Abstract
Gonorrhea may become untreatable, and new treatment options are essential. Verified resistance to spectinomycin is exceedingly rare. However, we describe a high-level spectinomycin-resistant (MIC, >1,024 μg/ml) Neisseria gonorrhoeae strain from Norway with a novel resistance mechanism. The resistance determinant was a deletion of codon 27 (valine) and a K28E alteration in the ribosomal protein 5S. The traditional spectinomycin resistance gene (16S rRNA) was wild type. Despite this exceedingly rare finding, spectinomycin available for treatment of ceftriaxone-resistant urogenital gonorrhea would be very valuable.
TEXT
Neisseria gonorrhoeae has developed resistance to all antimicrobials previously recommended for first-line empirical treatment of gonorrhea (1–5). In recent years, in vitro resistance and treatment failures with the currently recommended extended-spectrum cephalosporins (cefixime and ceftriaxone), the last remaining options for empirical antimicrobial monotherapy, have been verified (6–14). Gonorrhea may become untreatable, particularly in settings where dual antimicrobial therapy is not feasible or affordable (1, 3–5, 8, 11, 15). From a global public health perspective, an effective antimicrobial monotherapy remains crucial. Gentamicin (16–18), solithromycin (19), and ertapenem (20) have been suggested; however, none of these appear to be an effective long-term solution for single antimicrobial therapy of gonorrhea.
Nevertheless, spectinomycin remains an effective option for treatment, with the exception of pharyngeal gonorrhea (4, 21–25). Verified resistance to spectinomycin is exceedingly rare worldwide, e.g., in the U.S. Gonococcal Isolate Surveillance Project (GISP), established in 1986 (http://www.cdc.gov/std/gisp), and the European Gonococcal Antimicrobial Surveillance Programme (EURO-GASP), initiated in 2004 (26, 27), only five spectinomycin-resistant isolates (all between 1988 and 1990) and no isolates, respectively, have been identified. Unfortunately, spectinomycin is not available in many settings worldwide (4, 8, 24, 25, 28), and, in some settings, resistance emerged when it was widely used as a first-line drug in the 1980s (29).
Spectinomycin inhibits protein translation by binding to the bacterial 30S ribosomal subunit; i.e., it interacts directly with 16S rRNA and inhibits the elongation factor G (EF-G)-catalyzed translocation of the peptidyl-tRNA from the A site to the P site during polypeptide elongation (30, 31). The interaction with 16S rRNA is in helix 34, close to the base-paired nucleotides G1064-C1192 (31–33). In bacterial species, spectinomycin resistance has resulted from production of adenyltransferases that inactivate the drug, alterations of specific amino acids in loop 2 of 30S ribosomal protein S5 (encoded by rpsE), and mutations in the spectinomycin binding region of helix 34 encompassing the cross-linked positions 1063 to 1066 and 1190 to 1193 (Escherichia coli numbering) in 16S rRNA (30, 33–48). In N. gonorrhoeae, only a single nucleotide polymorphism (SNP), C1192U transition, in 16S rRNA has been verified to result in high-level spectinomycin resistance (39, 49).
This study describes an exceedingly rare N. gonorrhoeae strain with high-level spectinomycin resistance, due to a novel resistance mechanism (mutated ribosomal protein S5), identified in Norway.
The strain (SPT-R) was isolated in 2010 from a 32-year-old Norwegian man who had sex with men (MSM) after unprotected anal intercourse in Oslo, Norway, with an anonymous, untraceable Norwegian man. The patient had proctitis, and porA pseudogene PCR (50) and selective culture of rectal specimens were positive for gonococci. The patient was administered ceftriaxone (250 mg once intramuscularly). Five weeks later, the patient returned with resolved symptoms and porA pseudogene PCR (50) of a rectal sample was negative.
SPT-R was species confirmed using an oxidase test, microscopy (Gram staining), a sugar utilization test, and a Phadebact GC monoclonal test (Bactus AB, Solna, Sweden). SPT-R showed high-level resistance to spectinomycin (MIC, >1,024 μg/ml), intermediate susceptibility to ciprofloxacin, and susceptibility to cefixime, ceftriaxone, and azithromycin, and it was assigned to serovar Byvu and N. gonorrhoeae multiantigen sequence type (NG-MAST) ST918 (49) (Table 1).
Table 1.
Phenotypic and genetic characteristics of a Neisseria gonorrhoeae strain with high-level resistance to spectinomycin identified in Norway
| Characteristica | Resultb |
|---|---|
| MIC (resistance)c | |
| SPT | >1,024 (R) |
| CRO | 0.016 (S) |
| CFM | <0.016 (S) |
| AZM | 0.125 (S) |
| CIP | 0.125 (I) |
| Serovar | Byvu |
| NG-MAST | ST918 |
| 16S rRNA gene | WT |
| Ribosomal protein 5S | Deletion of valine (amino acid 25d) and an alteration of lysine to glutamic acid (amino acid 26d) |
For MIC data, Etest was used and only whole MIC dilutions are presented. NG-MAST, Neisseria gonorrhoeae multiantigen sequence typing; SPT, spectinomycin; CRO, ceftriaxone; CFM, cefixime; AZM, azithromycin; CIP, ciprofloxacin.
ST, sequence type; WT, wild type.
Susceptibility (S), intermediate susceptibility (I), and resistance (R) were determined based on the interpretative criteria stated by the Clinical and Laboratory Standards Institute (CLSI) (M100-S22).
Escherichia coli (GenBank accession no. AAA58100) numbering that corresponds to amino acids 27 and 28, respectively, in the ribosomal protein 5S of Neisseria gonorrhoeae.
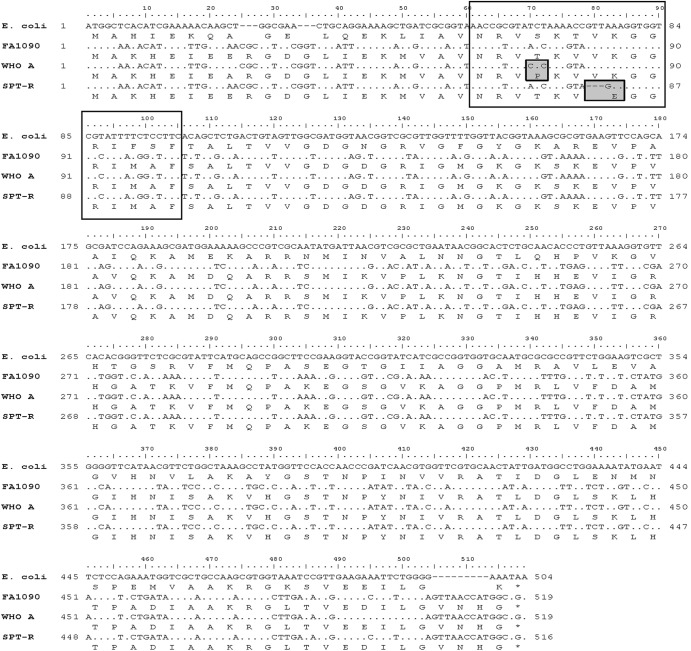
For elucidation of the spectinomycin resistance determinant, the full-length 16S rRNA gene (1,545 bp) was sequenced (51), which surprisingly revealed a wild-type gene. Consequently, the full-length rpsE gene (519 bp) was PCR amplified and sequenced with the primers 5S-F (5′-TGGCAAAACATGAAATTGAAG-3′) and 5S-R (5′-GCCATGGTTAACTCCCAAAA-3′), which were designed based on the genome sequence of the spectinomycin-susceptible gonococcal reference strain FA1090 (GenBank accession no. AE004969.1). Compared to rpsE genes in FA1090, the eight 2008 WHO gonococcal reference strains (49), and the old N. gonorrhoeae reference strain WHO A (a strain with low-level spectinomycin resistance), rpsE in SPT-R contained a deletion of nucleotides 79 to 81 (whole codon 27 encoding valine) and an A82G transition resulting in the amino acid alteration K28E (lysine to glutamic acid) in the ribosomal protein S5, which correspond to amino acids 25 and 26, respectively, in Escherichia coli (GenBank accession no. AAA58100). A nucleotide blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) did not find these alterations in any rpsE from gonococci or other bacterial species. Notable, WHO A contained a T22P amino acid alteration (E. coli numbering) compared to the spectinomycin-susceptible reference strains (Fig. 1). A transformation experiment (8, 52), using purified PCR-amplified full-length rpsE transformed to WHO M (49), verified that the rpsE alleles in SPT-R and WHO A resulted in high-level and low-level spectinomycin resistance, respectively. The spectinomycin MICs of the transformants increased to donor levels for both SPT-R (from 16 μg/ml to 1,024 μg/ml) and WHO A (from 16 μg/ml to 128 μg/ml). Both transformants contained full-length rpsE allele from the donor (identical sequence to rpsE in SPT-R and WHO A, respectively) and no changes in, e.g., the 16S rRNA gene sequence.
Fig 1.
Nucleotide and amino acid alignment of the rpsE genes and the corresponding amino acid sequences of ribosomal protein 5S in Escherichia coli (GenBank accession no. AAA58100), the international genome-sequenced N. gonorrhoeae reference strain FA1090 (GenBank accession no. AE004969.1), N. gonorrhoeae reference strain WHO A (low-level spectinomycin resistance; MIC, 128 μg/ml), and the N. gonorrhoeae strain with high-level spectinomycin resistance (MIC, >1,024 μg/ml) identified in Norway (SPT-R). The transparent boxes indicate the amino acids 19 to 33 in the N terminus of the ribosomal protein 5S that form a loop structure (loop 2) which nonspecifically binds to helix 34 of 16S rRNA and is within 50 nm of the spectinomycin-binding site. Amino acid alterations at this loop can disrupt the binding of spectinomycin to the ribosome, which results in spectinomycin resistance (31, 37, 42, 43). The shaded boxes indicate the mutations causing low-level spectinomycin resistance and high-level spectinomycin resistance in WHO A and SPT-R, respectively.
Herein, we describe a high-level spectinomycin-resistant (MIC, >1,024 μg/ml) gonococcal strain from Norway with a novel resistance mechanism (mutated ribosomal protein 5S). High-level MIC-verified spectinomycin resistance in N. gonorrhoeae has been exceedingly rare globally (2, 4, 24-27; http://www.cdc.gov/std/gisp). This may reflect its rare use in most settings; however, in some settings it has been relatively frequently used, and it is surprising that international spread of spectinomycin-resistant successful gonococcal clones has never been documented. This may indicate that, at least in many gonococcal clones, the characteristic spectinomycin resistance SNP in 16S rRNA (C1192U), and possibly other resistance determinants, results not only in high-level spectinomycin resistance but also in a decreased biological fitness, limiting the further spread of the resistant clone. A project addressing this issue is in progress. Recently, the first three extensively drug-resistant (XDR) (4) gonococcal strains with high-level ceftriaxone resistance were reported, and all those strains were susceptible to spectinomycin (8, 11, 53). Based on this fact and because spectinomycin resistance is exceedingly rare globally, it would be very valuable to have spectinomycin available worldwide for treatment of ceftriaxone-resistant anogenital gonorrhea and for the rare patients who cannot tolerate cephalosporins (4, 21-23, 25). Spectinomycin might also be appropriate in a dual antimicrobial treatment regimen, effectively treating also pharyngeal gonorrhea and inhibiting resistance development.
This study also verified a novel resistance determinant for gonococcal high-level spectinomycin resistance, i.e., a deletion of amino acid 25 and a K26E amino acid alteration (E. coli numbering) in the ribosomal protein 5S (Fig. 1). The amino acids 19 to 33 in the N terminus of the ribosomal protein 5S form a loop structure which nonspecifically binds to helix 34 of 16S rRNA, and this loop is also involved in the binding of spectinomycin to the ribosome and spectinomycin resistance (37). For example, in E. coli, mutations at amino acid positions 20 to 22 (31, 37, 38, 42, 43) and a G28D mutation (43) result in spectinomycin resistance. In the present study, a T22P alteration in WHO A was also verified to result in low-level spectinomycin resistance in gonococci. In Pasteurella multocida, deletion of the conserved lysine at position 23, which interacts directly with 16S rRNA (31), and deletion of phenylalanine at position 33 accompanied by a Ser32Ile alteration result in spectinomycin resistance (42). Likely, the deletion of valine (amino acid 25) accompanied by the alteration of the conserved lysine at position 26, which is proposed to interact with 16S rRNA (31), to glutamic acid (K26E) in SPT-R (Fig. 1) disrupts the binding to 16S rRNA and spectinomycin that results in high-level resistance to spectinomycin in gonococci.
In conclusion, this study describes an N. gonorrhoeae strain with verified high-level resistance to spectinomycin (MIC, >1,024 μg/ml) due to a novel spectinomycin resistance mechanism (mutated ribosomal protein 5S). Nevertheless, resistance to spectinomycin is exceedingly rare globally, spectinomycin is an effective alternative for treatment of urogenital gonorrhea, and spectinomycin should be available worldwide, in particular for emergent cases of multidrug resistance, including clinical resistance to cefixime and ceftriaxone.
Nucleotide sequence accession number.
The novel N. gonorrhoeae rpsE allele has been assigned the GenBank/EMBL/DDBJ accession number KC311362.
ACKNOWLEDGMENTS
This work was supported by the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Sweden.
Footnotes
Published ahead of print 26 November 2012
REFERENCES
- 1. Bolan GA, Sparling PF, Wasserheit JN. 2012. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 366:485–487 [DOI] [PubMed] [Google Scholar]
- 2. Lewis DA. 2010. The gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86:415–421 [DOI] [PubMed] [Google Scholar]
- 3. Stoltey JE, Barry PM. 2012. The use of cephalosporins for gonorrhea: an update on the rising problem of resistance. Expert Opin. Pharmacother. 13:1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev. Anti Infect. Ther. 7:821–834 [DOI] [PubMed] [Google Scholar]
- 5. Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 1230(1):E19–E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deguchi T, Yasuda M, Yokoi S, Ishida K, Ito M, Ishihara S, Minamidate K, Harada Y, Tei K, Kojima K, Tamaki M, Maeda S. 2003. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J. Infect. Chemother. 9:35–39 [DOI] [PubMed] [Google Scholar]
- 7. Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. 2011. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 16(14):pii=19833. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19833 [PubMed] [Google Scholar]
- 8. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tapsall J, Read P, Carmody C, Bourne C, Ray S, Limnios A, Sloots T, Whiley D. 2009. Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods. J. Med. Microbiol. 58(Part 5):683–687 [DOI] [PubMed] [Google Scholar]
- 10. Unemo M, Golparian D, Hestner A. 2011. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 16(6):pii=19792. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19792 [PubMed] [Google Scholar]
- 11. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unemo M, Golparian D, Potočnik M, Jeverica S. 2012. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 17(25):pii=20200. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20200 [PubMed] [Google Scholar]
- 13. Unemo M, Golparian D, Stary A, Eigentler A. 2011. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 16(43):pii=19998. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19998 [PubMed] [Google Scholar]
- 14. Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. 2010. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 15(47):pii=19721. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19721 [DOI] [PubMed] [Google Scholar]
- 15. Whiley DM, Goire N, Lahra MM, Donovan B, Limnios AE, Nissen MD, Sloots TP. 2012. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J. Antimicrob. Chemother. 67:2059–2061 [DOI] [PubMed] [Google Scholar]
- 16. Chisholm SA, Quaye N, Cole MJ, Fredlund H, Hoffmann S, Jensen JS, van de Laar MJ, Unemo M, Ison CA. 2011. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J. Antimicrob. Chemother. 66:592–595 [DOI] [PubMed] [Google Scholar]
- 17. Dowell D, Kirkcaldy RD. 23 August 2012, posting date Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex. Transm. Infect. [Epub ahead of print.] doi:10.1136/sextrans-2012-050604 [DOI] [PubMed] [Google Scholar]
- 18. Ross JD, Lewis DA. 2012. Cephalosporin resistant Neisseria gonorrhoeae: time to consider gentamicin? Sex. Transm. Infect. 88:6–8 [DOI] [PubMed] [Google Scholar]
- 19. Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob. Agents Chemother. 56:2739–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob. Agents Chemother. 56:3603–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bignell C, Fitzgerald M, Guideline Development Group, British Association for Sexual Health and HIV UK 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int. J. STD AIDS 22:541–547 [DOI] [PubMed] [Google Scholar]
- 22. Moran JS. 1995. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex. Transm. Dis. 22:39–47 [DOI] [PubMed] [Google Scholar]
- 23. Moran JS, Levine WC. 1995. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin. Infect. Dis. 20(Suppl 1):S47–S65 [DOI] [PubMed] [Google Scholar]
- 24. Newman LM, Moran JS, Workowski KA. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44(Suppl 3):S84–S101 [DOI] [PubMed] [Google Scholar]
- 25. Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC) 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59(RR-12):1–110 [PubMed] [Google Scholar]
- 26. Cole MJ, Unemo M, Hoffmann S, Chisholm SA, Ison CA, van de Laar MJ. 2011. The European gonococcal antimicrobial surveillance programme, 2009. Euro Surveill. 16(42):pii=19995. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19995 [PubMed] [Google Scholar]
- 27. European Centre for Disease Prevention and Control (ECDC) 2012. Gonococcal antimicrobial susceptibility surveillance in Europe, 2010. ECDC, Stockholm, Sweden: http://ecdc.europa.eu/en/publications/Publications/1206-Gonococcal-AMR.pdf Accessed 30 October 2012 [Google Scholar]
- 28. Anonymous 2006. Discontinuation of spectinomycin. MMWR 55:370 [Google Scholar]
- 29. Boslego JW, Tramont EC, Takafuji ET, Diniega BM, Mitchell BS, Small JW, Khan WN, Stein DC. 1987. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and penicillinase-producing Neisseria gonorrhoeae. N. Engl. J. Med. 317:272–278 [DOI] [PubMed] [Google Scholar]
- 30. Bilgin N, Richter AA, Ehrenberg M, Dahlberg AE, Kurland CG. 1990. Ribosomal RNA and protein mutants resistant to spectinomycin. EMBO J. 9:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramakrishnan V, White SW. 1992. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature 358:768–771 [DOI] [PubMed] [Google Scholar]
- 32. Moazed D, Noller HF. 1987. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 327:389–394 [DOI] [PubMed] [Google Scholar]
- 33. Sigmund CD, Ettayebi M, Morgan EA. 1984. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 12:4653–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Binet R, Maurelli AT. 2005. Fitness cost due to mutations in the 16S rRNA associated with spectinomycin resistance in Chlamydia psittaci 6BC. Antimicrob. Agents Chemother. 49:4455–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brink MF, Brink G, Verbeet MP, de Boer HA. 1994. Spectinomycin interacts specifically with the residues G1064 and C1192 in 16S rRNA, thereby potentially freezing this molecule into an inactive conformation. Nucleic Acids Res. 22:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Criswell D, Tobiason VL, Lodmell JS, Samuels DS. 2006. Mutations conferring aminoglycoside and spectinomycin resistance in Borrelia burgdorferi. Antimicrob. Agents Chemother. 50:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davies C, Bussiere DE, Golden BL, Porter SJ, Ramakrishnan V, White SW. 1998. Ribosomal proteins S5 and L6: high resolution crystal structures and roles in protein biosynthesis and antibiotic resistance. J. Mol. Biol. 279:873–888 [DOI] [PubMed] [Google Scholar]
- 38. Funatsu G, Schiltz E, Wittmann HG. 1971. Ribosomal proteins. XXVII. Localization of the amino acid exchanges in protein S5 from two Escherichia coli mutants resistant to spectinomycin. Mol. Gen. Genet. 114:106–111 [DOI] [PubMed] [Google Scholar]
- 39. Galimand M, Gerbaud G, Courvalin P. 2000. Spectinomycin resistance in Neisseria spp. due to mutations in 16S rRNA. Antimicrob. Agents Chemother. 44:1365–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johanson U, Hughes D. 1995. A new mutation in 16S rRNA of Escherichia coli conferring spectinomycin resistance. Nucleic Acids Res. 23:464–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kehrenberg C, Catry B, Haesebrouck F, de Kruif A, Schwarz S. 2005. Novel spectinomycin/streptomycin resistance gene, aadA14, from Pasteurella multocida. Antimicrob. Agents Chemother. 49:3046–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kehrenberg C, Schwarz S. 2007. Mutations in 16S rRNA and ribosomal protein S5 associated with high-level spectinomycin resistance in Pasteurella multocida. Antimicrob. Agents Chemother. 51:2244–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirthi N, Roy-Chaudhuri B, Kelley T, Culver GM. 2006. A novel single amino acid change in small subunit ribosomal protein S5 has profound effects on translational fidelity. RNA 12:2080–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LeBlanc DJ, Lee LN, Inamine JM. 1991. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1804–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Makosky PC, Dahlberg AE. 1987. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: an analysis of three mutants. Biochimie 69:885–889 [DOI] [PubMed] [Google Scholar]
- 46. Murphy E. 1985. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3″) (9). Mol. Gen. Genet. 200:33–39 [DOI] [PubMed] [Google Scholar]
- 47. O'Connor M, Dahlberg AE. 2002. Isolation of spectinomycin resistance mutations in the 16S rRNA of Salmonella enterica serovar Typhimurium and expression in Escherichia coli and Salmonella. Curr. Microbiol. 45:429–433 [DOI] [PubMed] [Google Scholar]
- 48. Suter TM, Viswanathan VK, Cianciotto NP. 1997. Isolation of a gene encoding a novel spectinomycin phosphotransferase from Legionella pneumophila. Antimicrob. Agents Chemother. 41:1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall JW. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63:1142–1151 [DOI] [PubMed] [Google Scholar]
- 50. Hjelmevoll SO, Olsen ME, Sollid JU, Haaheim H, Unemo M, Skogen V. 2006. A fast real-time polymerase chain reaction method for sensitive and specific detection of the Neisseria gonorrhoeae porA pseudogene. J. Mol. Diagn. 8:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hjelmevoll SO, Olsen ME, Sollid JU, Haaheim H, Melby KK, Moi H, Unemo M, Skogen V. 2008. Clinical validation of a real-time polymerase chain reaction detection of Neisseria gonorrheae porA pseudogene versus culture techniques. Sex. Transm. Dis. 35:517–520 [DOI] [PubMed] [Google Scholar]
- 52. Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, Shimuta K, Okazaki N, Nakayama S, Watanabe H. 2010. Spreading of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob. Agents Chemother. 54:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 67:1858–1860 [DOI] [PubMed] [Google Scholar]