Abstract
The declining efficacy of artemisinin derivatives against Plasmodium falciparum in western Cambodia is a major concern. The knowledge gap in the understanding of the mechanisms involved hampers designing monitoring tools. Here, we culture-adapted 20 isolates from Pailin and Ratanakiri (areas of artemisinin resistance and susceptibility in western and eastern Cambodia, respectively) and studied their in vitro response to dihydroartemisinin. No significant difference between the two sets of isolates was observed in the classical isotopic test. However, a 6-h pulse exposure to 700 nM dihydroartemisinin (ring-stage survival assay -RSA]) revealed a clear-cut geographic dichotomy. The survival rate of exposed ring-stage parasites (ring stages) was 17-fold higher in isolates from Pailin (median, 13.5%) than in those from Ratanakiri (median, 0.8%), while exposed mature stages were equally and highly susceptible (0.6% and 0.7%, respectively). Ring stages survived drug exposure by cell cycle arrest and resumed growth upon drug withdrawal. The reduced susceptibility to artemisinin in Pailin appears to be associated with an altered in vitro phenotype of ring stages from Pailin in the RSA.
INTRODUCTION
Recent scaling up of control efforts has reduced malaria morbidity and mortality rates in many areas of endemicity (1). However, Plasmodium falciparum drug resistance jeopardizes these successes. Resistance to quinolines and antifolates has disseminated worldwide during the last decades (2, 3), and the situation has recently worsened, with efficacy of artemisinin (ART) derivatives declining in western Cambodia (4–6) and Thailand (7).
In western Cambodia, especially along the border with Thailand, an area considered the world's hot spot for P. falciparum multidrug resistance (8), artemisinin resistance (ART-R) was first suspected in 2002 to 2005, with the observation of an increased number of day 3 (D3) positive cases following treatment with artemisinin-based combination therapies, the recommended first-line treatment for uncomplicated falciparum malaria. The prolonged parasite clearance phenotype after treatment with artemisinin derivatives was later confirmed by clinical studies conducted in Battambang, Pailin, and Pursat, three provinces in western Cambodia, in 2006, 2008, and 2009 to 2010 (5, 6). Importantly, although evidence exists that resistance in the country is not confined to a single western province, therapeutic efficacy studies conducted in Cambodia in 2009 to 2011 by the National Center for Parasitology, Entomology and Malaria Control (CNM) indicated that ART-R has not yet emerged or spread to eastern Cambodia. The PCR-corrected treatment failure rate following dihydroartemisinin-piperaquine (DHA-PIP) treatment was approximately 25% (95% confidence interval [95% CI], 10% to 51%) in Pailin in western Cambodia and 0% in Ratanakiri in eastern Cambodia, and the proportion of patients positive on day 3 was 32.8% in Pailin but nil in Ratanakiri (9).
The declining efficacy of artemisinin derivatives is evidenced by a prolonged parasite clearance half-life (4, 7, 10). Although recent studies indicated that the phenotype of prolonged parasite clearance half-life in vivo is genetically determined (4, 7, 11, 12), no reliable parasite molecular marker has been identified yet. The correlation between the altered in vivo infection parameters and the in vitro drug susceptibility profile in the standard radioactive chemosensitivity assay is unclear, with a slightly elevated 50% inhibitory concentration (IC50) for artemisinin derivatives in parasites collected from patients with prolonged parasite clearance times (PCTs) (5, 6) or half-life (4), but a substantial overlap in the distribution of IC50s rapidly cleared parasites. To date, there has been no consensus on the mechanism of action of artemisinins or on the mechanisms by which resistance operates in the field. Mathematical modeling predicted a reduced activity of artemisinins against ring-stage parasites (ring stages) (13). In vitro studies with artemisinin-resistant parasites isolated from the F32-Tanzania clone showed that quiescence of ring stages was involved in survival after exposure to high doses of artemisinin (14). This was deemed not responsible for resistance by Tucker and colleagues (15), as numerous sensitive lines are able to enter a dormant state and this depends on the genetic background (16). They proposed that resistant parasites tolerate more drug by exiting dormancy and resuming growth at a greater rate than susceptible parental strains; i.e., dormant resistant parasites have a higher survival rate after dormancy (15). In a study investigating the link between dormancy and the resistance of artelinic acid (AL)-resistant parasites, the AL resistance phenotype was associated with a decreased sensitivity of mature-stage parasites (mature stages), a decreased sensitivity of the ring stage to the induction of dormancy, and a faster recovery from dormancy (17). A reduced sensitivity of mature stages of ART-R laboratory lines was also reported by Cui et al. (18), who showed that resistance to artemisinin was not limited to ring-stage dormancy but also occurred in trophozoites and schizonts. This differs from the artemisinin-resistant F32-Tanzania mature stages, which are killed by artemisinin derivatives (14). How these different findings relate to the reduced susceptibility of field isolates in Cambodia is unknown.
To address this issue, we took advantage of the geographic partitioning of artemisinin resistance in Cambodia and culture-adapted P. falciparum field isolates from Pailin and Ratanakiri, areas of artemisinin resistance and susceptibility, respectively, and explored their in vitro susceptibility to dihydroartemisinin (DHA) using a panel of in vitro susceptibility assays. We show that ring stages from Pailin withstand artemisinin toxicity through developmental arrest or quiescence, while mature stages are fully susceptible.
MATERIALS AND METHODS
Sample collection.
P. falciparum isolates were obtained in 2010 to 2011 from febrile patients consulting at public health centers in Pailin province (Pailin reference hospital and Ou Chra health center) and in Ratanakiri province (Veurn Say health center) (see Fig. S1 in the supplemental material and Table 1). Giemsa-stained blood smears were examined to check for P. falciparum monospecies infection and to evaluate parasite density as described previously (19). Samples (i) were cryopreserved in glycerolyte (20) and stored in nitrogen liquid and (ii) were processed for in vitro culture adaptation, and (iii) red cell pellet aliquots were stored at −20°C.
Table 1.
Characteristics of patients and sample isolates, Cambodia, 2010 to 2011a
| IPC ID | Field ID | Mo of collection | Site | Project name | Age (yr) | Sex | D0 parasite density | Treatment received | MOI | Genotyping result (at D0 before antimalarial treatment and after culture adaptation) for ex vivo-adapted/culture-adapted isolates |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad20 | K1 | RO33 | 3D7 | FC27 | glurp | ||||||||||
| 4248 | KH004_003 | May 2011 | Pailin | TRAC | 19 | Female | 103,620 | AS*3 + DHA/PIP | 1 | 230 | 400 | 630 | |||
| 4970 | PLPF004 | September 2011 | Pailin | WHO | 15 | Male | 17,100 | A_M | 1 | 180 | 400 | 550 | |||
| 4971 | PLPF005 | September 2011 | Pailin | WHO | 32 | Male | 9,234 | A_M | 1 | 120 | 300 | 450 | |||
| 4992 | PLPF006 | September 2011 | Pailin | WHO | 30 | Male | 165,781 | A_M | 1 | 150 | 300 | 750 | |||
| 5035 | KH004_030 | September 2011 | Pailin | TRAC | 39 | Male | 63,554 | AS*3 + DHA/PIP | 1 | 180 | 380 | 750 | |||
| 5100 | KH004_033 | September 2011 | Pailin | TRAC | 46 | Female | 31,651 | AS*3 + DHA/PIP | 1 | 180 | 380 | 750 | |||
| 5145 | PLPF008 | September 2011 | Pailin | WHO | 30 | Male | 227,524 | A_M | 1 | 120 | 300 | 750 | |||
| 5160 | KH004_034 | September 2011 | Pailin | TRAC | 25 | Male | 57,776 | AS*3 + DHA/PIP | 1 | 230 | 400 | 630 | |||
| 5168 | KH004_035 | September 2011 | Pailin | TRAC | 24 | Male | 40,192 | AS*3 + DHA/PIP | 1 | 150 | 350 | 550 | |||
| 5208 | PLPF009 | October 2011 | Pailin | WHO | 38 | Male | 51,406 | A_M | 1 | 120 | 400 | 750 | |||
| 3592 | RTKPF033 | November 2010 | Ratanakiri | WHO | 15 | Male | 20,378 | AM_LUM | >1 | 200 | 160 | 320 | 320 | 680/750 | |
| 4880 | 04PF026 | August 2011 | Ratanakiri | Sentinel Network | 20 | Male | 3,585 | A_M | >1 | 200 | 160 | 280 | 680 | ||
| 4914 | 04PF029 | August 2011 | Ratanakiri | Sentinel Network | 14 | Male | 65,820 | A_M | 1 | 180 | 350 | 580 | |||
| 4974 | 04PF033 | September 2011 | Ratanakiri | Sentinel Network | 25 | Female | 15,358 | A_M | 1 | 160 | 420 | 580 | |||
| 5055 | 04PF035 | September 2011 | Ratanakiri | Sentinel Network | 46 | Male | 47,960 | A_M | >1 | 230/300 | 320 | 400 | 600/630 | ||
| 5150 | 04PF038 | September 2011 | Ratanakiri | Sentinel Network | 5 | Male | 35,629 | A_M | 1 | 230 | 320 | 680 | |||
| 5152 | 04PF040 | September 2011 | Ratanakiri | Sentinel Network | 7 | Female | 23,540 | A_M | 1 | 150 | 320 | 680 | |||
| 5159 | 04PF041 | September 2011 | Ratanakiri | Sentinel Network | 20 | Female | 55,100 | A_M | 1 | 150 | 320 | 750 | |||
| 5188 | 04PF042 | October 2011 | Ratanakiri | Sentinel Network | 23 | Male | 2,530 | A_M | 1 | 120 | 250 | 750 | |||
| 5207 | 04PF043 | October 2011 | Ratanakiri | Sentinel Network | 35 | Male | 5,318 | A_M | 1 | 200 | 470 | 680 | |||
ID IPC, Institut Pasteur in Cambodia identification number; MOI, multiplicity of infection. AS*3 + DHA/PIP: D0, artesunate at 4 mg/kg of body weight; D1, artesunate at 4 mg/kg; D2, artesunate at 4 mg/kg; D3, DHA/PIP (40 mg DHA plus 320 mg piperaquine) (Duo-cotecxin; Zhejiang Holley Nanhu Pharamaceutical Co. Ltd., Jiaxing City, China), 3 tablets; D4, DHA/PIP, 3 tablets; D5, DHA/PIP, 3 tablets. AM_LUM: D0, artemether at 20 mg and lumefantrine at 120 mg (Coartem; Novartis, Bale, Switzerland), 8 tablets; D1, 8 tablets; D2, 8 tablets. A_M: D0, artesunate at 12 mg/kg (Guilin, Shanghai, China) and mefloquine at 25 mg/kg (AECH, Basel, Switzerland); D1, artesunate at 12 mg/kg and mefloquine at 25 mg/kg; D2, artesunate at 12 mg/kg and mefloquine at 25 mg/kg. Parasite density is expressed as the number of parasites per microliter. NA, not available. Parasite isolates were selected according to the following criteria: date of collection and successful adaption to in vitro culture.
In vitro culture adaptation.
Culture adaptation was performed as described previously (21, 22). Briefly, after removal of plasma, the red blood cell (RBC) pellet was washed three times in RPMI 1640 supplemented with gentamicin (Gibco-Life Technologies SAS, France) and placed in culture medium (RPMI 1640, 0.5% AlbuMAX II [Gibco-Life Technologies SAS, France], 2% decomplemented B-positive [B+] human plasma [blood bank, Phnom Penh, Cambodia]) at 4% hematocrit at 37°C in 5% O2–5% CO2–90% N2. Parasitemia, checked daily, was kept below 2% by addition of fresh blood (blood bank, Phnom Penh, Cambodia).
Culture adaptation was considered successful after 3 weeks of uninterrupted culture for parasites that withstood two successive rounds of cryopreservation/thawing. Parasites used here were cultured after the third cryopreservation/thawing procedure.
Laboratory reference clones from the Malaria Research and Reference Reagent Resource Center (MR4) (MRA-102 [strain 3D7], MRA-157 [W2], MRA-152 [7G8], and MRA-155 [HB3]; ATCC, Manassas, VA) and the wWARN in vitro module (G15) were cultivated under the same conditions.
Multiplicity of infection (clonality).
Parasite DNA was extracted from fresh blood collected at h 0 (H0) before antimalarial treatment administration and from the matched culture-adapted isolates using a QIAamp DNA blood minikit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. Genotyping was carried out using allelic family-specific nested PCR (MAD20, K1, and RO33 for msp-1 and 3D7 and FC27 for msp-2 and glurp), as described previously (23). Paired ex vivo/culture-adapted isolates were analyzed on the same days and on the same agarose gels.
The multiplicity of infection (clonality) was calculated from the highest number of alleles detected at any of the three loci.
All PCR amplifications contained a positive control (genomic DNA from strains W2, HB3, and 3D7 Africa) and a negative control (no-target DNA).
Quantitative msp-1 PCR.
For the quantitative PCR targeting msp-1 (24), DNA extracts before exposure and daily from D1 to D6 were used. PCRs were carried out in 20-μl volumes in a 96-well plate containing 1× HOT FIREPol EvaGreen qPCR mix (Solis Biodyne, Taru, Estonia), 0.5 μM (each) primer (for K1, 3′-gaaattactacaaaaggtgcaagtg-5′ and 3′-agatgaagtatttgaacgaggtaaagtg-5′; for Mad20, 3′-gaacaagtsgaacagctgtta-5′ and 3′-tgaattatctgaaggatttgtacgtcttga-5′), and 2 μl of template DNA.
Amplifications were performed under conditions of 94°C for 15 min and 45 cycles of 94°C for 15 s, 59°C for 90 s, and 72°C for 60 s, followed by melt analysis (from 65°C to 90°C) to check the specificity of the PCR products. Detection of Mad20 and K1 msp-1 alleles was performed using a LightCycler 480 system (Roche Diagnostics, Basel, Switzerland).
Results were analyzed by the comparative threshold cycle (CT) method (25), based on the finding that the Mad20 and K1 alleles amplified with the same efficiency over a range of DNA concentrations. Differences in CT (ΔCt) values of Mad20 and K1 alleles in exposed and nonexposed cultures were calculated as follows: ΔCt = CtMad20 − CtK1. The change in ΔCt over time was computed as ΔΔCtt(n) = ΔCtt(n) − ΔCtt(0), where n is time (in days) and t(0) is day 0. Relative expression levels were then calculated as 2−δδCtt(n) to account for the exponential properties of PCR. All reactions were performed in triplicate.
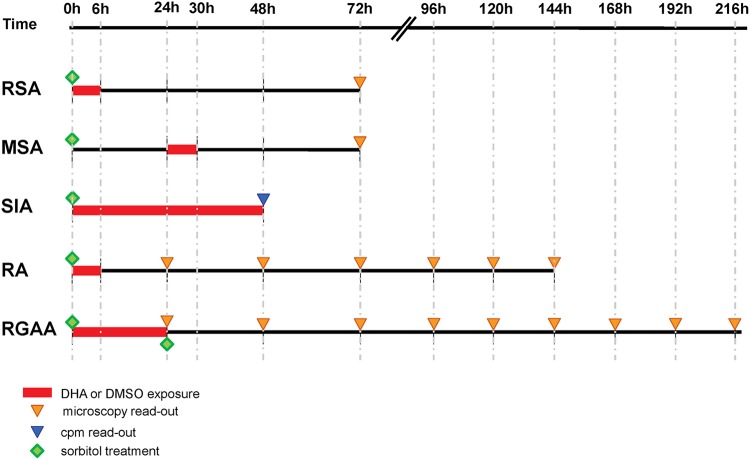
Experimental in vitro assays (Fig. 1). (i) Standard in vitro drug susceptibility assay (SIA).
Fig 1.
Schematic representation of the various in vitro phenotypes investigated with synchronous cultures. RSA, ring-stage survival assay; MSA, mature-stage survival assay; SIA, standard in vitro drug susceptibility assay; RA, recovery assay: RGAA, ring-stage growth arrest assay.
Parasite sensitivity to eight antimalarial drugs was assessed using the standard isotopic 48-h test (26) and 3D7 as the reference strain. Artesunate, dihydroartemisinin, doxycycline, chloroquine, mefloquine, piperaquine, and quinine were obtained from the WWARN QA/QC module (www.wwarn.org/research/tools/qaqc) and atovaquone from Sigma-Aldrich (Singapore). Stock solutions of antimalarial drugs were prepared in water (Biosedra, France) for chloroquine, 0.5% lactic acid for piperaquine, and methanol for the other drugs. The final plate concentrations ranged from 0.1 to 102.4 nM for artesunate, 0.0625 to 64 nM for dihydroartemisinin, 5 to 5,120 nM for chloroquine, 1 to 1,024 nM for mefloquine, 2 to 2,000 nM for piperaquine, 6.25 to 6,400 nM for quinine, 0.1 to 100 nM for atovaquone, and 1 to 500 μM for doxycycline.
Results were expressed as the 50% inhibitory concentration (IC50), defined as the concentration at which 50% of [3H]hypoxanthine incorporation was inhibited compared to the drug-free control well results, and determined by nonlinear regression using online ICEstimator software (www.antimalarial-icestimator.net).
(ii) Ring-stage survival assay (RSA) and mature-stage survival assay (MSA).
Parasite cultures were synchronized by three 5% sorbitol treatments (27). Parasites at the ring or mature stage (2 to 12 h and 24 to 36 h postreinvasion for the ring and mature stages, respectively) at 1% to 2% parasitemia, 2% hematocrit, and a 2-ml final volume were exposed for 6 h to 700 nM DHA or 0.1% dimethyl sulfoxide (DMSO) for the control cultures, washed, and resuspended in drug-free culture medium.
In the RSA and the MSA, susceptibility to DHA was assessed microscopically on thin films by estimating the percentage of viable parasites that had developed into a second generation of rings or trophozoites at 66 h and 42 h following exposure of ring- or mature-stage parasites, respectively (see Fig. 1).
The interassay repeatability of the RSA was assessed for 13 adapted lines on successive batches of cultures from the same thawed cryopreserved sample.
(iii) Recovery assay (RA).
A phenotype used in previous work with reference lines was time to recovery to initial parasitemia (14) or time to growth recovery, monitoring parasite counts for 5 days to several weeks postexposure (15, 16, 18). We monitored time to initial parasitemia after a 6-h 700 nM DHA pulse in the recovery assay. Giemsa-stained blood smears were made every 24 h after drug exposure for 6 consecutive days and examined by two independent microscopists. Recovery was expressed as the percentage of initial parasite density.
In mixed-culture experiments, synchronous ring-stage parasites with different levels of susceptibility in the RSA and distinct msp-1 alleles (PL4971 from Pailin and RT4974 from Ratanakiri) were mixed at a 1:1 ratio or cultivated separately, exposed for 6 h to 700 nM DHA, and cultivated for 6 days in the absence of drug.
(iv) Ring-stage growth arrest assay (RGAA).
Synchronized ring stages (1 to 2% parasitemia, 2% hematocrit, 10 ml final volume) were exposed for 24 h to 700 nM DHA or to 0.1% DMSO in the case of control cultures. After drug exposure, cultures were split in two flasks, and one was treated with 5% sorbitol to lyse mature stages (27). Cultures were resuspended in culture medium, and parasitemia was monitored by microscopy every day until day 10. Results were expressed as percentages of initial parasite density.
Statistical analysis.
Microsoft Excel software (Microsoft Office 2010) and MedCalc software (version 12; Mariakerke, Belgium) were used for data analysis. Data from the standard in vitro assays were analyzed after logarithmic transformation and expressed as medians with 95% confidence intervals. Categorical variables were compared by using the chi-square test or Fisher's exact test and continuous variables by using the Mann-Whitney U test. The degree of association between two variables was calculated using Spearman's correlation coefficient.
The interassay repeatability of the RSA was calculated using the average, the standard deviation (SD), and the relative standard deviation (RSD) (RSD = 100 × [SD/average], expressed as a percentage).
P values < 0.05 were considered to indicate statistically significant differences.
Ethical statement.
Ethical clearances for collection of patients' isolates were obtained from the Cambodian National Ethic Committee for Health Research (NECHR; institutional review board [IRB] no. 160, 28 October 2010, and IRB no. 007, 14 February 2011) and the Technical Review Group of the WHO Regional Office for the Western Pacific (no. ACTRN126000181808).
Informed written consent was provided by all patients or their parents or guardians before inclusion in the study.
RESULTS
Twenty isolates collected from patients with pure P. falciparum infection enrolled in clinical efficacy trials (10 patients from Pailin and 1 from Ratanakiri) or consulting in health centers (9 patients from Ratanakiri) were successfully adapted for long-term culture. Overall, 10/10 and 7/10 isolates from Pailin and Ratanakiri, respectively, were classified as monoclonal infections. Comparison of the genotyping profiles of the ex vivo sample and the respective culture-adapted line showed that there was no loss or modification of genotypes. We concluded that culture adaptation did not modify the genotyping pattern and hence clonality of the isolates studied (Table 1).
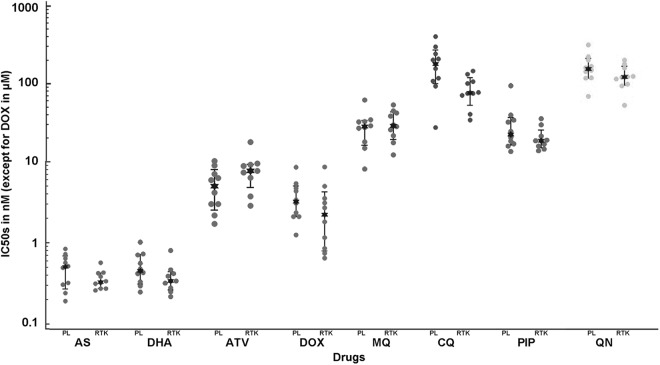
In the standard growth inhibition assay (SIA), the median values of the IC50 for artemisinin derivatives (AS and DHA), mefloquine, piperaquine, quinine, and doxycyclin were similar in the isolates from Pailin and Ratanakiri. However, the isolates from Pailin had a higher median IC50 for chloroquine than those from Ratanakiri (170 nM and 69 nM, respectively) (P = 0.01) and tended to have a lower median IC50 for atovaquone (6.9 nM and 11.2 nM, respectively) (P = 0.02) (Fig. 2).
Fig 2.
In vitro susceptibility to eight antimalarial drugs (expressed as IC50) of 20 adapted parasites collected in Pailin (n = 10) and Ratanakiri (n = 10) in 2010 to 2011 determined using the standard growth inhibition assay (SIA). PL, Pailin; RTK, Ratanakiri. Median IC50s: for artesunate (AS), 0.61 nM for PL and 0.38 nM for RTK (P = 0.26); for dihydroartemisinin (DHA), 0.47 nM for PL and 0.32 nM for RTK (P = 0.07); for atovaquone (ATV), 6.9 nM for PL and 11.2 nM for RTK (P = 0.02); for doxycycline (DOX), 5.7 μM for PL for PL and 4.8 μM for RTK (P = 0.22); for mefloquine (MQ), 30.4 nM for PL and 31.6 nM for RTK (P = 0.60); for chloroquine (CQ), 170 nM for PL and 69 nM for RTK (P = 0.01); for piperaquine (PIP), 37.6 nM for PL and 33.2 nM for RTK (P = 0.10); and for quinine (QN), 198 nM for PL and 160 nM for RTK (P = 0.25). The quality control data determined using 3D7 were as follows (median IC50 ± SD): 0.7 nM ± 0.3 nM (n = 21) for AS, 0.5 nM ± 0.2 nM (n = 21) for DHA, 19 nM ± 9 nM (n = 22) for ATV, 5.21 μM ± 1.8 nM (n = 21) for DOX, 24 nM ± 9 nM (n = 21) for MQ, 16 nM ± 4 nM (n = 22) for CQ, 36 nM ± 5 nM (n = 22) for PIP, and 74 nM ± 32 nM (N=22) for ON.
In vitro phenotype of reduced susceptibility to artemisinin derivatives.
We next explored the susceptibility of synchronous cultures of mature stages and ring stages to a 6-h pulse with 700 nM DHA, a physiologically relevant, high drug dose, in the MSA and RSA, respectively, and explored survival of the parasites (Fig. 1; see also Fig. S2 in the supplemental material).
The MSA showed that mature stages were fully susceptible, as more than 99% of the parasites were recorded as dead within 42 h following drug exposure. The high susceptibility of mature stages was observed for all parasite lines, irrespective of their geographic origin (Fig. 3). At 72 h, the median percentages of parasites in drug-exposed Pailin and Ratanakiri isolates were similar (medians, 0.6% and 0.7%, respectively; P = 0.76).
Fig 3.
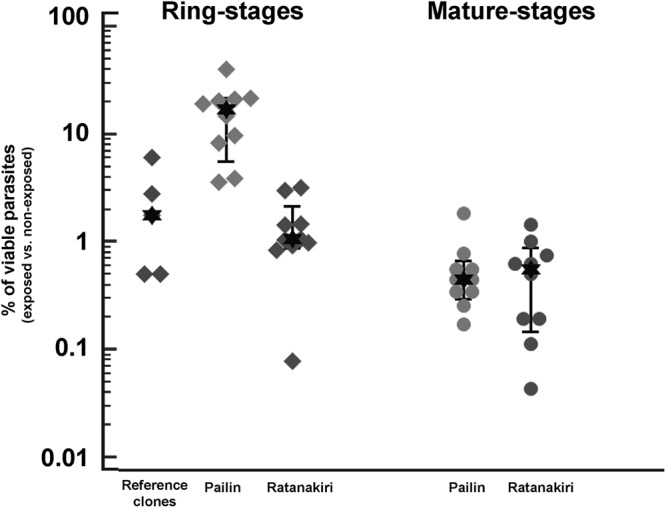
Results of the RSA and MSA expressed as the percentage of viable parasites following a 6-h exposure to 700 nM DHA of ring- and mature-stage parasites from Pailin and Ratanakiri and reference clones (W2, 3D7, HB3, G15, and 7G8).
In contrast, the RSA identified marked differences between ring stages from Pailin and Ratanakiri after exposure to a 6-h pulse of 700 nM DHA (Fig. 3). As a consequence, parasite viability was assessed at 72 h, i.e., after erythrocyte invasion and initiation of the subsequent erythrocytic cycle. The median percentage of viable parasites at 72 h was significantly higher (P = 0.0002) for parasites from Pailin (13.5% [95% CI, 4.9% to 17.0%; range, 3.9% to 19.7%]) than for parasites from Ratanakiri (0.8% [95% CI, 0.2% to 0.9%; range, 0.2% to 3.3%]) or laboratory reference clones (2.0% [range, 0.6% to 5.4%]) (Table 2).
Table 2.
In vitro phenotype of culture-adapted parasites from Pailin and Ratanakiri collected in 2010 to 2011 and reference clones determined using the standard in vitro drug susceptibility assay, the ring-stage survival assay, and the recovery assay at D3, D6, and D10a
| IPC ID | Site | SIA IC50 (nM) |
RSA (%) | RA (%) |
|||
|---|---|---|---|---|---|---|---|
| AS | DHA | D3 | D6 | D10 | |||
| 5145 | Pailin | 0.7 | 0.77 | 14.2 | 1.70 | 3.78 | 47.83 |
| 4970 | Pailin | 0.8 | 0.34 | 15.5 | NA | NA | NA |
| 4992 | Pailin | 0.35 | 0.5 | 13.8 | 0.0 | 2.5 | 14.54 |
| 4248 | Pailin | 0.27 | 0.25 | 7.1 | NA | NA | NA |
| 5035 | Pailin | 2.92 | 4.79 | 7.1 | NA | NA | NA |
| 5100 | Pailin | 0.63 | 0.44 | 4.2 | NA | NA | NA |
| 5160 | Pailin | 0.21 | 0.3 | 19.6 | NA | NA | NA |
| 5168 | Pailin | 0.37 | 0.45 | 14.2 | NA | NA | NA |
| 5208 | Pailin | 0.63 | 0.6 | 3.9 | NA | NA | NA |
| 4971 | Pailin | 1.08 | 1.13 | 17.4 | 0.87 | 3.37 | 77.71 |
| 5188 | Ratanakiri | 0.31 | 0.2 | 3.3 | NA | NA | NA |
| 3592 | Ratanakiri | 1.1 | 1,00 | 1.6 | 0.0 | 0.88 | 10.66 |
| 5150 | Ratanakiri | 0.51 | 0.45 | 0.7 | 0.0 | 0,0 | 2.5 |
| 5055 | Ratanakiri | 0.31 | 0.32 | 0.16 | NA | NA | NA |
| 4974 | Ratanakiri | 0.5 | 0.23 | 1.3 | 0.0 | 0.0 | 3.48 |
| 5152 | Ratanakiri | 0.29 | 0.37 | 0.6 | NA | NA | NA |
| 5159 | Ratanakiri | 0.45 | 0.4 | 0.6 | NA | NA | NA |
| 4880 | Ratanakiri | 0.39 | 0.26 | 1.3 | NA | NA | NA |
| 5207 | Ratanakiri | 0.36 | 0.3 | 1.1 | NA | NA | NA |
| 4914 | Ratanakiri | 0.37 | 0.32 | 2.5 | NA | NA | NA |
| 3D7 | Reference clone | 0.58 | 0.47 | 0.8 | NA | NA | NA |
| G15 | Reference clone | NA | NA | 3.5 | NA | NA | NA |
| W2 | Reference clone | 0.71 | 0.63 | 5.4 | NA | NA | NA |
| 7G8 | Reference clone | NA | NA | 1.7 | NA | NA | NA |
| HB3 | Reference clone | NA | NA | 0.6 | NA | NA | NA |
SIA, standard in vitro drug susceptibility assay; RSA, ring-stage survival assay; RA, recovery assay; AS, artesunate; DHA, dihydroartemisinin; IC50, 50% inhibitory concentration; RSA (%), percentage of viable parasites having developed into a second generation of rings or trophozoites at 66 h following exposure of ring stages; RA (%), percentage of recovery from the initial parasitemia.
The mean standard deviation (SD) and the mean relative standard deviation (RSD) of the RSA interassay repeatability (n = 13) were estimated as 1.8% (95% CI, 0.7% to 3.0%) and 22% (95% CI, 16% to 28%), respectively. Variations in parasite density evaluations assessed by microscopy readings were higher for exposed cultures (mean RSD, 34% [95% CI, 25% to 43%]) than for nonexposed cultures (mean RSD, 8% [95% CI, 6% to 9%]) (see Table S1 in the supplemental material).
In addition, no correlation was found between the RSA and MSA or between the RSA and IC50s for DHA in any parasite line.
Recovery to initial parasitemia level.
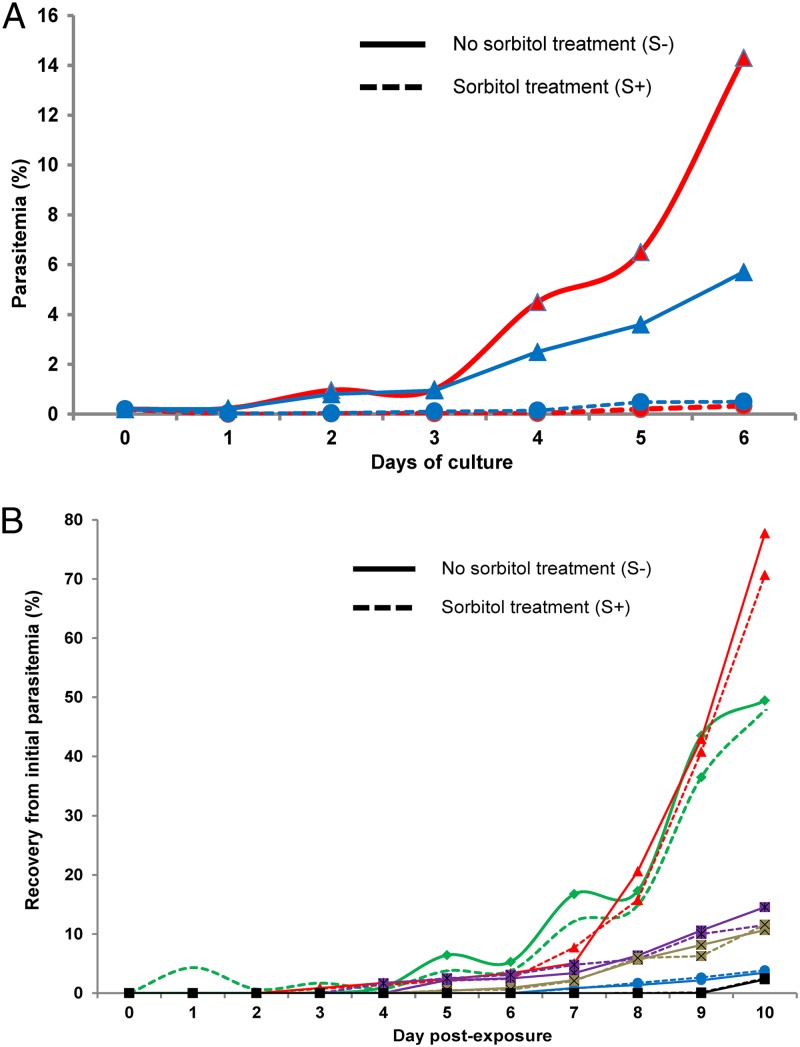
Recovery to the initial level of parasitemia after the 6-h 700 nM DHA pulse (RA; see Fig. 1) was monitored for two isolates, RT4974 (from Ratanakiri) and PL4971 (from Pailin). Figure 4A shows that RT4974 parasitemia dropped quickly to very low levels (<5% of initial parasitemia) and remained so for the next 5 days. In contrast, PL4971 parasitemia dropped more slowly during the first 2 days and rebounded thereafter, such that the initial values were reached again between day 4 and day 5. Overall, for the set of six isolates studied, significant correlations were observed between the percentages of viable parasites in the RSA and in the recovery assay monitored at D6 (n = 6, r = 0.95 [95% CI, 0.61 to 0.99; P = 0.003]) and at D10 (n = 6, r = 0.83 [95% CI, 0.06 to 0.98; P = 0.04]) (Table 2).
Fig 4.
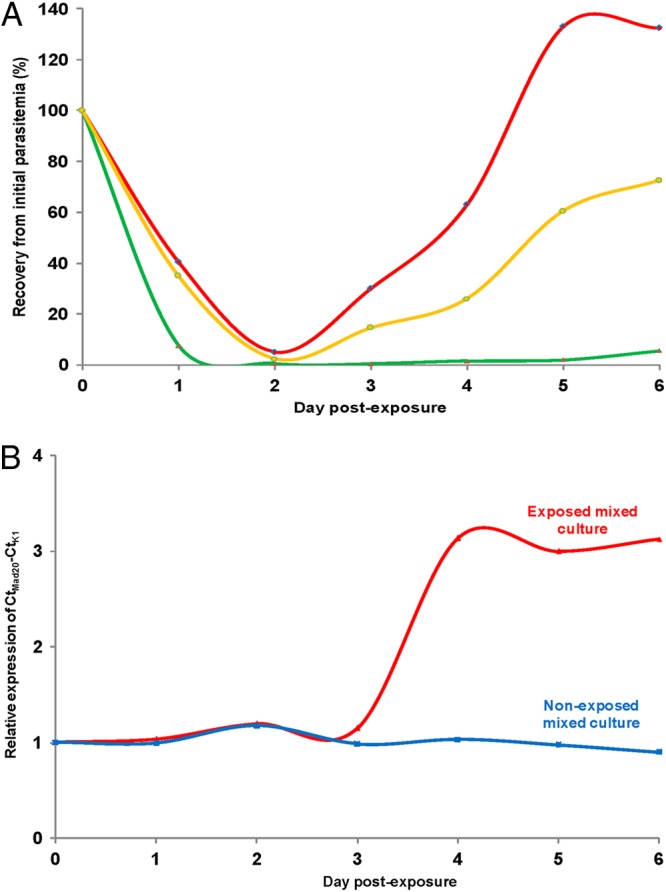
Results of the recovery assay (RA) following a 6-h exposure to 700 nM DHA of ring-stage parasites in separate and mixed cultures. (A) Recovery of the initial parasitemia for PL4971 from Pailin (red), RT4974 from Ratanakiri (green), and a mixed culture with an initial 1:1 ratio (orange). Recovery of the initial parasitemia from D2 to D6 of separate cultures (for PL4971, 5%, 30%, 63%, 133%, and 132.5%; for RT4974, 0.45%, 0.3%, 1.4%, 1.9%, and 5.5%) and mixed cultures (2.35%, 14.5%, 26%, 60.4%, and 72.5%) that were perfectly matched. (B) Relative expression of msp-1 alleles (CtMad20 for PL4971 and CtK1 for RT4974) in exposed cultures (red) and nonexposed cultures (blue).
To further document the in vitro phenotype of quicker (more efficient) recovery of ring stages from Pailin parasites to a 6-h exposure to 700 nM DHA, we performed a RT4974 plus PL4971 mixed-culture experiment. Recovery of the mixed culture matched the calculated 50% survival of the individual cultures (Fig. 4A). This was further documented by quantitative genotyping of the cultures. As PL4971 and RT4974 harbor distinct msp-1 block 2 alleles, a Mad20-type and a K1-type allele, respectively, we monitored the allele ratio during the next 6 days of cultivation. Whereas the 1/1 ratio of Mad20 to K1 msp-1 block2 alleles was stable in the mixed unexposed culture, there was a progressive increase in the level of the Mad20 allele harbored by PL4971 in the mixed drug-treated culture, consistent with a substantial loss of the RT4974 parasites after drug treatment (Fig. 4B).
Reduced ring-stage susceptibility to DHA and developmental arrest.
To substantiate that the reduced susceptibility to DHA of Pailin isolates in the RSA was mediated by developmental arrest at the ring stage, we extended the duration of the 700 nM DHA exposure to 24 h and carried out a sorbitol treatment immediately thereafter in order to eliminate any parasite(s) that had matured during drug exposure.
In the cultures that were not exposed to DHA, the 24-h incubation allowed maturation of the parasites and, as predicted, sorbitol treatment induced a massive infected red cell lysis and a dramatic drop of parasite counts (Fig. 5A). In contrast, sorbitol lysis had no impact on the growth curve of DHA-treated cultures, indicating developmental arrest of ring stages and minimal sorbitol-induced loss of mature parasites (Fig. 5B).
Fig 5.
Recrudescence following a 24-h exposure to 700 nM DHA of ring stages with or without sorbitol treatment after drug exposure. (A) Parasitemia of control cultures (no drug exposure) of PL4971 (red) and RT4974 (blue). Cultures received a sorbitol treatment at H24 (S+) (dotted line) or were mock treated (S−) (solid line). Sorbitol treatment induced, as predicted, massive lysis of infected red cells and a dramatic drop of parasite counts (at D6, PL4971 = 14.3% in S− versus 0.2% in S+ and RT4974 = 5.6% in S− versus 0.3% in S+). (B) Recovery of the initial parasitemia of DHA-exposed PL4971 (red), PL5145 (green), PL4992 (purple), RT3592 (brown), RT4974 (blue), and RT5150 (black). Cultures received a sorbitol treatment immediately after a 24-h exposure to 700 nM DHA (S+) (dotted line) or were mock treated (S−) (solid line). In DHA-treated cultures, sorbitol treatment had no impact on the growth curve of cultures (at D10, PL4971 = 77.7% in S− versus 70.6% in S+, PL5145 = 47.8% in S− versus 49.5% in S+, PL4992 = 14.5% in S− versus 11.5% in S+, RT3592= 10.6% in S− versus 11.6% in S+, RT4974 = 3.5% in S− versus 3.9% in S+, and RT5150 = 2.5% in S− versus 2.3% in S+).
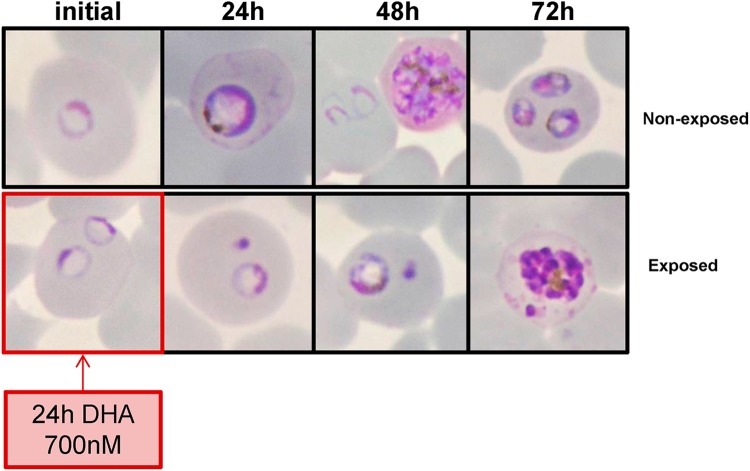
Examination of Giemsa-stained smears of DHA-exposed parasites showed that many ring stages became pyknotic within 24 h, while the surviving ring stages remained morphologically unaltered and apparently blocked in their development as they resumed growth thereafter. This is detailed in Fig. 6. In the control nonexposed culture (upper panels), viable parasites were detected at H24 (trophozoites), at H48 (schizonts/newly invaded rings), and at H72 (trophozoites). The lower panels of the figure illustrate the morphology of DHA-exposed parasites and show a typical example of doubly infected RBCs, with one surviving parasite displaying a normal morphology (H24 rings, H48 trophozoites, and H72 schizonts) and a second pyknotic intracellular parasite.
Fig 6.
Morphology of PL5145 parasites from H0 to H72 in nonexposed cultures and exposed cultures (24-h exposure to 700 nM DHA). The figure shows Giemsa-stained smears (100× magnification).
DISCUSSION
A better understanding of the mechanisms allowing parasites to withstand artemisinin toxicity in western Cambodia is urgently needed. We used here culture-adapted parasites originating from two geographically distant areas of Cambodia, Pailin, where in vivo artemisinin resistance is established and commonly observed, and Ratanakiri, where there is no sign of reduced artemisinin efficacy (9). We examined susceptibility to DHA using several in vitro assays in order to identify a robust test that would differentiate parasites from the two areas.
There was no evidence of differing susceptibilities to artemisinins among the various lines in the standard in vitro growth inhibition assay. Importantly, mature stages appeared equally susceptible to a 6-h pulse with 700 nM DHA in the two sets of cell lines (Fig. 3). However, ring-stage susceptibility in the RSA differed markedly between parasites from Pailin and Ratanakiri (Fig. 3 and Table 2). The Pailin parasite lines consistently displayed much higher survival rates (an approximately 17-fold increase) at 72 h than parasites from Ratanakiri or reference lines. Follow-up over a 6-day period (RA) showed a reduced initial slope of decaying parasitemia in lines with a high percentage of viable parasites in RSA compared to the slope seen with lines with a low percentage of viable parasites in RSA and a quicker recovery of initial parasitemia after drug removal (Fig. 4).
The most important information gathered from this study is that ring stages from Pailin showed an altered in vitro phenotype. They were able to survive a high-dose DHA exposure, unlike the mature stages, which were fully susceptible to DHA. It thus appears that the artemisinin resistance reported in Pailin is associated with an increased number of rings capable of entering a transient developmental arrest and resuming growth after drug removal (Fig. 5B). The in vitro phenotype consistently observed in all Pailin lines exposed to DHA thus differs from the usual drug resistance phenotype, in which essentially all parasites survive exposure and proceed unimpaired to schizogony. This is partially consistent with the observations made by Teuscher et al. using artelinic acid-resistant parasite lines where higher proportions of viable rings were observed in the resistant lines after treatment than in sensitive lines (17) and differs from the phenotype of the DHA-resistant laboratory lines derived from the multidrug-resistant Dd2 clone of W2 (18), which multiply in the presence of artemisinins and as a consequence display a higher IC50 for DHA than their sensitive parents. In both Pailin and Ratanakiri, DHA seems active in its effect on mature stages but has a reduced efficacy for ring stages, consistent with recent mathematical modeling (13).
Our observation that mature stages of artemisinin-resistant parasites from Pailin remain fully susceptible to DHA toxicity explains why these parasites score as susceptible in the standard in vitro test, which monitors DNA synthesis and maturation. We previously reported that parasites from western Cambodia (including Pailin) tended to show 2-to-3-fold-higher geometric mean IC50s for artesunate than parasites from eastern Cambodia (including Ratanakiri) (19). These observations derived from analysis of 820 parasite isolates (495 from western Cambodia and 325 from eastern Cambodia) collected from 2001 to 2007. When stratified by year, however, the geographic difference was significant only for the years 2003 to 2006. In 2007, the most recent year investigated, we did not observe any difference in the artesunate IC50s between western and eastern Cambodia (IC50 geometric mean [GMIC50] = 1.7 nM [range, 0.4 to 6.7 nM] and GMIC50 = 1.4 nM [range, 0.5 to 5.6 nM], respectively). This was confirmed by studies performed in Pailin and Ratanakiri from 2009 to 2011 (9). The artesunate and DHA IC50 medians for P. falciparum isolates from Pailin and Ratanakiri were not significantly different. Although the data observed here are not fully comparable, as they were obtained with a smaller sample size and with culture-adapted lines, the trend for similar IC50s for artesunate and DHA in Pailin and Ratanakiri in the recent years is in line with the data gathered in 2007 (19) and in 2009 to 2011 (9).
Use of a 24-h drug exposure to 700 nM instead of a 6-h course allowed us to convincingly document cell growth arrest, evidenced by essentially unaltered morphology of ring stages and resistance to sorbitol-induced lysis. The developmental block of DHA-treated ring stages observed here has been reported for numerous lines, including susceptible lines (14, 16, 17), but higher numbers of surviving ring stages were recorded for the artemisinin-resistant F32-ART clone (14) and Dd2-derived artemisinin-resistant clones (18). Here, a substantial fraction of rings from Pailin (3.9% to 20%) were quiescent (i.e., apparently arrested in their development) upon exposure to DHA, contrasting with the small fraction of parasites in reference laboratory lines (W2, HB3, 3D7, 7G8, and G15) (0.6% to 5.4%) and even smaller fraction in parasite lines from Ratanakiri (0.2% to 3.3%). These findings indicate that quiescence is an inherent phenotypic trait of P. falciparum parasites and that reduced susceptibility to artemisinin derivatives is associated with a higher proportion of ring stages entering developmental arrest upon exposure to the drug and subsequently exiting quiescence. This seems to account for the observed fundamental difference between the Pailin and Ratanakiri parasite populations, consistent with the evidence of artemisinin resistance being a heritable parasite-encoded trait (11).
After a 6-h exposure to 700 nM DHA and after a 24-h exposure to the same high dose, the quiescent, seemingly growth-arrested parasites were readily detected, as they retained typical ring morphology. In this regard, the phenotype of quiescent parasites observed here and by Witkowski et al. (14) differs from the phenotype of dormant parasites reported by Teuscher et al. (16) and Tucker et al. (15), who describe dormant parasites as having a smaller cytoplasm and more-condensed chromatin, i.e., resembling pyknotic forms more than ring forms.
Our results indicate that after a single dose of 700 nM DHA, all 10 lines from Pailin had a higher proportion of surviving rings than all 10 lines from Ratanakiri or reference laboratory clones. The counts of parasites that resumed growth upon drug removal matched the counts of the surviving ring forms, consistent with the interpretation that these forms were quiescent. This demonstrated that survival of ring-stage parasites from Pailin—and therefore their in vitro phenotype of reduced susceptibility to DHA—was mediated by cell growth arrest or by a physiological state of very slow, marginal progression in the cell cycle. This conclusion is substantiated by the positive correlation found between the RSA and the recovery assay at D6 and D10 after a 24-h DHA exposure. We therefore propose that Pailin parasites withstand DHA toxicity thanks to an increased number of rings able to enter quiescence and remain alive at high DHA concentrations. We think that this increased number of quiescent forms accounts for the ability to recover earlier than the susceptible strains and for the ability to recrudesce at higher numbers than susceptible parasites. Quicker recovery and a higher number of recrudescing parasites have been described in artelinic acid-resistant lines (17) and artemisinin-resistant lines (15), although they do not seem attributable to a greater number of dormant rings in these lines.
We have developed here an in vitro assay that provides phenotypic partitioning in a clear dichotomy between Pailin and Ratanakiri isolates, the latter exhibiting phenotypes close to those of ART-susceptible laboratory lines. The assay is simple, and it is much quicker than the recovery or recrudescence assay. Decline and resumed growth are captured in the RSA, which monitors the number of parasites having survived a short pulse of high-dose DHA. Detection of viable parasites was carried out by microscopy, and only morphologically viable parasites capable of producing a second generation of parasites were numerated, disregarding the parasites with “crisis” or “pyknotic” morphology (Fig. 6; see also Fig. S2 in the supplemental material). Control cultures (nonexposed to DHA) were processed under the same conditions to control for variations due to the fitness or reinvasion capacity of each parasite line. We used here microscopic examination in order to visually inspect and follow events occurring in DHA-treated cultures, including vacuolization and pyknosis or, alternatively, apparent unchanged morphology and subsequent development. Although microscopy remains cheap and reliable, the method is time-consuming and not adapted for high-throughput formats needed for large-scale epidemiological studies. Alternative methods such as fluorescence-activated cell sorter (FACS) analysis using specific viability markers (14, 18, 28) have to be developed or adapted for use with ring stages under field-based conditions. Despite their current limitations, RSA and RA formats provided in vitro evidence for the reduced susceptibility to artemisinin in P. falciparum ring-stage parasites from western Cambodia so far documented only in vivo studies and substantiated by parasite population genetic evidence (4, 11). These phenotypes should help analysis of the underlying genetic traits (12). Work is now warranted to investigate these in vitro phenotypes on a larger scale to understand their relationships with in vivo clearance and recrudescence data.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the patients and the field staff involved in conducting clinical trials and sample collections. We are grateful to the staff of the Ministry of Health of Cambodia for their collaboration, especially the provincial health directors. We thank the following individuals who provided valuable technical support: Steven Bjorge and Eva-Maria Christophel (WHO) and Sandie Ménard (UMR152 UPS-IRD, Université de Toulouse, Toulouse, France).
Sample collections were supported by the Global Fund Grant Malaria Programme Round 9 (CAM-S10-G14-M), USAID, the Bill and Melinda Gates Foundation, and the University of Oxford (http://clinicaltrials.gov/ct2/show/NCT01350856). Laboratory work was supported by grants from Institut Pasteur, Division International (ACIP A-10-2010, “Long-term culture adaptation of Plasmodium falciparum isolates with different genetic backgrounds: useful tools for studying artemisinin derivative resistance and screening new anti-malaria compounds”) and Natixis Banque (“Développer des marqueurs de la résistance de Plasmodium falciparum aux dérivés de artémisinine: répondre à une urgence”). B.W. is supported by a postdoctoral fellowship from the Division International, Institut Pasteur, and Didier Ménard by the French Ministry of Foreign Affairs.
B.W. contributed to the study design, performed experiments and data analysis, and wrote the paper. P.C., S. Ke, and N. Kloeung performed the in vitro assays. N. Khim and S.C. performed genetic polymorphism analyses. O.G. performed FACS analysis. S. Kim, S.D., R.L., P.R., A.M.D., and R.T. coordinated and supervised the clinical studies and provided P. falciparum isolates. F.B.-V., A.B., F.A., and J.-C.B. contributed to the study design. O.M.-P. contributed to the study design, the analysis of the data, and the writing of the manuscript. D.M. contributed to the study design and the writing of the manuscript and performed quality control of the data and data analysis. P.R. is a staff member of the World Health Organization.
We alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 3 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01868-12.
REFERENCES
- 1. World Health Organization 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. Mita T, Tanabe K, Kita K. 2009. Spread and evolution of Plasmodium falciparum drug resistance. Parasitol. Int. 58:201–209 [DOI] [PubMed] [Google Scholar]
- 3. Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209–218 [DOI] [PubMed] [Google Scholar]
- 4. Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect. Dis. 12:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 7. Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, Ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enserink M. 2010. Malaria's drug miracle in danger. Science 328:844–846 [DOI] [PubMed] [Google Scholar]
- 9. Leang R, Barrette A, Mey Bouth D, Menard D, Abdur R, Duong S, Ringwald P. 3 December 2012. Efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008–2010. Antimicrob. Agents Chemother. doi:10.1128/AAC.00686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar. J. 10:339 doi:10.1186/1475-2875-10-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson TJ, Nair S, Nkhoma S, Williams JT, Imwong M, Yi P, Socheat D, Das D, Chotivanich K, Day NP, White NJ, Dondorp AM. 2010. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J. Infect. Dis. 201:1326–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan JC, Al Saai S, Phyo AP, Moo CL, Lwin KM, McGready R, Ashley E, Imwong M, Stepniewska K, Yi P, Dondorp AM, Mayxay M, Newton PN, White NJ, Nosten F, Ferdig MT, Anderson TJ. 2012. A major genome region underlying artemisinin resistance in malaria. Science 336:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saralamba S, Pan-Ngum W, Maude RJ, Lee SJ, Tarning J, Lindegardh N, Chotivanich K, Nosten F, Day NP, Socheat D, White NJ, Dondorp AM, White LJ. 2011. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 108:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Witkowski B, Lelievre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. 2010. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. Agents Chemother. 54:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker MS, Mutka T, Sparks K, Patel J, Kyle DE. 2012. Phenotypic and genotypic analysis of in vitro-selected artemisinin-resistant progeny of Plasmodium falciparum. Antimicrob. Agents Chemother. 56:302–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. 2010. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J. Infect. Dis. 202:1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teuscher F, Chen N, Kyle DE, Gatton ML, Cheng Q. 2012. Phenotypic changes in artemisinin-resistant Plasmodium falciparum lines in vitro: evidence for decreased sensitivity to dormancy and growth inhibition. Antimicrob. Agents Chemother. 56:428–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui L, Wang Z, Miao J, Miao M, Chandra R, Jiang H, Su XZ, Cui L. 2012. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol. Microbiol. 86:111–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim P, Wongsrichanalai C, Chim P, Khim N, Kim S, Chy S, Sem R, Nhem S, Yi P, Duong S, Bouth DM, Genton B, Beck HP, Gobert JG, Rogers WO, Coppee JY, Fandeur T, Mercereau-Puijalon O, Ringwald P, Le Bras J, Ariey F. 2010. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrob. Agents Chemother. 54:2135–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diggs C, Joseph K, Flemmings B, Snodgrass R, Hines F. 1975. Protein synthesis in vitro by cryopreserved Plasmodium falciparum. Am. J. Trop. Med. Hyg. 24:760–763 [DOI] [PubMed] [Google Scholar]
- 21. Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. 1997. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91:363–365 [DOI] [PubMed] [Google Scholar]
- 22. Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675 [DOI] [PubMed] [Google Scholar]
- 23. Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, Brown KN, Viriyakosol S. 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:369–374 [DOI] [PubMed] [Google Scholar]
- 24. Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. 2003. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am. J. Trop. Med. Hyg. 68:133–139 [PubMed] [Google Scholar]
- 25. Beshir KB, Hallett RL, Eziefula AC, Bailey R, Watson J, Wright SG, Chiodini PL, Polley SD, Sutherland CJ. 2010. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malar. J. 9:312 doi:10.1186/1475-2875-9-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420 [PubMed] [Google Scholar]
- 28. Klonis N, Crespo-Ortiz MP, Bottova I, Abu-Bakar N, Kenny S, Rosenthal PJ, Tilley L. 2011. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. U. S. A. 108:11405–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.