Abstract
We studied whether addition of enfuvirtide (ENF) to a background combination antiretroviral therapy (cART) would improve the CD4 cell count response at week 24 in naive patients with advanced HIV disease. ANRS 130 Apollo is a randomized study, conducted in naive HIV-1-infected patients, either asymptomatic with CD4 counts of <100/mm3 or stage B/C disease with CD4 counts of <200/mm3. Patients received tenofovir-emtricitabine with lopinavir-ritonavir (LPV/r) or efavirenz and were randomized to receive ENF for 24 weeks (ENF arm) or not (control arm). The primary endpoint was the proportion of patients with CD4 counts of ≥200/mm3 at week 24. A total of 195 patients were randomized: 73% had stage C disease, 78% were male, the mean age was 44 years, the median CD4 count was 30/mm3, and the median HIV-1 RNA load was 5.4 log10 copies/ml. Eighty-one percent of patients received LPV/r. One patient was lost to follow-up, and eight discontinued the study (four in each arm). The proportions of patients with CD4 counts of ≥200/mm3 at week 24 were 34% and 38% in the ENF and control arms, respectively (P = 0.53). The proportions of patients with HIV-1 RNA loads of <50 copies/ml were 74% and 58% at week 24 in the ENF and control arms, respectively (P < 0.02), and the proportion reached 79% in both arms at week 48. Twenty (20%) and 12 patients (13%) in the ENF and control arms, respectively, experienced at least one AIDS event during follow-up (P = 0.17). Although inducing a more rapid virological response, addition of ENF to a standard cART does not improve the immunological outcome in naive HIV-infected patients with severe immunosuppression.
INTRODUCTION
Combination antiretroviral therapy (cART) has substantially reduced mortality and morbidity in HIV-1-infected patients since its introduction in 1996 (1, 2). Even patients who initiate treatment relatively late in the course of the disease have been shown to benefit from cART (3, 4). However, mortality and morbidity rates remain high in patients who enter HIV care with severe immunodeficiency (5–8), with most progressions to AIDS or death occurring in the first 6 months following the initiation of cART (2, 9). Several studies have shown that the magnitude of the CD4 cell count response at 6 months is associated with a reduction in the rate of clinical progression (10–12), including crude mortality (13).
Current recommendations support initiating cART with two nucleoside reverse transcriptase inhibitors (NRTIs) plus efavirenz or a boosted protease inhibitor (PI). Optimization of ART in naive patients presenting with advanced HIV disease is needed to improve their immunological response and reduce the rate of clinical progression, especially in the first months following the initiation of treatment.
The aim of the randomized trial described here was to assess whether the addition of a fourth antiretroviral drug, namely, enfuvirtide, to a standard cART therapy would improve the increase in CD4 cell count in naive HIV-1-infected patients with advanced HIV disease.
MATERIALS AND METHODS
Study design.
The ANRS 130 Apollo trial was an open-label, randomized, multicenter, nationwide, French clinical trial of three versus four antiretroviral drugs at the initial phase of antiretroviral treatment in severely immunosuppressed HIV-infected patients. Immunosuppression was defined as a CD4 cell count of <100/mm3 if patients were asymptomatic or <200/mm3 if patients were stage B or C, according to the 1993 Centers for Disease Control and Prevention classification. The trial statistician prepared the randomization list, and the codes were known only to him and the data services programmers and were unknown to the investigators and coordinators. Randomized treatment assignment was done using a central computer following the generated list, after eligibility criteria had been checked centrally at the clinical trials unit.
Patients from 38 HIV clinics in France were eligible if they met the following inclusion criteria: men or women aged ≥18 years old; HIV-1 infected, as assessed by a positive Western blot assay result; and naive for any ART with a CD4 cell count of <100/mm3 if they were asymptomatic or <200/mm3 if they had a past or present history of stage B or C disease, whatever the plasma HIV-1 RNA value. Patients were not eligible if they had HIV-2 infection or a cancer requiring radio- or chemotherapy, if they had received any immunomodulator therapy or HIV vaccine, or if they were pregnant, breast-feeding, or of childbearing age without effective contraception. Other exclusion criteria included creatinine clearance level of <60 ml/min, hemoglobin level of <8 g/dl, absolute neutrophil count of <750/mm3, platelet count of <50,000/mm3, and alkaline phosphatase or transaminases level ≥3 times the upper limit of the normal range (ULN). Patients with chronic hepatitis B or C were eligible if they did not have cirrhosis with a Child-Pugh score of B or C.
Written informed consent was obtained from all patients. The protocol was reviewed and approved by an ethics committee (Comité de Protection des Personnes) and competent health authority (Agence Française de Sécurité Sanitaire des Produits de Santé). The trial was conducted in accordance with the Declaration of Helsinki.
Study treatment regimens.
All patients received a standard therapy of emtricitabine (FTC) coformulated with tenofovir disoproxil fumarate (Truvada; Gilead) combined with either lopinavir-ritonavir (400/100 mg; Kaletra; Abbott) twice daily or efavirenz (600 mg; Sustiva; Bristol-Myers Squibb) once daily, as per the choice of the local investigator. Standard therapy was defined before randomization.
Patients were centrally randomized (1:1) to receive either a standard therapy alone (control arm) or an intensified therapy through the addition of enfuvirtide (Fuzeon; Roche) at 90 mg subcutaneously twice daily to the standard therapy (enfuvirtide intensification arm). The duration of the enfuvirtide therapy in the intensification group was 24 weeks. All subjects were followed for 48 weeks. Randomization was stratified by clinical center, backbone therapy (efavirenz or boosted lopinavir), and clinical stage of HIV infection (stage C yes or no). If a patient had had a recent infection, no minimal delay between infection and initiation of ART was required.
Changes in the standard therapy were permitted throughout the duration of the study to control for the toxic effects of antiretroviral drugs, provided that patients continued to receive a combination of two nucleosides with either efavirenz or a boosted protease inhibitor. All patients received prophylaxis for Pneumocystis jirovecii pneumonia and toxoplasmosis until their CD4 cell count increased, per current guidelines (14).
Outcome measures.
The primary endpoint was the proportion of patients with a CD4 cell count of ≥200/mm3 at week 24. We chose an immunological endpoint since the CD4 response is associated with a reduction in clinical progression, which was our main goal in this population of patients with advanced HIV disease. Secondary endpoints included the proportion of patients who reached plasma HIV-1 viral loads of <50 copies/ml at weeks 12, 24, and 48; the proportion of patients with a CD4 cell count of ≥200/mm3 at week 48; the changes in CD4 cell count and plasma HIV-1 RNA levels between baseline and week 48; drug tolerability; the incidence of clinical HIV- and non-HIV-related events, death, and immune reconstitution and inflammatory syndrome (IRIS) reactions; and the proportion of patients who interrupted the therapeutic strategy allocated by randomization. Therapeutic strategy interruptions were defined as (i) interruption of enfuvirtide for more than 1 month in the intensification arm, (ii) addition of enfuvirtide in the control arm, or (iii) changes in the backbone therapy leading to administration of a combination other than two nucleosides and a boosted PI or two nucleosides and efavirenz.
Local laboratories assessed HIV-1 RNA levels and CD4 cell counts at screening, at enrollment, and at weeks 2, 4, 8, 12, 24, 36, and 48. An independent data and safety monitoring board regularly reviewed available safety and efficacy data. The severity of clinical and laboratory abnormalities was graded according to the ANRS scale for grading the severity of adverse events in adults. Investigators evaluated the association between the study regimen and each adverse event. Results of additional secondary endpoints (change in drug susceptibility and HIV-1 tropism) will be reported elsewhere.
IRIS case definition.
IRIS was defined as evidence of symptoms temporally related to the initiation of cART with an increase in CD4 cell counts and/or a decrease in plasma HIV-1 RNA levels, consistent with an inflammatory condition, that could not be explained by a newly acquired infection, the failure of treatment of a previously diagnosed infection, or side effects of cART itself (15). All cases of IRIS required review by an event review board.
Statistical analysis.
We assumed that in the control arm, 30% of subjects would reach the primary efficacy endpoint. Thus, a sample size of 103 patients in each arm was necessary to provide at least 80% power, with an alpha risk of 5%, to detect a proportion of 50% of patients reaching the primary endpoint in the enfuvirtide arm. Primary efficacy analyses were conducted on an intent-to-treat basis. They included all enrolled patients, regardless of whether they received at least one dose of the study medication or whether they completed their allocated treatment. When CD4 cell count values were missing, they were considered treatment failures (i.e., CD4 count, <200/mm3) in the primary efficacy analyses. Changes in CD4 cell count and HIV-1 RNA level were calculated on the basis of the available data. Differences were assessed using the chi-square test for comparing proportions and the Mann-Whitney/Wilcoxon test for comparing medians. In all analyses, the baseline was defined as the week 0 visit date. In the case of discontinuation of the trial, data were included up to the last visit date.
All statistical analyses were performed with SAS, version 9.1.3, service pack 2, software (SAS Institute).
RESULTS
Patient disposition and baseline characteristics.
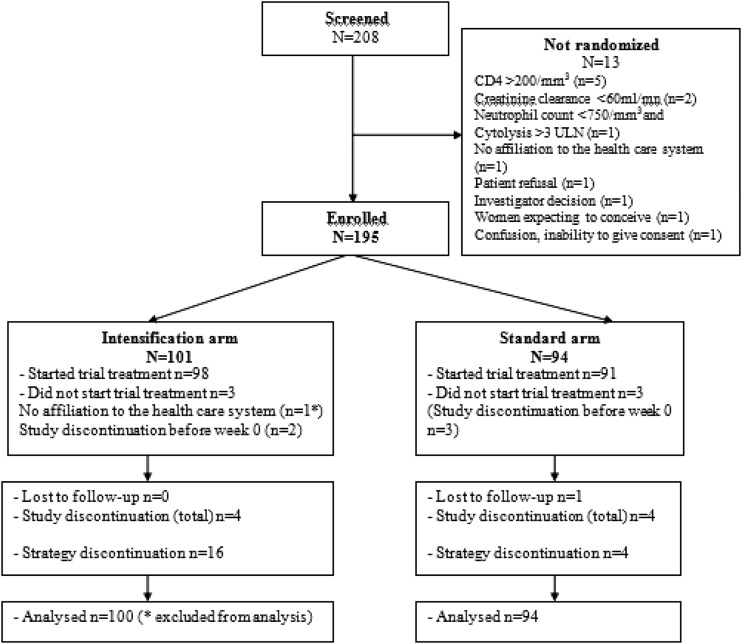
From March 2006 to December 2008, 208 patients were screened and 195 patients were randomized. Among the 13 patients who were not randomized into the study, 5 had a CD4 cell count of >200 cells/mm3 at baseline. Other reasons are described in the trial profile (Fig. 1). One hundred one patients were randomized in the enfuvirtide group and 94 were randomized in the control one. One patient was not affiliated with the French social security system and had to be excluded from the analysis. One patient was lost to follow-up, eight patients (four in each arm) discontinued the study, including five who discontinued before week 0 (mostly because of patient demotivation) and did not receive study medication.
Fig 1.
ANRS 130 Apollo trial profile.
Patient baseline characteristics were well-balanced across the arms (Table 1). Patients were primarily men (78%), and the mean age was 44 years. Most patients (82%) presented with a late HIV diagnosis (delay between diagnosis and study entry, less than 6 months). The baseline median CD4 cell count was 30/mm3; 66% of the patients entered the study with a CD4 cell count of <50 cells/mm3. The baseline plasma HIV-1 RNA level was 5.4 log10 copies/ml. Lopinavir-ritonavir was prescribed to 81% of patients, and efavirenz was prescribed to 19%. Most patients were stage C (73%), and 108 patients (56%) had an active AIDS event at baseline. Twenty-nine out of these 108 patients entered the study with multiple active AIDS events. The most common opportunistic infections were Pneumocystis jirovecii pneumonia (45 patients) and toxoplasmosis (16 patients).
Table 1.
Baseline characteristics of the patients in the ANRS 130 Apollo trial
| Characteristic | Enfuvirtide group (n = 100) | Control group (n = 94) | Total (n = 194) |
|---|---|---|---|
| No. (%) male | 74 (74) | 77 (82) | 151 (78) |
| Mean (SD) age (yr) | 44 (9) | 43 (10) | 44 (10) |
| No. (%) of patients by mode of infection | |||
| Heterosexual contact | 55 (55) | 51 (54) | 106 (55) |
| Men having sex with men | 33 (33) | 30 (32) | 63 (32) |
| Injecting drug user | 2 (2) | 1 (1) | 3 (2) |
| Transfusion | 0 (0) | 1 (1) | 1 (1) |
| Unknown | 10 (10) | 11 (12) | 21 (11) |
| No. (%) of patients by clinical stage | |||
| A | 10 (10) | 11 (12) | 21 (11) |
| B | 16 (16) | 16 (17) | 32 (16) |
| C | 74 (74) | 67 (71) | 141 (73) |
| No. (%) of patients with active AIDS event at wk 0 | 55 (55) | 53 (56) | 108 (56) |
| No. (%) of patients with delay from HIV diagnosis to wk 0 of ≤6 mo | 84 (84) | 75 (80) | 159 (82) |
| Baseline CD4 count (no. of cell/mm3) | |||
| Mean (SD) | 49 (50) | 54 (62) | 51 (56) |
| Median (IQR) | 35 (14, 69) | 29 (11, 88) | 30 (12, 72) |
| Baseline HIV-1 RNA load (log10 no. of copies/ml) | |||
| Mean (SD) | 5.3 (0.6) | 5.4 (0.6) | 5.4 (0.6) |
| Median (IQR) | 5.4 (5–5.8) | 5.4 (5–5.8) | 5.4 (5–5.8) |
| No. (%) of patients receiving standard treatment of: | |||
| Lopinavir-ritonavir | 78 (78) | 79 (84) | 157 (81) |
| Efavirenz | 22 (22) | 15 (16) | 37 (19) |
Therapeutic strategy interruptions.
Therapeutic strategy interruptions occurred in 20 patients, 16 in the intensification arm (15.8%) and 4 in the control arm (4.3%). In the intensification arm, 15 patients interrupted enfuvirtide (mostly definitely and due to poor convenience or local discomfort), and 1 patient switched from lopinavir to raltegravir because of diarrhea. In the control arm, one patient received enfuvirtide and raltegravir due to primary viral resistance at baseline, and three patients simultaneously received a nonnucleoside reverse transcriptase inhibitor and a PI with an NRTI because of a poor virologic response; these were combined with raltegravir in one patient or with enfuvirtide in another patient.
CD4 cell count and HIV-1 RNA responses.
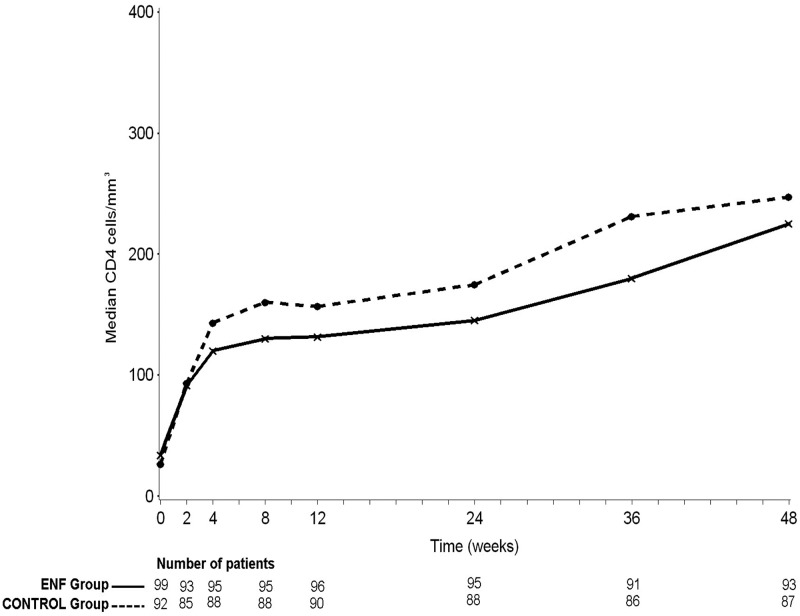
The proportions of patients with a CD4 cell count of ≥200/mm3 at week 24 were 38% in the control arm and 34% in the intensification arm (P = 0.53). Applying an extreme case analysis to the missing CD4 values at week 24 (six in the control arm and five in the intensification arm) did not change the direction of the result. The median increase in CD4 count at week 24 was 113 cells/mm3 (interquartile ratio [IQR], 70, 171 cells/mm3) in the enfuvirtide arm versus 129 cells/mm3 (IQR, 76, 194 cells/mm3) in the control arm (P = 0.35). The median CD4 cell count at week 24 was 145 cells/mm3 (IQR, 103, 242 cells/mm3) in the enfuvirtide arm versus 175 cells/mm3 (IQR, 116, 268 cells/mm3) in the control arm (P = 0.19). CD4 trends from week 0 to week 48 according to randomization are depicted in Fig. 2. The lack of difference between the two arms persisted when considering different subgroups of patients according to their absolute CD4 cell count at baseline, i.e., ≤50, >50, and ≤100 or >100 cells/mm3.
Fig 2.
Median CD4 cell count from week 0 to week 48, ANRS 130 Apollo trial (ENF, enfuvirtide; data points are medians, and errors bars are omitted for clarity).
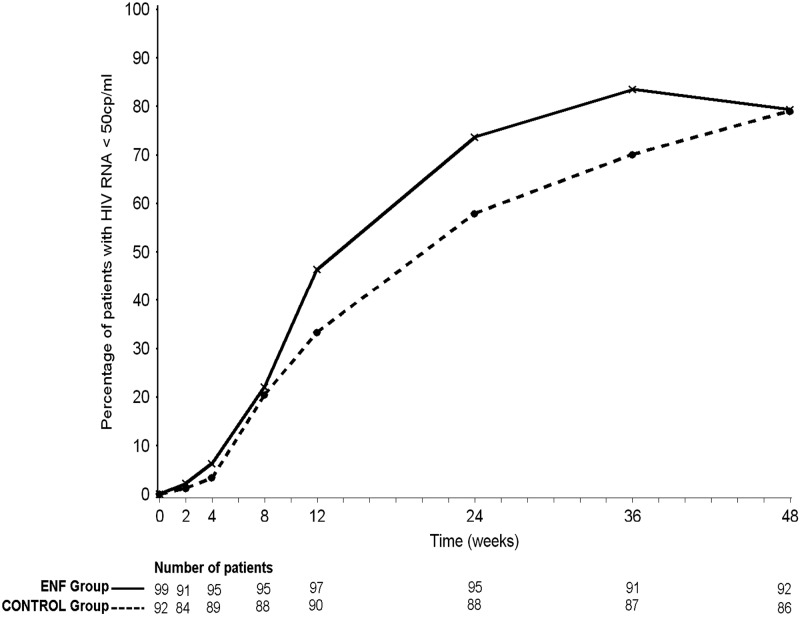
The proportion of patients with HIV-1 RNA loads of <50 copies/ml is depicted in Fig. 3. At week 12, 45 patients (46%; 95% confidence interval [CI], 37 to 56%) in the enfuvirtide arm and 30 patients (33%; 95% CI, 24 to 43%) in the control arm had a plasma HIV-1 RNA load of <50 copies/ml (P = 0.07). At week 24, 70 patients (74%; 95% CI, 65 to 83%) in the enfuvirtide arm and 51 patients (58%; 95% CI, 48 to 68%) in the control arm had a plasma HIV-1 RNA load of <50 copies/ml (P = 0.02). Finally, similar proportions of patients in both arms reached HIV-1 RNA loads of <50 copies/ml at week 48 (79% [95% CI, 71 to 88%] in both arms). A lack of an early virological response, defined as a decrease in the HIV-1 RNA measure of less than 2 log10 copies/ml at week 4, was reported in 12 patients (12%) and 16 patients (17%) in the enfuvirtide arm and in the control arm, respectively (P = 0.31).
Fig 3.
Proportion of patients with HIV-1 RNA loads of <50 copies/ml, ANRS 130 Apollo trial (ENF, enfuvirtide; data points are medians, and errors bars are omitted for clarity).
Clinical progression and adverse events.
Clinical adverse events reported throughout the duration of the study are shown in Table 2. There was no difference between arms in the percentage of patients reporting at least one clinical adverse event, a grade 3 or 4 clinical event, a serious event, or a study treatment-related event. Enfuvirtide toxicity was limited to nodular or local inflammatory reactions, mostly grade 1 and 2, reported by 38 patients. Twenty patients (20%) and 12 patients (13%) in the enfuvirtide arm and in the control arm, respectively, experienced at least one AIDS event during follow-up (P = 0.17). AIDS events are depicted in Table 3. Of note, two-thirds of these events were new AIDS-defining diseases. Four deaths occurred, two in the intensification arm and two in the control arm. Three out of the four deaths were related to severe bacterial infections.
Table 2.
Clinical adverse events in the ANRS 130 Apollo trial
| Adverse event | No. (%) of patients |
||
|---|---|---|---|
| Enfuvirtide group (n = 100) | Control group (n = 94) | Total (n = 194) | |
| Clinical adverse events | |||
| Any grade | 92 (92) | 85 (90) | 177 (91) |
| Any grade and trial treatment related | 61 (61) | 52 (55) | 113 (58) |
| Grades 3 and 4 | 23 (23) | 28 (30) | 51 (26) |
| Grades 3 and 4 and trial treatment related | 7 (7) | 8 (9) | 15 (8) |
| Serious | 28 (28) | 27 (29) | 55 (28) |
| Serious and study treatment related | 9 (9) | 13 (14) | 22 (11) |
| AIDS events | 20 (20) | 12 (13) | 32 (16) |
| IRIS | 15 (15) | 20 (21) | 35 (18) |
| Deaths | 2 (2) | 2 (2) | 4 (2) |
Table 3.
AIDS events in the ANRS 130 Apollo trial
| AIDS event | No. of events (no. of new AIDS events) |
||
|---|---|---|---|
| Enfuvirtide group | Control group | Total | |
| Total | 31 (19) | 17 (11) | 48 (30) |
| Mycobacterial infection | 7 (6) | 4 (3) | 11 (9) |
| Esophageal candidiasis | 4 (3) | 4 (2) | 8 (5) |
| Cytomegalovirus infection | 5 (3) | 2 (2) | 7 (5) |
| Kaposi's sarcoma | 5 (1) | 1 (1) | 6 (2) |
| Recurrent pneumonia | 2 (1) | 1 (1) | 3 (2) |
| Cryptosporidiosis | 2 (2) | 2 (2) | |
| Cryptococcus infection | 2 (1) | 2 (1) | |
| Pneumocystis pneumonia | 1 (1) | 1 | 2 (1) |
| Toxoplasmosis | 2 | 2 | |
| HIV encephalitis | 2 (1) | 2 (1) | |
| Progressive multifocal leukoencephalitis | 1 (1) | 1 (1) | |
| Lymphoma | 1 (1) | 1 (1) | |
| Salmonella septicemia | 1 | 1 | |
An IRIS event was reported in 15 patients (15%) and 20 patients (21%) in the enfuvirtide arm and in the control arm, respectively (P = 0.26). The most frequent manifestations were mycobacterial infections (16 cases), folliculitis (9 cases), and nonrecurrent varicella-zoster (6 cases). The probability of developing an IRIS event was not different between the two arms (P = 0.18).
Grade 3 or 4 laboratory abnormalities were found in 21% of patients, with no difference between arms (P = 0.45) (Table 4). Among 57 grade 3 or 4 laboratory abnormalities, only 7 were considered by the investigator to be related to study drugs (mostly hypophosphatemia, hypertriglyceridemia, or proteinuria related to emtricitabine-tenofovir) (Table 5).
Table 4.
Laboratory abnormalities in the ANRS 130 Apollo trial
| Laboratory abnormality | No. (%) of patients |
||
|---|---|---|---|
| Enfuvirtide group (n = 100) | Control group (n = 94) | Total (n = 194) | |
| Any grade | 92 (92) | 87 (93) | 179 (92) |
| Grade 2 | 58 (58) | 57 (61) | 115 (59) |
| Grade 3 or 4 | 19 (19) | 22 (23) | 41 (21) |
Table 5.
Grade 3 and 4 laboratory abnormalities in the ANRS 130 Apollo trial
| Grade 3 or 4 abnormality | No. of events |
||
|---|---|---|---|
| Enfuvirtide group (n = 28) | Control group (n = 29) | Total (n = 57) | |
| Leukopenia/neutropenia (<2,000/750 cells/mm3) | 13 | 13 | 26 |
| ASAT, ALAT, PAL, bilirubin >5× ULNa | 11 | 4 | 15 |
| Related to antiretroviral drugs | 1 | 1 | |
| Triglycerides (≥8.6 mmol/liter) | 1 | 3 | 4 |
| Related to lopinavir | 1 | 1 | |
| Related to lopinavir and emtricitabine-tenofovir | 1 | 1 | |
| Phosphatemia (≤1.4 mg/dl) | 1 | 3 | 4 |
| Related to emtricitabine-tenofovir | 1 | 2 | 3 |
| Platelets (<50,000 cells/mm3) | 1 | 2 | 3 |
| Hemoglobin (<7 g/dl) | 1 | 1 | 2 |
| Proteinuria | 2 | 2 | |
| Related to emtricitabine-tenofovir | 1 | 1 | |
ASAT, aspartate transaminase; ALAT, alanine transaminase; PAL, alkaline phosphatase.
DISCUSSION
Even in Western countries, late diagnosis of HIV infection is frequent. In a British cohort of patients diagnosed with HIV-1 infection between 1996 and 2003, 15.3% had a CD4 cell count below 50 cells/mm3 (8). In a Spanish prospective cohort of antiretroviral-naive HIV-1-infected individuals, 18% had reached the AIDS stage and 32% had a CD4 cell count of <200 cells/mm3 at enrollment (16). Initial entry to medical care in the later stages of HIV disease is mainly due to late testing, particularly in populations with poor access to health care services, such as communities of color, young adults, immigrants, or poor city dwellers (17–19). In a cross-sectional study conducted in North Carolina among patients who initiated HIV care for the first time, Dennis et al. reported that more than 40% of patients presented with a CD4 cell count of <200/mm3 or an AIDS-defining event (18). In a study at the Veterans Health Administration, more than half of the HIV-infected patients presented with AIDS (19). In France, several studies have shown the same types of results. Among the 1,077 patients enrolled in the ANRS-EN12-VESPA survey, a representative sample of the French HIV-infected population, one-third of patients were classified as late testers, presenting with either clinical AIDS events or CD4 counts of <200/mm3 at diagnosis (20). The overall prevalence of delayed access to care (CD4 cell count of <200/mm3 or an AIDS-defining event) was 35.7% in the French Hospital Database on HIV (ANRS CO4 FHDH) (21).
Mortality and morbidity remain high in these late testers, despite cART. Indeed, in the FHDH, the mortality hazard ratio in patients with delayed access to care was 13.9 in the first 6 months and remained significantly higher than 1 during the subsequent 4 years, despite initiation of cART (21). Thus, improving therapeutic efficacy is needed, and it is appropriate to evaluate combinations of more than three drugs to initiate treatment in this population of patients.
Few randomized studies have compared a four-antiretroviral-drug combination to standard cART. In three studies of PI-containing cART (22–24), no beneficial effect was observed in the four-drug arm, but the PI regimen was probably not optimal (nelfinavir or unboosted indinavir). In the ACTG A5095 study, a combination of three NRTIs plus efavirenz was not more effective in reducing the plasma HIV load or increasing the CD4 cell count than two NRTIs plus efavirenz (25). However, patients were not severely immunosuppressed in this trial, (i.e., baseline CD4 cell count, 240 cells/mm3). In the present study, we chose enfuvirtide for several reasons. First, when the trial was designed, enfuvirtide was the only approved entry inhibitor and it seemed that it would be interesting to evaluate a combination including a drug interacting with targets other than reverse transcriptase or protease. Second, enfuvirtide appeared to be appropriate for patients with a poor clinical condition, considering its excellent systemic safety profile, the lack of drug interactions, and its parenteral administration, preventing a high pill burden in this context of four-drug therapy.
The Apollo trial is the first randomized comparative clinical trial evaluating the effect of intensification of standard cART with enfuvirtide in naive HIV-1-infected patients with advanced disease. This strategy trial did not show a difference between the two arms in the proportion of patients with a CD4 cell count of ≥200/mm3 after 6 months of therapy. There was no trend of a better CD4 response in the intensified arm whatever the category of CD4 level at baseline (≤50, 50 to 100, and >100/mm3). Our results differ from those of the pilot study reported by Bonora et al. (26), in which addition of enfuvirtide to cART at the initial phase of treatment resulted in an improved CD4 cell count. However, the latter study had two major limitations: the small sample size (11 patients per arm) and the observational nature of the comparison. In our study, although intensification of backbone cART did not result in immunological benefit, it induced a more rapid decline in plasma HIV-1 RNA levels, and the proportion of patients with HIV-1 loads below 50 copies/ml was significantly higher in the enfuvirtide arm. However, the more rapid decline of HIV-1 RNA at week 24 in the enfuvirtide arm did not translate into an improved CD4 cell count response or improved antiviral efficacy after week 48. The improved antiviral activity in the intensified arm suggests that the lack of an enhanced CD4 response did not result from impaired adherence to treatment. A virological substudy reporting resistance and viral tropism data did not show any difference in viral resistance at baseline. There was a trend for a more frequent switch from CCR5 to CXCR4 in the enfuvirtide arm; whether this could participate in the lack of an immunological benefit with enfuvirtide remains to be discussed (27). There was no difference between arms in terms of new AIDS-defining events and/or death. The rate of clinical progression was similar to data from other studies performed in such a population (12, 28), and our findings reflect the spectrum of opportunistic infections reported in resource-rich countries. As reported in the ACTG A5164 trial (12), the most frequent opportunistic infections occurring as new AIDS-defining events under therapy were mycobacterial infections (nine events), esophageal candidiasis (five events), and cytomegalovirus infections (five events).
Interruption of the strategy occurred mainly in the intensification arm, with 15 patients stopping enfuvirtide mostly due to poor convenience or local discomfort. However, in spite of advanced HIV disease and constraints related to twice-daily subcutaneous administration, enfuvirtide appeared to be well accepted. As it could be anticipated by toxicity data available from previous studies, the incidence of grade 3 or 4 laboratory abnormalities was similar in both arms, confirming the biological safety of enfuvirtide.
The proportion of patients with IRIS was 18%. This is consistent with the incidence of IRIS in similar cohorts, in which 17 to 25% of patients initiating cART develop manifestations of the syndrome (29–31). As reported in other studies, IRIS cases were most often dermatological or associated with mycobacterial infections. As expected, considering the CD4 cell count pattern over time, IRIS cases were not more frequent in the intensification arm, in spite of an improved early virological response.
The trial was powered to detect differences in an immunological surrogate marker of AIDS progression and survival, and our primary endpoint was based on the CD4 cell count response at 6 months. Several studies have shown that the impact of cART is primarily seen on morbidity and mortality events in the first 6 months of therapy (10–12), suggesting that early improvement in immune responsiveness is critical to prevent clinical progression. In a retrospective analysis in urban Zambia, a CD4 cell count increase of less than 100/mm3 at 6 months was associated with an increased risk of death after 6 months (13). Our primary endpoint was analyzed at the time point that, furthermore, matched with the end of enfuvirtide administration.
The backbone cART was a combination of tenofovir-emtricitabine with boosted lopinavir or efavirenz and was in agreement with the recommendations current at the time that the trial was conducted. In fact, boosted darunavir or atazanavir was not yet recommended as first-line therapy during this period. It has to be stressed that 80% of patients received boosted PI therapy; in this clinical setting, physicians tended to prefer the boosted PI-based antiretroviral regimen, probably because of its stronger genetic barrier, thus avoiding the selection of resistant viral strains that could affect potential choices for further lines of therapy (32).
In conclusion, intensification of standard cART through the addition of enfuvirtide in naive HIV-1-infected patients with advanced disease did not improve the CD4 cell count response to treatment up to 48 weeks, although it induced a faster virological response. These results suggest that this therapeutic strategy would not decrease the risk of clinical progression following initiation of treatment in these subjects. However, intensification of cART merits further investigation with compounds from other classes, such as integrase inhibitors or CCR5 inhibitors.
ACKNOWLEDGMENTS
We thank Roche, which provided enfuvirtide, and Gilead, which provided tenofovir-emtricitabine.
We thank all the patients, investigators, collaborators, virologists, and pharmacists of the clinical sites who participated in the study. We are grateful to all members of the Scientific Committee (O. Lortholary, G. Peytavin, F. Brun-Vezinet, and A. Certain), members of the Data and Safety Monitoring Board (P. Flandre, M. L. Chaix, and P. Galanaud), members of the IRIS Review Board (G. Breton, E. Caumes, and T. May), the trial clinical research assistants (A. Beuscart and N. Agher), the informatics programmer (A. Frosch), the representatives of ANRS, and the sponsor (S. Couffin-Cadiergues, M. J. Commoy, and A. Diallo).
The study was supported by ANRS (French National Agency for Research on AIDS and Viral Hepatitis).
Apollo Study Group participants in France are as follows: Saint Jean Hospital, Perpignan. H. Aumaître, M. Medus, S. Neuville, and M. Saada; Avicenne Hospital, Bobigny, S. Abgrall, M. Bentata, O. Bouchaud, J. Cailhol, H. Cordel, R. Dhote, H. Gros, P. Honoré-Berlureau, T. Huynh, A. Krivitzky, R. Mansouri, M. Poupard, V. Prendki, D. Radia, F. Rouges, F. Touam, and B. Warde; Saint Louis Hospital, Paris, N. de Castro, N. Colin de Verdière, J. Delgado, S. Ferret, S. Gallien, T. Kandel, M. Lafaurie, M. Lagrange, C. Lascoux-Combe, D. Le, J. M. Molina, J. Pavie, C. Pintado, D. Ponscarme, A. Rachline, W. Rozenbaum, D. Sereni, and O. Taulera; Saint Jacques Hospital, Besançon, J. M. Estavoyer, J. F. Faucher, A. Foltzer, B. Hoen, and L. Hustache-Mathieu; Pellegrin Hospital, Bordeaux, M. Dupon, H. Dutronc, D. Neau, J. M. Ragnaud, and I. Raymond; Necker Hospital, Paris, S. Boucly, O. Lortholary, and J. P. Viard; Bicêtre Hospital, Le Kremlin Bicêtre, C. Bechara, J. F. Delfraissy, J. Ghosn, C. Goujard, W. Kamouh, M. Môle, and Y. Quertainmont; Lariboisière Hospital, Paris, J. F. Bergmann, E. Boulanger, H. Castillo, M. Parrinello, A. Rami, and P. Sellier; Henri Duffaut Hospital, Avignon, G. Lepeu and G. Pichancourt; Raymond Poincaré Hospital, Garches, L. Bernard, H. Berthé, J. Clarissou, M. Gory, J. C. Melchior, C. Perronne, S. Stegman, and P. de Truchis; Paul Brousse Hospital, Villejuif, O. Derradji, M. Malet, E. Teicher, and D. Vittecoq; Tenon Hospital, Paris, C. Chakvetadze, C. Fontaine, T. Lukiana, G. Pialloux, and L. Slama; Bichat Claude-Bernard Hospital, Paris, D. Bonnet, S. Boucherit, N. El Alami Talbi, I. Fournier, A. Gervais, V. Joly, L. Iordache, J. J. Laurichesse, C. Leport, G. Pahlavan, B. C. Phung, and P. Yeni; Cochin Hospital, Paris, N. Bennamar, A. Brunet, L. Guillevin, D. Salmon-Ceron, and T. Tahi; Henri Mondor Hospital, Creteil, C. Chesnel, S. Dominguez, P. Jouve, J. D. Lelièvre, Y. Levy, G. Melica, and A. Sobel; Pitié Salpêtrière Hospital, Paris, S. Ben Abdallah, M. Bonmarchand, F. Bricaire, S. Herson, M. Iguertsira, C. Katlama, H. Kouadio, L. Schneider, A. Simon, and M. A. Valantin; Pierre Zobda Quitman Hospital, Fort de France, S. Abel, V. Beaujolais, A. Cabié, B. Liauthaud, and S. Pierre François; CHU Angers, P. Abgueguen, J. M. Chennebault, J. Loison, E. Pichard, and V. Rabier; Saint André Hospital, Bordeaux, J. Delaune, I. Louis, P. Morlat, and M. C. Pertusa; Edouard Herriot Hospital, Lyon, F. Brunel-Delmas, P. Chiarello, F. Jeanblanc, J. J. Jourdain, J. M. Livrozet, D. Makhloufi, and J. L. Touraine; Hôtel Dieu Hospital, Lyon, C. Augustin-Normand, F. Bailly, N. Benmakhlouf, C. Brochier, L. Cotte, V. Gueripel, K. Koffi, P. Lack, B. Lebouché, M. Maynard, P. Miailhes, S. Radenne, I. Schlienger, V. Thoirain, and C. Trepo; Sainte Marguerite Hospital, Marseille, M. P. Drogoul, G. Fabre, O. Faucher, V. Frixon-Marin, J. A. Gastaut, E. Peyrouse, and I. Poizot-Martin; Gui de Chauliac Hospital, Montpellier, J. M. Jacquet, G. Le Facher, C. Merle de Boever, J. Reynes, and C. Tramoni; Hôtel Dieu Hospital, Nantes, C. Allavena, E. Billaud, C. Biron, B. Bonnet, S. Bouchez, D. Boutoille, C. Brunet-François, H. Hüe, O. Mounoury, F. Raffi, and V. Reliquet; Les Oudairies CHD, La Roche sur Yon, O. Aubry, J. L. Esnault, S. Leautez-Nainville, P. Perré, and I. Suaud; Archet I Hospital, Nice, S. Bréaud, C. Ceppi, P. Dellamonica, F. De Salvador, J. Durant, S. Ferrando, J. G. Fuzibet, A. Leplatois, V. Mondain, I. Perbost, P. Pugliese, V. Rahelinirina, E. Rosenthal, F. Sanderson, and M. Vassalo; Pontchaillou Hospital, Rennes, C. Arvieux, J. M. Chapplain, C. Michelet, M. Ratajczak, M. Revest, F. Souala, and P. Tattevin; Civil Hospital, Strasbourg, C. Chéneau, P. Fischer, J. M. Lang, M. Partisani, and D. Rey; Bretonneau Hospital, Strasbourg, F. Bastides, J. M. Besnier, P. Le Bret, P. Choutet, J. F. Dailloux, P. Guadagnin, P. Nau, J. Rivalain, and A. Soufflet; Gustave Dron Hospital, Tourcoing, E. Aïssi, H. Melliez, S. Pavel, Y. Mouton, and Y. Yazdanpanah; De Brabois Hospital, Nancy, L. Boyer, C. Burty, L. Letranchant, T. May, and S. Wassoumbou; René Dubos Hospital, Pontoise, L. Blum and O. Danne; and Aubenas Hospital, Aubenas, M. A. Arthus and P. Dion.
Footnotes
Published ahead of print 19 November 2012
REFERENCES
- 1. Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, HIV Outpatient Study Investigators 2006. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 43:27–34 [DOI] [PubMed] [Google Scholar]
- 2. Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, Rickenbach M, Malinverni R, Vernazza P, Battegay M. 1997. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ 315:1194–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch MS, Steigbigel RT, Staszewski S, McMahon D, Fischl MA, Hirschel B, Squires K, Dinubile NJ, Harvey CM, Chen J, Leavitt RY, Protocol 039 Study Team 2003. Long-term efficacy, safety, and tolerability of indinavir-based therapy in protease inhibitor-naive adults with advanced HIV infection. Clin. Infect. Dis. 37:1119–1124 [DOI] [PubMed] [Google Scholar]
- 4. Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, Sun E, Henry D, Rode R, Potthoff A, Leonard J. 1998. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet 35:543–549 [DOI] [PubMed] [Google Scholar]
- 5. Egger M, May T, Chêne G, Phillips AN, Lederberger B, Dabis F, Costagliola D, D'Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA, Cohort Collaboration ART 2002. Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360:119–129 [DOI] [PubMed] [Google Scholar]
- 6. Lanoy E, Mary-Krause M, Tattevin P, Perbost I, Poizot-Martin I, Dupont C, Costagliola D, ANRS C004 French Hospital Database on HIV Clinical Epidemiological Group 2007. Frequency, determinants and consequences of delayed access to care for HIV infection in France. Antivir. Ther. 12:89–96 [DOI] [PubMed] [Google Scholar]
- 7. Girardi E, Sabin CA, Monforte AA. 2007. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J. Acquir. Immune Defic. Syndr. 46(Suppl 1):S3–S8 [DOI] [PubMed] [Google Scholar]
- 8. Sabin CA, Smith CJ, Gumley H, Murphy G, Lampe FC, Phillips AN, Prinz B, Youle M, Johnson MA. 2004. Late presenters in era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS 18:2145–2151 [DOI] [PubMed] [Google Scholar]
- 9. Dheda K, Lampe FC, Johnson MA, Lipman FC. 2004. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J. Infect. Dis. 190:1670–1676 [DOI] [PubMed] [Google Scholar]
- 10. Chêne G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, Dabis F, Lundgren J, D'Arminio Monforte A, de Wolf F, Hogg R, Reiss P, Justice A, Leport C, Staszewski S, Gill J, Fatkenheuer G, Egger ME, Antiretroviral Therapy (ART) Cohort Collaboration 2003. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet 362:679–686 [DOI] [PubMed] [Google Scholar]
- 11. Grabar S, Le Moing V, Goujard C, Egger M, Leport C, Kazatchkine MD, Weiss L, Costagliola D. 2000. Clinical outcomes of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann. Intern. Med. 133:401–410 [DOI] [PubMed] [Google Scholar]
- 12. Zolopa AR, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hogg E, Komarow L. 2009. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 4:e5575 doi:10.1371/journal.pone.0005575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koethe JR, Limbada MI, Giganti MJ, Nyirenda CK, Mulenga L, Wester CW, Chi BH, Stringer JS. 2010. Early immunologic response and subsequent survival among malnourished adults receiving antiretroviral therapy in urban Zambia. AIDS 24:2117–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan JE, Masur H, Holmes KK, USPHS, Infectious Disease Society of America 2002. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recommend. Rep. 51(RR8):1–52 [PubMed] [Google Scholar]
- 15. French MA, Price P, Stone SF. 2004. Immune restoration disease after antiretroviral therapy. AIDS 18:1615–1627 [DOI] [PubMed] [Google Scholar]
- 16. Sobrino Vegas P, Garcia San Miguel L, Caro Murillo AM, Miró JM, Viciana P, Tural C, Saumoy M, Santos I, Sola J, del Amo J, Moreno S, CoRIS 2009. Delayed diagnosis of HIV infection in a multicenter cohort: Prevalence, risk factors, response to HAART and impact on mortality. Curr. HIV Res. 7:224–230 [DOI] [PubMed] [Google Scholar]
- 17. Girardi E, Aloisi MS, Arici C, Pezzotti P, Serraino D, Balzano R, Vigevani G, Alberici F, Ursitti M, D'Alessandro M, D'Arminio Monforte A, Ippolito G, for the ICoNA Behavioural Epidemiology Study Group 2004. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J. Acquir. Immune Defic. Syndr. 36:951–959 [DOI] [PubMed] [Google Scholar]
- 18. Dennis AM, Napravnik S, Sena AC, Eron JJ. 2011. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin. Infect. Dis. 53:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. 2007. Delayed presentation for human immunodeficiency virus (HIV) care among veterans. Med. Care 45:1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delpierre C, Dray-Spira R, Cuzin L, Marchou B, Massip P, Lang T, Lert F, Study Group VESPA 2007. Correlates of late HIV diagnosis: implications for testing policy. Int. J. STD AIDS 18:312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lanoy E, Mary-Krause M, Tattevin P, Dray-Spira R, Duvivier C, Fischer P, Obadia Y, Lert F, Costagliola D, Clinical Epidemiology Group of French Hospital Database on HIV Infection 2006. Predictors identified for losses to follow-up among HIV-seropositive patients. J. Clin. Epidemiol. 59:829–835 [DOI] [PubMed] [Google Scholar]
- 22. Shafer RW, Smeaton LM, Robbins De Gruttola GK V, Snyder SW, D'Aquila RT, Johnson VA, Morse GD, Nokta MA, Martinez AI, Gripshover BM, Kaul P, Haubrich R, Swingle M, McCarty SD, Vella S, Hirsch MS, Merigan TC, AIDS Clinical Trials Group 384 Team 2003. Comparison of four-drugs regimen and pairs of sequential three-drugs regimens as initial therapy for HIV-1 infection. N. Engl. J. Med. 349:2304–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeni P, Cooper DA, Aboulker JP, Babiker AG, Carey D, Darbyshire JH, Floridia M, Girard PM, Goodall RL, Hooker MH, Mijch A, Meiffredy V, Salzberger B, for the INITIO Trial International Co-ordinating Committee 2006. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITO: open-label randomized trial. Lancet 368:287–298 [DOI] [PubMed] [Google Scholar]
- 24. Fischl MA, Ribaudo HJ, Collier AC, Erice A, Giuliano M, Dehlinger M, Eron JJ, Jr, Saag MS, Hammer SM, Vella S, Morse GD, Feinberg JE, Demeter LM, Eshleman SH, Adult AIDS Clinical Trials Group 388 Study Team 2003. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen in advanced human immunodeficiency virus disease. J. Infect. Dis. 188:625–634 [DOI] [PubMed] [Google Scholar]
- 25. Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Schackman BR, Meyer WA, III, Acosta EP, Schouten J, Squires KE, Pilcher CD, Murphy RL, Koletar SL, Carlson M, Reichman RC, Bastow B, Klingman KL, Kuritzkes DR, AIDS Clinical Trials Group (ACTG) A5095 Study Team 2006. Three- versus four-drugs antiretroviral regimens for the initial treatment of HIV-1 infection. A randomized controlled trial. JAMA 296:769–781 [DOI] [PubMed] [Google Scholar]
- 26. Bonora S, Calcagno C, Cometto C, Fontana S, Aguilar D, D'Avolio A, Gonzalez de Requena D, Maiello A, Dal Conte I, Lucchini A, Di Perri G. 2012. Short-term additional enfuvirtide therapy is associated with a greater immunological recovery in HIV very late presenters: a controlled pilot study. Infection 40:69–75 [DOI] [PubMed] [Google Scholar]
- 27. Charpentier C, Joly V, Larrouy L, Fagard C, Visseaux B, Colin de Verdière N, Raffi F, Yeni P, Descamps D. on behalf the ANRS 130 APOLLO Trial Study Group Role and evolution of viral tropism in patients with advanced HIV disease receiving intensified initial regimen in the ANRS 130 APOLLO trial. J. Antimicrob. Chemother., in press [DOI] [PubMed] [Google Scholar]
- 28. Miro JM, Manzardo C, Pich J, Domingo P, Ferrer E, Arribas JR, Ribera E, Arrizabalaga J, LoncÁ M, Cruceta A, de Lazzari E, Fuster M, Podzamczer D, Plana M, Gatell JM, Advanz Study Group 2010. Immune reconstitution in severely immunosuppressed antiretroviral-naïve HIV type 1-infected patients using a nonnucleoside reverse transcriptase inhibitor-based or a boosted protease inhibitor-based antiretroviral regimen: three-year results (The Advanz Trial): a randomized, controlled trial. AIDS Res. Hum. Retroviruses 26:747–757 [DOI] [PubMed] [Google Scholar]
- 29. French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, Price P, Flexman JP, Tay-Kearney ML. 2000. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 1:107–115 [DOI] [PubMed] [Google Scholar]
- 30. Jevtovic DJ, Salemovic D, Ranin J, Pesic I, Zeriav S, Djurkovic-Djakovic O. 2005. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 6:140–143 [DOI] [PubMed] [Google Scholar]
- 31. Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. 2006. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin. Infect. Dis. 42:418–427 [DOI] [PubMed] [Google Scholar]
- 32. Manzardo C, Zacarelli M, Aguero F, Antinori A, Miro JM. 2007. Optimal timing and best antiretroviral regimen in treatment-naïve HIV-infected individuals with advanced disease. J. Acquir. Immune Defic. Syndr. 46(Suppl 1):S9–S18 [DOI] [PubMed] [Google Scholar]