Abstract
In an effort to maximize outcomes, recent expert guidelines recommend more-intensive vancomycin dosing schedules to maintain vancomycin troughs between 15 and 20 mg/liter. The widespread use of these more-intensive regimens has been associated with an increase in vancomycin-induced nephrotoxicity reports. The purpose of this systematic literature review is to determine the nephrotoxicity potential of maintaining higher troughs in clinical practice. All studies pertaining to vancomycin-induced nephrotoxicity between 1996 and April 2012 were identified from PubMed, Embase, Cochrane Controlled Trial Registry, and Medline databases and analyzed according to Cochrane guidelines. Of the initial 240 studies identified, 38 were reviewed, and 15 studies met the inclusion criteria. Overall, higher troughs (≥15 mg/liter) were associated with increased odds of nephrotoxicity (odds ratio [OR], 2.67; 95% confidence interval [CI], 1.95 to 3.65) relative to lower troughs of <15 mg/liter. The relationship between a trough of ≥15 mg/liter and nephrotoxicity persisted when the analysis was restricted to studies that examined only initial trough concentrations (OR, 3.12; 95% CI, 1.81 to 5.37). The relationship between troughs of ≥15 mg/liter and nephrotoxicity persisted after adjustment for covariates known to independently increase the risk of a nephrotoxicity event. An incremental increase in nephrotoxicity was also observed with longer durations of vancomycin administration. Vancomycin-induced nephrotoxicity was reversible in the majority of cases, with short-term dialysis required only in 3% of nephrotoxic episodes. The collective literature indicates that an exposure-nephrotoxicity relationship for vancomycin exists. The probability of a nephrotoxic event increased as a function of the trough concentration and duration of therapy.
INTRODUCTION
Since its discovery in the 1950s, vancomycin has been a mainstay of therapy for serious Staphylococcus aureus infections. Although vancomycin was a second-line therapy early in its life cycle, it emerged as a first-line agent for infections due to methicillin-resistant S. aureus (MRSA) in the 1970s (1). Over the next several decades, its usage dramatically increased due to the explosion of MRSA in both the community and health care settings (2–4). Despite the recent availability of alternative agents, vancomycin still remains the treatment of choice for serious MRSA infections (5).
Despite its widespread use, there are growing concerns about the future role of vancomycin, particularly among patients who have invasive MRSA infections with vancomycin MICs of >1 mg/liter (6). Although host- and pathogen-related factors have been implicated as a cause, suboptimal vancomycin dosing has been suggested as an alternative explanation for the poorer outcomes among these patients. To counteract some of these concerns and to maximize the likelihood of achieving a 24-h ratio of area under the curve to MIC (AUC/MIC) of greater than 400, expert guidelines now recommend more-intensive vancomycin dosing and maintenance of troughs between 15 mg/liter and 20 mg/liter for serious MRSA infections (7–9).
The recommendation to maintain troughs between 15 and 20 mg/liter for serious MRSA infections has been widely integrated into clinical practice. Despite its adoption, there are (10) limited data to suggest that maintenance of vancomycin trough values between 15 and 20 mg/liter improves outcomes (8, 10). Furthermore, the widespread use of the more-intensive vancomycin dosing schedules advocated by recent guidelines has been associated with increasing reports of vancomycin-induced nephrotoxicity. Nephrotoxicity is a long-standing, yet highly debated, adverse effect associated with vancomycin administration (1). Initial reports of vancomycin-induced nephrotoxicity were largely attributed to impurities in the original formulation. Following modern fermentation methods and purification, nephrotoxicity was considered to be infrequent (5 to 7%) and reversible (1, 11, 12). With increasing reports of vancomycin-induced nephrotoxicity in the “15-to 20-mg/liter” vancomycin trough era, there is a renewed interest in evaluating the relationship between vancomycin trough concentrations and incidence of nephrotoxicity (13–16). Although there are a few recent review articles on this topic, no group has attempted to quantify systematically the risk associated with increased vancomycin trough levels (17–19). The purpose of this systematic literature review and meta-analysis is to determine the nephrotoxicity potential of maintaining higher troughs (>15 mg/liter) in clinical practice.
MATERIALS AND METHODS
Search strategy and selection criteria.
Studies were retrieved from the PubMed, Embase, Cochrane Controlled Trial Registry, and Medline databases from January 1995 to April 2012. Search terms included “vancomycin” in combination with “nephrotoxicity” or “renal toxicity” or “renal injury.” References were also identified from the bibliographies of studies retrieved from the literature search. Studies written in languages other than English and those presented solely as abstracts at scientific conferences were not considered in this analysis.
Study selection.
The abstracts of all studies were reviewed. A study was considered eligible for inclusion if the observed nephrotoxicity rates could be extracted and stratified by vancomycin troughs (<15 versus ≥15 mg/liter). Studies in which vancomycin was administered by continuous infusion were excluded to ensure uniformity in vancomycin administration across studies. When data pertinent to our review were missing, authors were contacted (whenever possible) to provide further details.
Data extraction, outcomes, and data analysis.
Data extracted from the identified studies included clinical setting, number of patients studied, inclusion and exclusion criteria, definitions of nephrotoxicity, and concomitant risk factors for nephrotoxicity (age, residence in intensive care unit [ICU], severity of illness, and receipt of concomitant nephrotoxins as defined in each study). Additional information extracted included vancomycin treatment duration, vancomycin trough levels, and patient outcomes, if available.
Analyzed outcomes.
The primary outcome was incidence of vancomycin nephrotoxicity among patients with high vancomycin troughs (≥15 mg/liter) relative to those with low vancomycin troughs (<15 mg/liter). Since the definition of nephrotoxicity varied slightly across the studies, the nephrotoxicity definition employed in the reviewed study was used in the primary outcome analysis. In addition to the primary analysis, several restricted analyses were performed. These restricted analyses included studies (i) that were limited to adults (≥18 years of age), (ii) that evaluated the relationship between initial trough values (high versus low) and nephrotoxicity, (iii) that examined the risk of nephrotoxicity across more demarcated trough strata (<10, 10 to 15, 15 to 20, and >20 mg/liter), (iv) that assessed the effect of duration of therapy on incidence of nephrotoxicity, and (v) that only had authors with no declared conflicts of interest with pharmaceutical companies. Secondary outcomes examined included mortality, hospital length of stay, reversibility of the nephrotoxicity, and need for renal replacement therapy following a nephrotoxic event.
Data analysis and statistical methods.
Data analysis was performed using Review Manager (RevMan), version 5.1 (The Cochrane Collaboration, 2011, The Nordic Cochrane Centre, Copenhagen). Odds ratios (OR) and 95% confidence intervals (CI) for dichotomous variables were calculated. Meta-analysis was performed using fixed-effects models, unless significant heterogeneity was observed, in which case random-effects models were used. Heterogeneity was assessed using the chi-square test with the extent of inconsistency assessed using I2 statistics. A P value of 0.05 was regarded as significant.
RESULTS
Our literature search identified 240 studies. Of the 240 studies, 38 studies were reviewed and 15 were included in the meta-analysis (8, 13–16, 20–52). Twenty-three studies were excluded for the following reasons: nephrotoxicity data were not presented by vancomycin trough strata (<15 versus ≥15 mg/liter; 20 studies) (16, 20, 24, 25, 27–29, 33–35, 37, 41, 42, 44–47, 49, 52); nephrotoxicity data were stratified by alternative vancomycin trough strata (<10 versus ≥10 mg/liter; 1 study) (26); the study was a case report (1 study) (40); and vancomycin was administered as a continuous infusion (2 studies) (14, 48).
The remaining 15 studies were included in the meta-analysis (Table 1) (8, 13, 15, 21–23, 30–32, 36, 38, 39, 43, 50, 51). Of these, 14 studies were conducted with adults and 1 involved children (38) (Table 1). The average trough value over the duration of therapy was examined in 6 studies (13, 30, 38, 39, 43, 50), and the initial trough value was considered in 8 studies (8, 15, 21–23, 32, 36, 51). Although timing differed between studies, most initial trough levels were obtained at the time or shortly after steady state was achieved (i.e., after the 3rd dose) but not greater than 4 days into therapy (Table 2). In patients with multiple levels during this initial period, the highest trough (22, 36) or the average trough (21) was used to define the trough exposure in the parent analysis. Four studies categorized vancomycin into more precisely defined trough strata (8, 22, 36, 50), while four studies provided data for nephrotoxicity as a function of duration of vancomycin therapy (13, 15, 39, 43).
Table 1.
Details of studies examining vancomycin-associated nephrotoxicitya
| Study authors and type (reference) | Study population | Exclusions | Definition of NTb (no., % of patients with NT) | Clinical characteristics for patients with and without NT |
Outcomes including renal | |||
|---|---|---|---|---|---|---|---|---|
| Age (yr) | ICU residence | Severity score | Receipt of other NTsb | |||||
| Bosso et al., prospective observational study (n = 291) (21) | Patients were ≥18 yr, with MRSA infections treated with VAN for ≥72 h, and had one steady-state VAN trough measurement 2–4 days into therapy | Patients on concomitant AmB or NSAID, renal replacement therapy, neutropenia, or cystic fibrosis | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 55, 19%) | Not stated | NT, 42%; nNT, 31% | Not stated | NT, 47%; nNT, 39% | Not stated |
| Cano et al., retrospective analysis of IMPACT-HAP database (n = 188) (23) | Patients had HAP, VAP, or HCAP and a baseline SCR of <2.0 mg/dl, were treated with VAN, and had at least one trough measurement taken | SCR of ≥2 mg/dl on admission or history of end stage renal disease or dialysis at baseline | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 29, 15%) | NT, 60; nNT, 58 | All patients | APACHE II score: NT, 20; nNT, 19 | NT, 55%; nNT, 29% (P < 0.01) | Patients with NT had longer ICU LOS than nNT patients (17 versus 12 days, P = 0.03). Mortality and hospital LOS were similar in the two groups |
| Chung et al., retrospective cohort study (n = 73) (21) | Patients were ≥18 yr, had nosocomial pneumonia in ICU, and were treated with VAN for ≥72 h | Patients who developed AKINc within 48 h of VAN initiation | AKIN classification (n = 28, 38%) | Not stated | All patients | Not stated | Not stated | VAN failure and mortality not associated with VAN trough concentrations |
| Hermsen et al., retrospective cohort study (n = 55) (30) | Patients were >19 yr, had MRSA infections, and were treated with VAN for ≥5 days | Patients with neutropenia and CrCl of <30 ml/min | Increase in SCR of ≥0.5 mg/dl from baseline for two consecutive measurements (n = 9, 16%) | Not stated | Not stated | Not stated | Not stated | |
| Hidayat et al., prospective cohort study (n = 95) (13) | Patients were ≥18 yr, had nosocomial MRSA infections, and were treated with VAN for ≥72 h | None | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 12, 19%) | NT, 72; nNT, 74 | Not stated | Not stated | NT, 91%; nNT, 20% (P < 0.01) | |
| Jeffres et al., retrospective cohort study (n = 94) (15) | All patients had MRSA HCAP and were treated with VAN for ≥72 h | Polymicrobial infections and acute renal failure requiring dialysis | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 40, 43%) | NT 60; nNT, 58 | Not stated | APACHE II: NT, 23; nNT, 18 (P < 0.01) | Dependent on agent, NT, 5–63%; nNT, 5–44% | SCR returned to baseline in 73% of patients prior to discharge, with no patients requiring dialysis |
| Kralovicova et al., retrospective cohort study (n = 198) (31) | Cancer patients receiving VAN for a Gram-positive bloodstream infection | Increase in SCR of ≥110 μmol/liter or >20% from baseline or decrease of CrCl of <0.7 ml/s (n = 50, 25%) | Not stated | Not stated | Not stated | 68% | Receipt of concomitant NT agents in the prior 30 days was not associated with increased NT | |
| Kullar et al., retrospective cohort study (n = 280) (8) | Adult patients with MRSA bloodstream infections, treated with VAN for ≥72 h | VAN therapy <3 days and with end stage renal failure | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 50, 19%) | Not stated | Not stated | Not stated | Not stated | Increased VAN failure (including mortality) with VAN trough concentrations of <15 mg/liter compared to trough levels of ≥15 mg/liter. LOS in hospital was longer in patients who developed NT (20 versus 13 days, P = 0.001) |
| Kullar et al., prospective multicenter study (n = 200) (32) | Adult patients with Gram-positive infections requiring targeted VAN therapy | Transplant recipient within the past 6 months; receipt of concurrent vasopressors; wt, >110 kg; baseline CrCl, <30 ml/min | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 9, 5%) | Not stated | Not stated | Not stated | NT, 67%; nNT, 5% | |
| Lodise et al., retrospective cohort study (n = 166) (36) | Nonneutropenic (neutrophil count, ≥1,000 cells/mmd) patients, who were ≥18 yr, with a baseline SCR of <2.0 mg/dl and a Gram-positive infection, received VAN for >48 h, and had a VAN trough measurement taken within the first 96 h of therapy | Patients with cystic fibrosis or who received contrast dye or vasopressors at start of VAN therapy | Increase in SCR level of 0.5 mg/dl or 50% from baseline on 2 consecutive days (n = 21, 13%) | NT, 56; nNT, 56 | NT, 67%; nNT, 39% (P = 0.02) | APACHE II score: NT, 12; nNT, 11 | NT, 29%; nNT, 38% | The mean duration that SCR remained >50% above baseline was 7 days |
| McKamy et al., retrospective cohort study (n = 167) (38) | Patients were between >1 week and 19 yr with age-appropriate normal baseline SCR and were treated with VAN for ≥48 h for a Gram-positive infection | Premature infants | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 24, 14%) | Not stated | NT, 92%; nNT 54% (P < 0.01) | Not stated | Dependent on agent, NT, 8–75%; nNT, 1–24% | SCR returned to baseline in 75% of patients prior to discharge, with no patients requiring dialysis |
| Minejima et al., prospective cohort study (n = 227) (39) | Patients were >17 yr, treated with VAN for ≥5 days, and had ≥1 VAN trough measurements taken | SCR of ≥2 mg/dl on admission or history of chronic kidney disease ≥stage III | RIFLEd criteria (n = 43; 19%) | NT, 68; nNT, 70 | NT, 29%; nNT,15% (P = 0.041) | APACHE II score: NT, 9; nNT, 8 | NT, 22%; nNT, 10% (P = 0.017) | NT was reversible in 56% of patients by the time of discharge. No patients required dialysis |
| Prabaker et al., retrospective cohort study (n = 348) (43) | Inpatients who received VAN for ≥5 days as treatment for various infections | SCR of ≥2 mg/dl prior to VAN therapy, NT occurring prior to 5 days, and concomitant AmB therapy | Increase in SCR level of 0.5 mg/dl or 50% from baseline on 2 consecutive days (n = 31, 9%) | NT, 64; nNT, 60 | Not stated | Not stated | NT, 68%; nNT, 55% | NT was reversible in 78% of patients within 72 h of VAN discontinuation. One patient required dialysis and died following withdrawal of active treatment |
| Wunderink et al., prospective randomized triale (n = 333) (50) | Adult patients treated with VAN for MRSA nosocomial pneumonia | Patients treated with any MRSA-active antibiotic >48 h within or 72 h before the prestudy period | Increase in SCR level of 0.5 mg/dl or 50% from baseline on 2 consecutive days (n = 50, 15%) | Not stated | Not stated | Not stated | Not stated | |
| Zimmermann et al., retrospective cohort study (n = 45) (51) | Patients were ≥18 yr, with Gram-positive bloodstream infections, were treated with VAN for ≥48 h, and had two VAN trough measurements taken | Acute or chronic renal impairment, receipt of nephrotoxin vasopressors | Increase in SCR of ≥0.5 mg/dl or 50% increase from baseline for two consecutive measurements (n = 9, 5%) | NT, 58; nNT, 60 | NT, 25%; nNT, 5% | Not stated | Excluded | Following dosage adjustment, SCR returned to baseline in all patients within 3–5 days |
Abbreviations: VAN, vancomycin; AmB, amphotericin B; MRSA, methicillin-resistant S. aureus; NT, nephrotoxic/nephrotoxicity; nNT, nonnephrotoxic/no nephrotoxicity; SCR, serum creatinine; CrCl, creatinine clearance; GFR, glomerular filtration rate; ICU, intensive care unit; APACHE II, acute physiology and chronic health evaluation score; SAPS, simplified acute physiology score; HAP, hospital-acquired pneumonia; HCAP, health-care associated pneumonia; VAP, ventilator-associated pneumonia; NSAID, nonsteroidal anti-inflammatory drugs; LOS, length of stay.
Nephrotoxins include any or all of the following: aminoglycosides, amphotericin B, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, colistin, contrast dye, cyclosporine, cisplatin, diuretics, nonsteroidal anti-inflammatory drugs, tacrolimus, and vasopressors.
AKIN, Acute Kidney Injury Network classification, which classifies acute renal injury into 1 of 3 stages: stage 1, increase in SCR of >0.3 mg/dl or a 1.5× increase from baseline; stage 2, 2× increase in SCR from baseline; stage 3, increase in SCR of ≥4 mg/dl (with an acute rise of ≥0.5 mg/dl) or 3× increase from baseline (62).
RIFLE, Risk-Injury-Failure-Loss End-stage renal disease criteria, which classify acute renal injury into 5 groups: (i) risk of renal dysfunction, 1.5× increase in SCR or 25% decrease in GFR; (ii) renal injury, 2× increase in SCR or 50% decrease in GFR; (iii) failure, 3× increase in SCR or acute increase of ≥0.5 mg/dl or ≥75% in GFR; (iv) complete loss of renal function persisting for >4 weeks; and (v) end stage renal disease (63).
These data, obtained from the Zephyr study (vancomycin versus linezolid for MRSA nosocomial pneumonia) and supplied by Pfizer, represent the intention to treat vancomycin-treated arm in cases where vancomycin trough data were measured or available (50).
Table 2.
Risk of nephrotoxicity following vancomycin exposurea
| Study | Incidence of NT (no. of patients with NT/total no.) | VAN trough measurement | NT incidence (no. of patients with NT/total no.) with VAN trough of: |
Duration of VAN exposure | Independent predictors of NT on multivariate analysis (if performed) | ||
|---|---|---|---|---|---|---|---|
| <15 mg/liter | ≥15 mg/liter | P value | |||||
| Bosso et al. (21) | 19% (55/288) | Initial (single level taken 2–5 days into therapy). If multiple levels taken during this period, avg initial level | 9% (13/146)b | 30% (42/142)b | <0.01 | Avg duration of VAN prior to NT was 10.6 days | VAN trough of ≥15 mg/liter (OR, 3.6; 95% CI, 1.8–7.6), black race (OR, 2.6; 95% CI, 1.3–5.2), and heart failure (OR, 3.7; 95% CI, 1.0–13.2) were independent predictors of NT. Incremental increase in rate of NT from 32 to 45 and 50% with rising VAN troughs of ≥20, ≥25, and ≥30 mg/liter, respectively |
| Cano et al. (22) | 15% (29/188) | Initial (highest level in the first 4 days of VAN therapy) | 7% (7/99) | 25% (22/89) | <0.01 | Median duration of VAN prior to NT was 8 days | VAN trough of ≥15 mg/liter (OR, 5.2; 95% CI, 1.9–13.9), duration of VAN (OR, 1.1 for each additional day; 95% CI, 1.0–1.23), and concomitant aminoglycoside use (OR, 2.7; 95% CI, 1.1–6.5) were independent predictors of NT. Incremental increase in rates of NT from 7% to 34% for rising VAN trough levels from <10 to >20 mg/liter |
| Chung et al. (23) | 38% (28/73) | Initial (level taken after 3–5 doses of VAN therapy) | 33% (16/48)b | 48% (12/25)b | 0.21 | Not stated | Not performed |
| Hermsen et al. (30) | 16% (9/55) | Avg | 10% (4/39) | 31% (5/16) | 0.04 | Median duration of VAN prior to NT was 12 days | Not performed |
| Hidayat et al. (13) | 12% (11/95) | Mean | 0% (0/32) | 17% (11/63) | 0.01 | Median duration of VAN prior to NT was 17 days. Increased NT (6, 21, and 30%) with longer durations of VAN (7, 8–14, and >14 days, respectively) | Not performed. In patients not receiving concurrent nephrotoxins, VAN trough concentrations of ≥15 mg/liter remained significantly associated with NT: 2% (1/44), versus 0% (0/24) for troughs of <15 mg/liter |
| Jeffres et al. (15) | 43% (40/94) | Initial (level taken immediately following the 3rd dose) | 29% (13/45) | 55% (27/49) | 0.01 | Not stated. NT was 20% and 45% for <14 and >14 days, respectively, of VAN | VAN trough of ≥15 mg/liter (OR, 2.82; 95% CI, 1.02–7.74) was the only independent predictor of NT |
| Kralovicova et al. (31) | 25% (50/198) | Not stated | 21% (29/138) | 35% (21/60) | NS | Not stated | Not performed |
| Kullar et al. (8) | 18% (50/280) | Initial (level taken immediately before the 4th dose) | 16% (23/141) | 19% (27/139) | NS | Not stated | Not performed |
| Kullar et al. (32) | 5% (9/200) | Initial (level taken immediately before the 4th or 5th dose) | 1% (1/84) | 7% (8/116) | Median duration of VAN prior to NT was 8 days | Not performed | |
| Lodise et al. (36) | 13% (21/166) | Initial (highest trough within the first 4 days of VAN therapy) | 10% (14/139) | 26% (7/27) | <0.05 | Mean duration of VAN prior to NT was 6 days | VAN trough (adjusted OR, 1.1 for each unit increase in trough; 95% CI, 1.1–1.2) and residence in ICU (OR, 3.25; 95% CI, 1.2–9.0) were independent predictors of NT |
| McKamy et al. (38) | 14% (24/167) | Avg | 7% (8/110) | 28% (16/57) | <0.01 | Mean durations of VAN prior to NT were 4.3 and 6.7 days for patients with troughs of <15 and ≥15 mg/liter, respectively | VAN trough of ≥15 mg/liter (OR, 3.3; 95% CI, 1.2–9.0) and receipt of furosemide in ICU (OR, 9.5; 95% CI, 3.4–26) were independent predictors of NT |
| Minejima et al. (39) | 19% (43/227) | Avg | 16% (25/155) | 24% (17/72) | 0.27 | Mean duration of VAN prior to NT was 9 days. NT, 13% and 25% for ≤7 and >7 days, respectively, of VAN | VAN trough of ≥15 mg/liter was not an independent predictor of NT (OR, 1.1, 95%; CI, 0.5–2.5), while malignancy (OR, 2.8; 95% CI, 1.2–6.3), residence in ICU (OR, 2.5; 95% CI, 1.2–5.4), and previous AKI were (OR, 6.3; 95% CI, 1.6–24.6) |
| Prabaker et al. (43) | 9% (31/348) | Mean | 8% (24/294) | 13% (7/54) | 0.11 | Median duration of VAN prior to NT was 7 days | VAN trough of ≥15 mg/liter (OR, 2.1; 95% CI, 0.9–4.6) was not an independent predictor of NT, while receipt of intravenous contrast dye was (OR, 3.6; 95% CI, 1.5–8.7) |
| Wunderink et al. (50) | 15% (50/333) | Median | 11% (24/215)c | 22% (26/118)c | Not stated | Not performed | |
| Zimmermann et al. (51) | 18% (8/45) | Initial (first level obtained after the 4th dose) | 0% (0/33) | 67% (8/12) | Avg duration of VAN prior to NT was 8.1 days | Not performed | |
Abbreviations: VAN, vancomycin; NT, nephrotoxic/nephrotoxicity; nNT, non-nephrotoxic/nephrotoxicity; AKI, acute kidney injury.
Represents the subset of original patients that had vancomycin trough data recorded.
These data, obtained from the Zephyr study (vancomycin versus linezolid for MRSA nosocomial pneumonia) and supplied by Pfizer, represent the intention to treat the vancomycin-treated arm in cases where vancomycin trough data were measured or available (50).
Nephrotoxicity.
The incidence of nephrotoxicity varied between studies from 5% to 43%. On average, nephrotoxicity occurred between 4.3 and 17 days after initiation of vancomycin therapy (13, 21, 22, 30, 32, 36, 38, 39, 43, 51). The definition of nephrotoxicity was identical (increase in serum creatinine [SCR] of 0.5 mg/dl, equivalent to 44.2 μmol/liter or 50% from baseline on 2 consecutive measurements) in all but three studies (Table 1). Of the three dissimilar studies, one used the Acute Kidney Injury Network classification of nephrotoxicity (23), one employed the Risk-Injury-Failure-Loss-End-stage-renal disease (RIFLE) classification of nephrotoxicity (39), and one used a nonstandardized definition of nephrotoxicity (increase in SCR by 20% from baseline or ≥110 μmol/liter or a decrease of creatinine clearance by <0.7 ml/s, equivalent to 42 ml/min) (31).
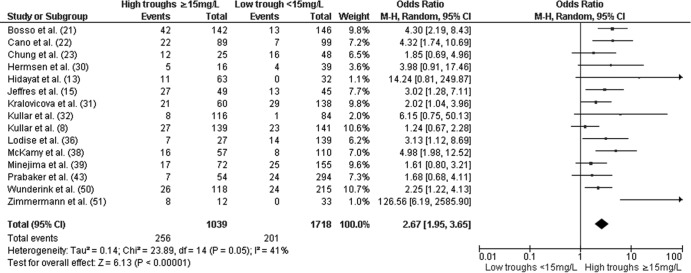
In the primary analysis, higher troughs (≥15 mg/liter) were associated with increased nephrotoxicity (OR, 2.67; 95% CI, 1.95 to 3.65; P <0.01) relative to low troughs (<15 mg/liter) (Fig. 1). The results did not change considerably when the analysis was restricted to adult patients (OR, 2.54; 95% CI, 1.84 to 3.50; P < 0.01) (38). The odds of nephrotoxicity among patients with troughs of ≥15 mg/liter remained increased (OR, 3.12; 95% CI, 1.81 to 5.37; P < 0.01) when the data were limited to studies that examined initial vancomycin trough values (8, 15, 21–23, 32, 36, 51) (Fig. 2). In addition, troughs of ≥15 mg/liter remained significantly associated with nephrotoxicity when articles (8, 13, 21, 22, 30, 36, 43) in which authors declared potential conflicts of interests with pharmaceutical companies were excluded (OR, 2.84; 95% CI, 1.65 to 4.87; P < 0.01).
Fig 1.
Forest plot (using Mantel-Haenszel [M-H] analysis) of events denoting nephrotoxicity associated with vancomycin, comparing rates for trough levels of ≥15 mg/dl and <15 mg/dl. Squares indicate point estimates, and the size of the square indicates the weight of each study.
Fig 2.
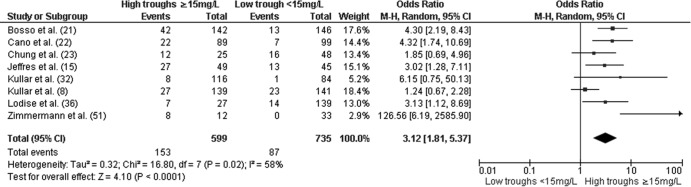
Forest plot (using Mantel-Haenszel [M-H] analysis) of events denoting nephrotoxicity associated with vancomycin, comparing rates for initial trough levels of ≥15 mg/dl and <15 mg/dl. Squares indicate point estimates, and the size of the square indicates the weight of each study. All initial trough levels were obtained at the time or shortly after steady state was achieved (i.e., after the 3rd dose) and not greater than 4 days into therapy (see Table 2 for more details).
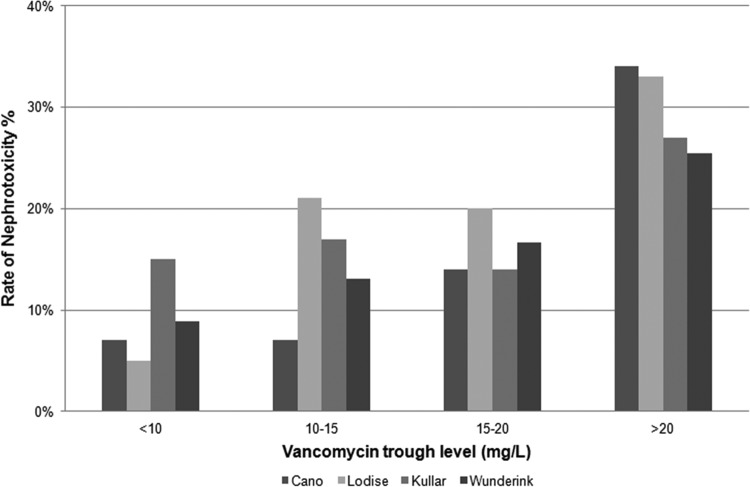
Only four studies provided nephrotoxicity rates as a function of incremental vancomycin trough values that confirmed the presence of an exposure-toxicity gradient (8, 22, 36, 50) (Fig. 3). All three found the highest rates of nephrotoxicity among patients with troughs of >20 mg/liter and the lowest rates among patients with troughs of <10 mg/liter. Two of the four studies found similar risks of nephrotoxicity between patients with troughs of 15 to 20 mg/liter and 10 to 15 mg/liter. In studies in which the duration of vancomycin therapy was analyzed, an incremental increase in nephrotoxicity (6% to 45%) was noted with longer durations (7 to 14 days) of therapy relative to shorter durations (Table 2).
Fig 3.
Incidence of vancomycin nephrotoxicity with rising trough levels (8, 22, 36, 50).
Three studies stratified baseline clinical characteristics by high (≥15 mg/liter) and low (<15 mg/liter) vancomycin troughs (13, 30, 38). A number of nephrotoxicity risk factors (e.g., increased severity of illness, receipt of concomitant nephrotoxins, and source of infection) were more pronounced in patients with high troughs than in those with low troughs. Patients in the ICU (OR, 2.57; 95% CI, 1.44 to 4.58; P < 0.01) were more likely to develop vancomycin-induced nephrotoxicity than were patients in non-ICU wards (Fig. 4). Conversely, patients receiving concomitant nephrotoxins (OR, 3.30; 95% CI, 1.30 to 8.39; P = 0.01) were more likely to develop vancomycin-induced nephrotoxicity than were patients who did not receive nephrotoxins (Fig. 5). These findings, however, were not uniform across studies. To adjust for potential confounders, several groups performed multivariate analyses (15, 21, 22, 36, 38, 39, 43). In five of the seven studies, high vancomycin troughs (≥15 mg/liter) remained an independent predictor of nephrotoxicity (Table 2).
Fig 4.
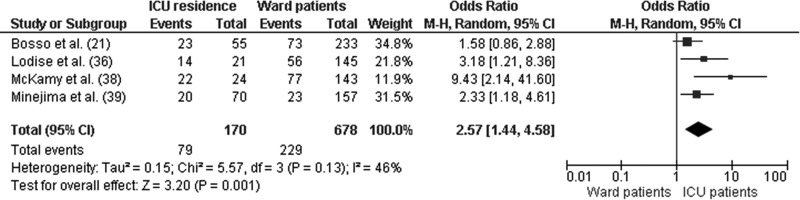
Forest plot (using Mantel-Haenszel [M-H] analysis) of events denoting nephrotoxicity associated with vancomycin, comparing rates for patients residing in ICU or the ward at the time of diagnosis. Squares indicate point estimates, and the size of the square indicates the weight of each study.
Fig 5.
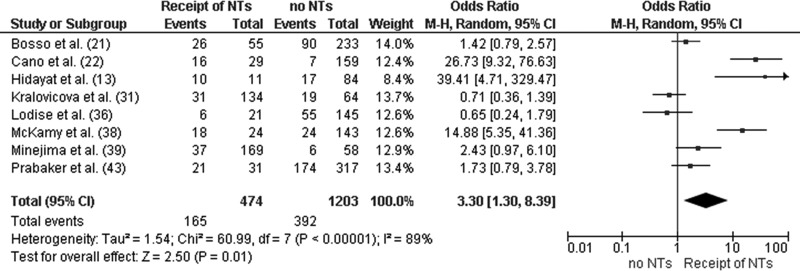
Forest plot (using Mantel-Haenszel [M-H] analysis) of events denoting nephrotoxicity associated with vancomycin, comparing rates for patients receiving and not receiving concomitant nephrotoxins at the time of diagnosis. Squares indicate point estimates, and the size of the square indicates the weight of each study. NT, nephrotoxins.
Renal and clinical outcomes.
In studies that reported on the clinical course of patients with vancomycin-induced nephrotoxicity, SCR levels returned to baseline or below predefined toxicity thresholds despite continuation of vancomycin in a substantial proportion of cases (35% to 46%) (36, 38, 43). In situations where vancomycin was discontinued, most episodes (44% to 75%) were reported to resolve within a week or less (15, 38, 39, 43, 51). In contrast, one study reported more-prolonged renal injury with SCR levels remaining toxic (≥50% of baseline value) for ≥7 days in over 50% of episodes (36). Short-term dialysis was required in a minority of patients (3%; 6 of 192 patients), and no patient was reported to require long-term dialysis (26, 36, 38, 43, 51). Nephrotoxicity was associated with increased overall mortality (39) and prolonged hospital (8, 39) and ICU (22) lengths of stay.
Heterogeneity and publication bias.
There was significant heterogeneity between the studies with different patient populations studied, nephrotoxicity definitions used, durations of vancomycin treatment required for inclusion, and measurements of trough level used in the analyses. As such, the random-effects model was required in all analyses.
DISCUSSION
The collective findings of this systematic review strongly suggest that adherence to the vancomycin trough recommendations in recent expert guidelines may result in an elevated risk of vancomycin-induced nephrotoxicity. Overall, maintaining troughs in excess of 15 mg/liter was found to substantially increase the risk of a nephrotoxic event (Fig. 2). The relationship between troughs of ≥15 mg/liter and nephrotoxicity persisted when the analyses were restricted to studies that examined initial trough values. Interestingly, data from 4 of the 15 studies suggest that a trough-toxicity gradient exists, with the greatest risk observed among individuals with troughs of >20 mg/liter (Fig. 3). The probability of a nephrotoxic event was also found to increase as a function of treatment duration, with most episodes occurring after 7 days of therapy. Lastly, data, albeit limited, may be applicable to children.
While the association between vancomycin trough and nephrotoxicity was largely uniform across studies, the incidence of vancomycin-induced nephrotoxicity was highly variable. Nephrotoxicity rates ranged between 5% and 43% and were highly dependent on the population evaluated. Not surprisingly, the highest nephrotoxicity rates were observed in studies that included a high percentage of critically ill patients that resided in the ICU and received concomitant nephrotoxins. It is well known that these populations have an elevated baseline risk of nephrotoxicity, independent of vancomycin exposure. While this suggests that vancomycin may not be responsible for the observed toxicity rates, a high vancomycin trough, >15 mg/liter, was still independently associated with a higher odds of nephrotoxicity in most studies that accounted for these variables in a multivariate analysis. Together, these findings imply that high vancomycin troughs augment the risk of nephrotoxicity, especially in the presence of conditions known to be independently associated with nephrotoxicity. Conversely, these medical factors likely affect the vancomycin trough concentration threshold at which the risk of nephrotoxicity is likely to increase.
The multifactorial nature of vancomycin-induced nephrotoxicity is best highlighted by the findings of two recent randomized clinical trials (50, 53). In the ceftaroline fosamil versus vancomycin/aztreonam for complicated skin and skin structure infections (cSSSI) clinical trial, the incidence of nephrotoxicity in the vancomycin arm was 1.3%, only 0.9% greater than in the ceftaroline arm (53). Of note, patients in this study rarely resided in the ICU, generally had troughs of <10 mg/liter, and typically received therapy for <10 days. In comparison, the incidence of nephrotoxicity was 9.6% greater with vancomycin than with linezolid in the recent phase IV vancomycin versus linezolid for nosocomial MRSA pneumonia clinical trial. In this study, patients were generally in poorer health (the mean Acute Physiology and Chronic Health Evaluation [APACHE II] score was 17.6), with 64% of patients requiring ventilation (50).
While the incidence of nephrotoxicity is concerning, it appears to be largely reversible in the majority of cases following vancomycin discontinuation (36, 38, 43, 51). Short-term dialysis was required in only approximately 3% of cases, and no patient needed long-term dialysis. All patients who required dialysis also received concomitant nephrotoxins (43). This finding supports the notion that certain clinical factors augment the severity of vancomycin-induced renal impairment. Although vancomycin-induced nephrotoxicity was generally reversible, it should be noted that the nephrotoxic events were associated with increased lengths of stay and poorer outcomes (39, 54).
Several issues should be considered when interpreting these results. First, it is a challenge to completely establish that exposure-nephrotoxicity relationships exist for drugs that are renally eliminated. Since vancomycin is eliminated predominantly by glomerular filtration, a decrease in renal function from any cause will increase vancomycin serum concentrations (13, 15). Cognizant of this, we performed an analysis limited to studies that examined only initial troughs. The odds of nephrotoxicity remained increased, at 3.12 (95% CI, 1.81 to 5.37; P < 0.01) for patients attaining initial trough levels of ≥15 mg/liter (8, 13, 21, 22, 30, 36, 43). The presence of a vancomycin trough-nephrotoxicity relationship is further substantiated when one considers that most nephrotoxic events occurred after 7 days of therapy (13, 15, 39, 43). This exposure-toxicity relationship is biologically plausible and supported by recent animal and human data that suggest vancomycin acts as an oxidative stressor in proximal renal tubular cells (55–61).
Second, the results of the analysis that categorized vancomycin into more precisely defined trough strata (<10, 10 to 15, 15 to 20, and >20 mg/liter) suggest that vancomycin-induced nephrotoxicity is similar among patients with troughs between 10 and 20 mg/liter and greatest among patients with troughs in excess of 20 mg/liter. Due to the small demarcation in trough values between 10 to 15 and 15 to 20 mg/liter, there was a high potential for vancomycin stratum misclassification error, especially as these data are derived from retrospective cohort studies. While it is possible that vancomycin trough values of >20 mg/liter may be driving the nephrotoxicity in the >15-mg/liter strata, caution should be exercised before drawing definitive conclusions from these data (8, 22, 36, 50). Until more data are available to adequately define the vancomycin exposure-toxicity curve, clinicians should rely on the collective results of the 15 studies included in this meta-analysis, which suggest that an augmented risk of toxicity occurs in individuals with troughs of >15 mg/liter (8, 13–15, 21–23, 30–32, 36, 38, 39, 43, 48, 50, 51).
Third, it was difficult to fully quantify the role of concomitant nephrotoxins in the observed results. It is quite possible that the role of concomitant nephrotoxins may have been underestimated. The number of concomitant nephrotoxins listed in each study varied, and some studies provided only examples of nephrotoxic agents rather than an exhaustive list (8, 36). No data on the relationship between the number of concurrent nephrotoxins and observed nephrotoxicity results were provided. Although definitions of concomitant use were similar across all studies except one (31), the effects of duration and timing of concomitant nephrotoxins on vancomycin-associated nephrotoxicity were not quantifiable.
Fourth, we could not exclude the presence of publication bias; positive studies are more likely to be published than negative ones. In addition, a targeted analysis taking into account all confounders was not possible; this limited the ability of this meta-analysis to definitively establish the presence of a causal relationship. As most studies included were retrospective observational cohorts, treatment selection bias and management decisions with respect to dosage adjustments could not be analyzed.
In conclusion, the findings from this systematic literature review strongly suggest that a relationship exists between the vancomycin trough value and nephrotoxicity. Patients with vancomycin troughs in excess of 15 mg/liter were found to have a greater risk of nephrotoxicity than did patients with troughs of <15 mg/liter. The incidence of toxicity increased as a function of therapy duration, with the highest rates observed among critically ill individuals that resided in the ICU and received concomitant nephrotoxins. These observed results have important clinical implications and suggest that initial trough concentrations can serve as a prognostic indicator for nephrotoxicity and to identify patients that require careful monitoring. Based on collective findings, clinicians should intently monitor the renal function of patients receiving vancomycin, especially those patients being maintained at a trough value in excess of 15 mg/liter. Unfortunately, data on practical management decisions with respect to how and when to adjust the dose or cease vancomycin therapy remain lacking, and these issues require urgent study.
ACKNOWLEDGMENTS
S.J.V.H. has received grant support from Novartis, Pfizer, Merck, and Gilead, and T.P.L. is a consultant for Pfizer, Cubist Pharmaceuticals, Astellas, and Forest. T.P.L. is a speaker for Pfizer, Forest, and Cubist Pharmaceuticals. T.P.L. has received grant support from Astellas, Cubist, and Pfizer. D.L.P. is a consultant for Leo Pharmaceuticals, Novartis, Johnson & Johnson, Merck, and AstraZenica.
Footnotes
Published ahead of print 19 November 2012
REFERENCES
- 1. Levine DP. 2006. Vancomycin: a history. Clin. Infect. Dis. 42(Suppl 1):S5–S12 [DOI] [PubMed] [Google Scholar]
- 2. Popovich KJ, Weinstein RA, Hota B. 2008. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin. Infect. Dis. 46:787–794 [DOI] [PubMed] [Google Scholar]
- 3. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984 [DOI] [PubMed] [Google Scholar]
- 4. Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 5. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan M JRDA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 6. van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin. Infect. Dis. 54:755–771 [DOI] [PubMed] [Google Scholar]
- 7. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925–942 [DOI] [PubMed] [Google Scholar]
- 8. Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52:975–981 [DOI] [PubMed] [Google Scholar]
- 9. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, JR, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98 [DOI] [PubMed] [Google Scholar]
- 10. Kullar R, Davis SL, Taylor TN, Kaye KS, Rybak MJ. 2012. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy 32:195–201 [DOI] [PubMed] [Google Scholar]
- 11. Moellering RC., Jr 2006. Vancomycin: a 50-year reassessment. Clin. Infect. Dis. 42(Suppl 1):S3–S4 [DOI] [PubMed] [Google Scholar]
- 12. Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl 1):S35–S39 [DOI] [PubMed] [Google Scholar]
- 13. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138–2144 [DOI] [PubMed] [Google Scholar]
- 14. Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J. Antimicrob. Chemother. 62:168–171 [DOI] [PubMed] [Google Scholar]
- 15. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. 2007. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin. Ther. 29:1107–1115 [DOI] [PubMed] [Google Scholar]
- 16. Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta A, Biyani M, Khaira A. 2011. Vancomycin nephrotoxicity: myths and facts. Neth. J. Med. 69:379–383 [PubMed] [Google Scholar]
- 18. Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. 2010. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am. J. Med. 123:182e1–182e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong-Beringer A, Joo J, Tse E, Beringer P. 2011. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int. J. Antimicrob. Agents 37:95–101 [DOI] [PubMed] [Google Scholar]
- 20. Aston JL, Dortch MJ, Dossett LA, Creech CB, May AK. 2010. Risk factors for treatment failure in patients receiving vancomycin for hospital-acquired methicillin-resistant Staphylococcus aureus pneumonia. Surg. Infect. (Larchmt.) 11:21–28 [DOI] [PubMed] [Google Scholar]
- 21. Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. 2011. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob. Agents Chemother. 55:5475–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, Kett DH. 2012. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP database. Clin. Ther. 34:149–157 [DOI] [PubMed] [Google Scholar]
- 23. Chung J, Oh JM, Cho EM, Jang HJ, Hong SB, Lim CM, Koh YS. 2011. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth. Intensive Care 39:1030–1037 [DOI] [PubMed] [Google Scholar]
- 24. Cohen E, Dadashev A, Drucker M, Samra Z, Rubinstein E, Garty M. 2002. Once-daily versus twice-daily intravenous administration of vancomycin for infections in hospitalized patients. J. Antimicrob. Chemother. 49:155–160 [DOI] [PubMed] [Google Scholar]
- 25. Colares VS, Oliveira RB, Abdulkader RC. 2006. Nephrotoxicity of vancomycin in patients with normal serum creatinine. Nephrol. Dial. Transplant. 21:3608 doi:10.1093/ndt/gfl426 [DOI] [PubMed] [Google Scholar]
- 26. Elting LS, Rubenstein EB, Kurtin D, Rolston KV, Fangtang J, Martin CG, Raad II, Whimbey EE, Manzullo E, Bodey GP. 1998. Mississippi mud in the 1990s: risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer 83:2597–2607 [DOI] [PubMed] [Google Scholar]
- 27. Fisher BT, Zaoutis TE, Leckerman KH, Localio R, Aplenc R. 2010. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: a retrospective cohort study. Pediatr. Blood Cancer 55:655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frymoyer A, Guglielmo BJ, Wilson SD, Scarpace SB, Benet LZ, Hersh AL. 2011. Impact of a hospitalwide increase in empiric pediatric vancomycin dosing on initial trough concentrations. Pharmacotherapy 31:871–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahn-Ast C, Glasmacher A, Arns A, Muhling A, Orlopp K, Marklein G, Von Lilienfeld-Toal M. 2008. An audit of efficacy and toxicity of teicoplanin versus vancomycin in febrile neutropenia: is the different toxicity profile clinically relevant? Infection 36:54–58 [DOI] [PubMed] [Google Scholar]
- 30. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. 2010. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin. Drug Saf. 9:9–14 [DOI] [PubMed] [Google Scholar]
- 31. Kralovicova K, Spanik S, Halko J, Netriova J, Studena-Mrazova M, Novotny J, Grausova S, Koren P, Krupova I, Demitrovicova A, Kukuckova E, Krcmery V., Jr 1997. Do vancomycin serum levels predict failures of vancomycin therapy or nephrotoxicity in cancer patients? J. Chemother. 9:420–426 [DOI] [PubMed] [Google Scholar]
- 32. Kullar R, Leonard SN, Davis SL, Delgado G, Jr, Pogue JM, Wahby KA, Falcione B, Rybak MJ. 2011. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy 31:441–448 [DOI] [PubMed] [Google Scholar]
- 33. Lahoti A, Kantarjian H, Salahudeen AK, Ravandi F, Cortes JE, Faderl S, O'Brien S, Wierda W, Mattiuzzi GN. 2010. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 116:4063–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemaire X, Loiez C, Valette M, Migaud H, Dubreuil L, Yazdanpanah Y, Senneville E. 2011. Comparison of vancomycin and teicoplanin trough serum levels in patients with infected orthopedic devices: new data for old therapies. J. Infect. Chemother. 17:370–374 [DOI] [PubMed] [Google Scholar]
- 35. Li J, Udy AA, Kirkpatrick CM, Lipman J, Roberts JA. 2012. Improving vancomycin prescription in critical illness through a drug use evaluation process: a weight-based dosing intervention study. Int. J. Antimicrob. Agents 39:69–72 [DOI] [PubMed] [Google Scholar]
- 36. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49:507–514 [DOI] [PubMed] [Google Scholar]
- 37. Marinho DS, Huf G, Ferreira BL, Castro H, Rodrigues CR, Sousa VP, Cabral LM. 2011. The study of vancomycin use and its adverse reactions associated to patients of a brazilian university hospital. BMC Res. Notes 4:236 doi:10.1186/1756-0500-4-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. 2011. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J. Pediatr. 158:422–426 [DOI] [PubMed] [Google Scholar]
- 39. Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. 2011. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob. Agents Chemother. 55:3278–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moffett BS, Kim S, Edwards M. 2011. Vancomycin nephrotoxicity may be overstated. J. Pediatr. 158:865–866 (Letter.) [DOI] [PubMed] [Google Scholar]
- 41. Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. 2012. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin. Infect. Dis. 54:51–58 [DOI] [PubMed] [Google Scholar]
- 42. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. 2011. Vancomycin: we can't get there from here. Clin. Infect. Dis. 52:969–974 [DOI] [PubMed] [Google Scholar]
- 43. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. 2012. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J. Hosp. Med. 7:91–97 [DOI] [PubMed] [Google Scholar]
- 44. Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. 2010. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am. J. Med. 123:1143–1149 [DOI] [PubMed] [Google Scholar]
- 45. Roberts JA, Taccone FS, Udy AA, Vincent JL, Jacobs F, Lipman J. 2011. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 55:2704–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME. 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin. Infect. Dis. 52:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen WC, Chiang YC, Chen HY, Chen TH, Yu FL, Tang CH, Sue YM. 2011. Nephrotoxicity of vancomycin in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Nephrology (Carlton) 16:697–703 [DOI] [PubMed] [Google Scholar]
- 48. Spapen HD, Janssen van Doorn K, Diltoer M, Verbrugghe W, Jacobs R, Dobbeleir N, Honore PM, Jorens PG. 2011. Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann. Intensive Care 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. 2011. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 66:2386–2392 [DOI] [PubMed] [Google Scholar]
- 50. Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin. Infect. Dis. 54:621–629 [DOI] [PubMed] [Google Scholar]
- 51. Zimmermann AE, Katona BG, Plaisance KI. 1995. Association of vancomycin serum concentrations with outcomes in patients with gram-positive bacteremia. Pharmacotherapy 15:85–91 [PubMed] [Google Scholar]
- 52. Hutschala D, Kinstner C, Skhirdladze K, Thalhammer F, Muller M, Tschernko E. 2009. Influence of vancomycin on renal function in critically ill patients after cardiac surgery: continuous versus intermittent infusion. Anesthesiology 111:356–365 [DOI] [PubMed] [Google Scholar]
- 53. Corrado ML. 2010. Integrated safety summary of CANVAS 1 and 2 trials: phase III, randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl 4):iv67–iv71 [DOI] [PubMed] [Google Scholar]
- 54. Cruz DN, Ricci Z, Ronco C. 2009. Clinical review: RIFLE and AKIN—time for reappraisal. Crit. Care 13:211 doi:10.1186/cc7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. King DW, Smith MA. 2004. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol. In Vitro 18:797–803 [DOI] [PubMed] [Google Scholar]
- 56. Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH. 2005. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin. Pharmacol. Toxicol. 97:325–332 [DOI] [PubMed] [Google Scholar]
- 57. Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233 [DOI] [PubMed] [Google Scholar]
- 58. Cetin H, Olgar S, Oktem F, Ciris M, Uz E, Aslan C, Ozguner F. 2007. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin. Exp. Pharmacol. Physiol. 34:1181–1185 [DOI] [PubMed] [Google Scholar]
- 59. Hodoshima N, Nakano Y, Izumi M, Mitomi N, Nakamura Y, Aoki M, Gyobu A, Shibasaki S, Kurosawa T. 2004. Protective effect of inactive ingredients against nephrotoxicity of vancomycin hydrochloride in rats. Drug Metab. Pharmacokinet. 19:68–75 [DOI] [PubMed] [Google Scholar]
- 60. Toyoguchi T, Takahashi S, Hosoya J, Nakagawa Y, Watanabe H. 1997. Nephrotoxicity of vancomycin and drug interaction study with cilastatin in rabbits. Antimicrob. Agents Chemother. 41:1985–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Le Moyec L, Racine S, Le Toumelin P, Adnet F, Larue V, Cohen Y, Leroux Y, Cupa M, Hantz E. 2002. Aminoglycoside and glycopeptide renal toxicity in intensive care patients studied by proton magnetic resonance spectroscopy of urine. Crit. Care Med. 30:1242–1245 [DOI] [PubMed] [Google Scholar]
- 62. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 8:R204–R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11:R31 doi:10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]