, Kolling GL, Freire R, Alcantara Warren C, Liu B, Sartor B, Guerrant RL. 2010. Murine model of Clostridium difficile infection using gnotobiotic aged C57Bl/6 mice and a BI strain. J. Infect. Dis. 202:1708–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47:367–373 doi:10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- 15. Salyers AA. 1984. Bacteroides of the human lower intestinal tract. Annu. Rev. Microbiol. 38:293–313 doi:10.1146/annurev.mi.38.100184.001453 [DOI] [PubMed] [Google Scholar]
- 16. Herpers BL, Vlaminckx B, Burkhardt O, Blom H, Biemond-Moeniralam HS, Hornef M, Welte T, Kuijper EJ. 2009. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin. Infect. Dis. 48:1732–1735 doi:10.1086/599224 [DOI] [PubMed] [Google Scholar]
- 17. Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. 2007. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin. Infect. Dis. 44:846–848 doi:10.1086/511870 [DOI] [PubMed] [Google Scholar]
- 18. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. 2009. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin. Infect. Dis. 48:e41–e46 doi:10.1086/596552 [DOI] [PubMed] [Google Scholar]
- 19. McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. 1999. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect. Control Hosp. Epidemiol. 20:43–50 doi:10.1086/501553 [DOI] [PubMed] [Google Scholar]
- 20. Sun X, Wang H, Zhang Y, Chen K, Davis B, Feng H. 2011. Mouse relapse model of Clostridium difficile infection. Infect. Immun. 79:2856–2864 doi:10.1128/IAI.01336-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 51:2716–2719 doi:10.1128/AAC. 01623-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baines SD, O'Connor R, Saxton K, Freeman J, Wilcox MH. 2009. Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J. Antimicrob. Chemother. 63:520–525 doi:10.1093/jac/dkn502 [DOI] [PubMed] [Google Scholar]
- 23. Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL. 2012. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis. 55(Suppl 2):S162–S169 doi:10.1093/cid/cis453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kyne L, Warny M, Qamar A, Kelly CP. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390–397 [DOI] [PubMed] [Google Scholar]
- 25. Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193 doi:10.1016/S0140-6736(00)03592-3 [DOI] [PubMed] [Google Scholar]
- 26. Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD, Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362:197–205 doi:10.1056/NEJMoa0907635 [DOI] [PubMed] [Google Scholar]
- 27. Weiss K. 2009. Toxin-binding treatment for Clostridium difficile: a review including reports of studies with tolevamer. Int. J. Antimicrob. Agents 33:4–7 [DOI] [PubMed] [Google Scholar]
- 28. Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE. 1997. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin. Infect. Dis. 25:729–732 [DOI] [PubMed] [Google Scholar]
- 29. Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. 2009. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob. Agents Chemother. 53:261–263 doi:10.1128/AAC.01443-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. 2010. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 156:3354–3359 doi:10.1099/mic.0.042010-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.