Abstract
We and others recently identified copper resistance as important for virulence of Mycobacterium tuberculosis. Here, we introduce a high-throughput screening assay for agents that induce a copper hypersensitivity phenotype in M. tuberculosis and demonstrate that such copper-boosting compounds are effective against replicating and nonreplicating M. tuberculosis strains.
TEXT
The finding that the innate immune system employs copper ions to eliminate bacterial infections and the establishment of copper resistance as a virulence factor of Mycobacterium tuberculosis (1–3) lead us to consider a drug screening system for compounds that induce copper hypersensitivity in M. tuberculosis.
In a first step, we identified Hartmans-de Bont (HdB) minimal medium (4) supplemented with 0.5% glucose as the carbon source and 0.02% tyloxapol as the anticlumping agent as the optimal medium for this drug screening effort. Direct bactericidal effects of copper ions against M. tuberculosis in HdB medium were observed at copper concentrations between 15 and 25 μM (Fig. 1A). This concentration range correlates well with physiologically achievable copper levels observed in humans (15 to 25 μM) (5). Optimal growth of M. tuberculosis in HdB medium was achieved at copper concentrations between 7.5 and 12.5 μM (Fig. 1A). To conduct the screen under biosafety level 2 (BSL2) conditions, we used M. tuberculosis mc26230 (ΔRD1, ΔpanCD) (6), an attenuated mutant of M. tuberculosis H37Rv. The attenuated strain showed the same tolerance toward copper as the virulent strain (data not shown), and none of the deleted genes are known or suspected to influence copper resistance. In the final assay setup, selectivity for copper-boosting compounds was achieved by screening compounds simultaneously in the presence and absence of copper.
Fig 1.
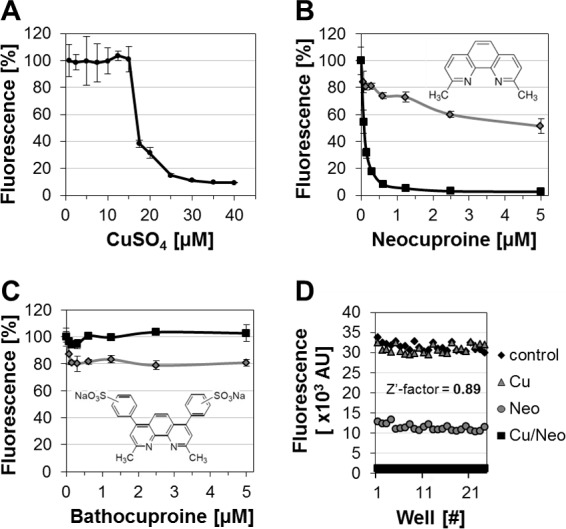
Establishment of screening parameters and molecular probes. (A) Susceptibility of M. tuberculosis to copper (added as CuSO4) in HdB medium. Copper-dependent activity of neocuproine (B) and bathocuproine (C) against M. tuberculosis in copper-free (gray line) or copper-supplemented (12.5 μM; black line) HdB medium. Molecular structures are depicted within the graphs. (D) Assay-specific Z′-factor of 0.89 was determined using untreated (control) and copper-neocuproine (Cu/Neo; 10 μM/3 μM)-treated cells. Copper only (Cu; 10 μM)- and neocuproine only (Neo; 3 μM)-treated cells were included as controls.
In the optimized assay format, all compounds were mixed with copper prior to the addition of medium and cells to enable the formation of potential copper complexes. The final cell density (optical density at 600 nm [OD600]) was 0.02 in a total volume of 200 μl. The 96-well plates were incubated for 7 days at 37°C. Then, 40 μl alamarBlue dye mixture (50% alamarBlue, 5% [vol/vol] Tween 80) was added, and incubation was continued for another 24 h before plates were analyzed. Conversion of the indicator dye resazurin into resorufin with a fluorescence emission peak at 590 nm served as a marker for bacterial growth and viability (7–9).
Next, we looked for chemical probes to optimize our assay. We distinguish at least two functional classes of such compounds: (i) copper resistance pathway inhibitors which target specific proteins that mediate copper resistance and (ii) copper complexing agents that cross the mycobacterial outer membrane barrier and thereby possibly increase the intracellular copper content. Because both compound classes enhance the bactericidal properties of copper ions, we henceforth refer to them as copper-boosting compounds. While specific inhibitors of copper resistance proteins have yet to be identified, we reasoned that known copper complexing agents may provide an exploitable resource for assay validation purposes. Neocuproine and the structurally related compound bathocuproine represent copper complexing agents with opposing membrane permeability profiles (10). Our assay revealed that the membrane-permeable neocuproine exhibited growth-inhibiting activity against M. tuberculosis in a copper-dependent manner (Fig. 1B). In contrast, the membrane-impermeable derivative bathocuproine failed to inhibit growth of M. tuberculosis in the absence or presence of copper (Fig. 1C). Thus, neocuproine and bathocuproine provided excellent positive and negative controls that were used to determine the quality of the developed assay. The assay-specific Z′-factor (11) was determined using untreated and neocuproine-copper (3 μM/10 μM)-treated cells. Copper only (10 μM)- and neocuproine only (3 μM)-treated cells were included as controls to access edge and drift effects, signal uniformity, and overall assay performance. The assay was characterized by a Z′-factor of 0.89, which indicates excellent assay performance and reliability (Fig. 1D). Although neocuproine served as an important tool during assay development, an unfavorable cytotoxicity profile prevents its use in human therapy (10).
To test the M. tuberculosis-based assay under automated conditions on a high-throughput screening platform, we performed a limited pilot screen using a random selection of compounds from our in-house small molecule library (Chembridge, Inc.). As copper chelation was identified as a potential mechanism to enhance the antibacterial properties of copper ions against M. tuberculosis, we included 20 reported copper chelators in the pilot screen to increase the likelihood of identifying hits.
Our pilot screen identified the membrane-permeable bis-thiosemicarbazones ATSM and GTSM (12) as novel copper-boosting compounds (Fig. 2A). ATSM has been developed for tumor imaging and diagnosis and is currently in clinical trials (13–15). In the absence of copper, ATSM at concentrations of up to 10 μM had no apparent inhibitory effect on M. tuberculosis. However, with 10 μM copper, ATSM had a 50% inhibitory concentration (IC50) of ∼0.6 μM (∼0.16 μg/ml) and an IC90 of ∼2.5 μM (∼0.65 μg/ml). The anti-M. tuberculosis activity of ATSM was confirmed in detailed experiments using virulent M. tuberculosis H37Rv (Fig. 2B). GTSM, an ATSM analogue, already exhibited anti-M. tuberculosis activity in regular HdB medium. Importantly, copper supplementation enhanced this activity even further. The copper-dependent IC50 for GTSM was ∼60 nM (∼0.014 μg/ml), and its IC90 was experimentally determined as ∼300 nM (∼0.07 μg/ml) (Fig. 2C). Using a previously published in vitro model for latent M. tuberculosis (16), we found that GTSM, but not ATSM, killed nonreplicating M. tuberculosis at a concentration of 2.5 μM (∼0.58 μg/ml) in the presence of 10 μM copper (Fig. 2D). GTSM, tested at 0.3 and 2.5 μM, had no activity in the absence of copper (Fig. 2D).
Fig 2.
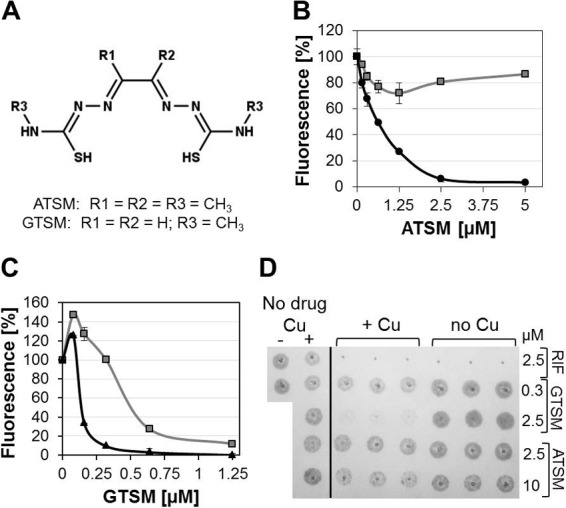
Copper-dependent activity of bis-thiosemicarbazones against M. tuberculosis. (A) Molecular structure of ATSM [diacetylbis(N(4)-methyl-3-thiosemicarbazone); MW, ∼260.38 g/mol] and GTSM [glyoxalbis(N(4)-methyl-3-thiosemicarbazone); MW, ∼232.33 g/mol]. Copper-dependent activity of ATSM (B) and GTSM (C) in the absence (gray lines) or presence (10 μM; black lines) of copper. (D) Activity against nonreplicating M. tuberculosis was evaluated 8 days after compound exposure by spotting 5 μl of each well on Middlebrook 7H10 medium plates. Growth was assessed after 16 days at 37°C. Rifampin (RIF) was included as the copper-independent inhibitor control (16).
Tolerability of ATSM and GTSM is implied by their use in various clinical settings and animal experiments (14, 17) and was confirmed by us on mouse peritoneal macrophages (Fig. 3), which were obtained according to published protocols (18). The in vitro therapeutic indices on peritoneal mouse macrophages were >20 for both compounds, indicating that such compounds could potentially advance into drug candidate status.
Fig 3.
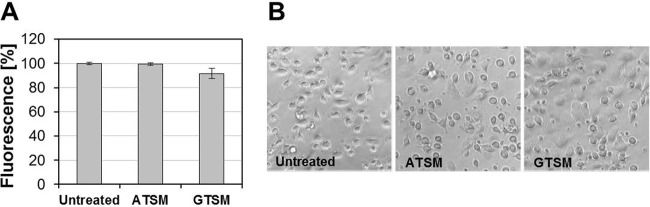
Cytotoxicity of ATSM and GTSM. Mouse peritoneal macrophages were exposed to 10 μM ATSM or GTSM in 96-well plates. Viability was accessed 24 h posttreatment by alamarBlue (A) according to the recommendations of the manufacturer (AbD Serotec) and by light microscopy (B). Magnification, 400-fold.
In summary, we provide a drug screening assay that specifically distinguishes between copper-dependent and copper-independent anti-M. tuberculosis activities. We demonstrate that small molecules can induce copper hypersensitivity in M. tuberculosis and identified copper complexation as a potential mechanism by which this can be achieved. The discovery of ATSM and GTSM is the first step toward the development of research tools that may enable us to evaluate if copper resistance mechanisms of M. tuberculosis provide for an exploitable new drug target. As copper binding drugs are in clinical use to treat Wilson's disease (penicillamine) or alcohol dependence (disulfiram) and are investigated as anticancer and anti-HIV drugs (19–21), the here-described copper-dependent anti-M. tuberculosis activities of ATSM and GTSM may provide a novel opportunity to therapeutically exploit the bactericidal properties of copper ions.
ACKNOWLEDGMENTS
We thank Bill Jacobs for providing the M. tuberculosis strain mc26230 and Jennifer Rowland for critically reading the manuscript.
This research was supported by the University of Alabama at Birmingham (UAB) Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027767-24) that was made possible by the following NIH Institutes: NIAID, NIMH, NIDA, NICHD, NHLDI, NIA. Further support was provided by NIH grant R01 AI083632 to M.N. and the National Science Foundation grant ECCS 1128570 to S.H.B.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284:33949–33956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. 2010. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 77:1096–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. 2011. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 108:1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber FJ, van Berkel WJ, Hartmans S, de Bont JA. 1992. Purification and properties of the NADH reductase component of alkene monooxygenase from Mycobacterium strain E3. J. Bacteriol. 174:3275–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. 2007. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87:1011–1046 [DOI] [PubMed] [Google Scholar]
- 6. Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, Chen M, Kim J, Porcelli SA, Chan J, Morris SL, Jacobs WR., Jr 2006. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine 24:6309–6320 [DOI] [PubMed] [Google Scholar]
- 7. Primm TP, Franzblau SG. 2007. Recent advances in methodologies for the discovery of antimycobacterial drugs. Curr. Bioact. Comp. 3:201–208 [Google Scholar]
- 8. Danilchanka O, Mailaender C, Niederweis M. 2008. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 52:2503–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ananthan S, Faaleolea ER, Goldman RC, Hobrath JV, Kwong CD, Laughon BE, Maddry JA, Mehta A, Rasmussen L, Reynolds RC, Secrist JA, III, Shindo N, Showe DN, Sosa MI, Suling WJ, White EL. 2009. High-throughput screening for inhibitors of Mycobacterium tuberculosis H37Rv. Tuberculosis (Edinb.) 89:334–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen SH, Lin JK, Liu SH, Liang YC, Lin-Shiau SY. 2008. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol. Sci. 102:138–149 [DOI] [PubMed] [Google Scholar]
- 11. Iversen P, Beck B, Chen YF, Dere W, Devanarayan V, Eastwood BJ, Farmen MW, Iturria SJ, Iversen PW, Montrose C, Moore RA, Weidner JR. 2004. HTS assay validation. In Sittampalam GS, Gal-Edd N, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Lemmon V, Li Z, McGee J, McManus O, Minor L, Napper A, Riss T, Trask OJ, Jr, Weidner J. (ed), Assay guidance manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda, MD [Google Scholar]
- 12. Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. 1997. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 38:1155–1160 [PubMed] [Google Scholar]
- 13. Lewis J, Laforest R, Buettner T, Song S, Fujibayashi Y, Connett J, Welch M. 2001. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): an agent for radiotherapy. Proc. Natl. Acad. Sci. U. S. A. 98:1206–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hung LW, Villemagne VL, Cheng L, Sherratt NA, Ayton S, White AR, Crouch PJ, Lim S, Leong SL, Wilkins S, George J, Roberts BR, Pham CL, Liu X, Chiu FC, Shackleford DM, Powell AK, Masters CL, Bush AI, O'Keefe G, Culvenor JG, Cappai R, Cherny RA, Donnelly PS, Hill AF, Finkelstein DI, Barnham KJ. 2012. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease. J. Exp. Med. 209:837–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, Tan JL, Lim NK, Lam L, Bica L, Lim S, Hickey JL, Morizzi J, Powell A, Finkelstein DI, Culvenor JG, Masters CL, Duce J, White AR, Barnham KJ, Li QX. 2011. Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J. Biol. Chem. 286:44035–44044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717–731 [DOI] [PubMed] [Google Scholar]
- 17. Kositwattanarerk A, Oh M, Kudo T, Kiyono Y, Mori T, Kimura Y, Maruyama R, Fujibayashi Y, Fujieda S, Okazawa H. 2012. Different distribution of (62)Cu ATSM and (18)F-FDG in head and neck cancers. Clin. Nucl. Med. 37:252–257 [DOI] [PubMed] [Google Scholar]
- 18. Zhang P, Katz J, Michalek SM. 2009. Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol. Immunol. 46:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmitt SM, Frezza M, Dou QP. 2012. New applications of old metal-binding drugs in the treatment of human cancer. Front. Biosci. (Schol. Ed.) 4:375–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W. 2012. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br. J. Cancer 107:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. 2013. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog (PTEN). AIDS 27:F7–F11 [DOI] [PubMed] [Google Scholar]


