Abstract
The need for novel antibacterial strategies and the awareness of the importance of quorum sensing (QS) in bacterial infections have stimulated research aimed at identifying QS inhibitors (QSIs). However, clinical application of QSIs identified so far is still distant, likely due to their unsuitability for use in humans. A promising way to overcome this problem is searching for anti-QS side activity among the thousands of drugs approved for clinical use in the treatment of different diseases. Here, we applied this strategy to the search for QSIs, by screening a library of FDA-approved compounds for their ability to inhibit the QS response in the Gram-negative pathogen Pseudomonas aeruginosa. We found that the anthelmintic drug niclosamide strongly inhibits the P. aeruginosa QS response and production of acyl-homoserine lactone QS signal molecules. Microarray analysis showed that niclosamide affects the transcription of about 250 genes, with a high degree of target specificity toward the QS-dependent regulon. Phenotypic assays demonstrated that niclosamide suppresses surface motility and production of the secreted virulence factors elastase, pyocyanin, and rhamnolipids, and it reduces biofilm formation. In accordance with the strong antivirulence activity disclosed in vitro, niclosamide prevented P. aeruginosa pathogenicity in an insect model of acute infection. Besides the finding that an FDA-approved drug has a promising antivirulence activity against one of the most antibiotic-resistant bacterial pathogens, this work provides a proof of concept that a lateral anti-QS activity can be detected among drugs already used in humans, validating a new approach to identify QSIs that could easily move into clinical applications.
INTRODUCTION
The introduction of antibiotics into clinical practice at the middle of the 20th century was a milestone in the history of medicine. However, the original expectation that all bacterial infections could be defeated one day by antibiotics was soon diminished by the emergence of antibiotic-resistant strains, prompting the still-ongoing race for the discovery of new antibacterial agents. While the treatment of infections sustained by antibiotic-resistant bacteria has high socio-economic costs and represents a major health problem worldwide, the pharmaceutical industry has dramatically reduced investments in antibiotics research. As traditional antibiotic research appears to be helpless in coping with the emergence of antibiotic-resistant strains, novel scientifically sound, and cost-effective approaches should be undertaken in order to identify new drugs (1).
Selective optimization of side activities of drug molecules (the SOSA approach) is a smart strategy for the identification of new potential drugs (2). A limited number of highly diverse drugs whose use in humans has already been approved are screened for side activities against unrelated diseases. Once a hit compound has been found, it can be either tested directly in clinical studies or used as the lead for drug optimization programs. This strategy has a high probability of yielding safe and bioavailable drug-like compounds, and it is thus expected to reduce the time and cost generally associated with standard drug discovery processes (2–4).
An innovative strategy to combat bacterial infections relies on specific inhibition of bacterial virulence, hence the ability to cause disease rather than bacterial growth (5). The use of “antivirulence drugs” could have the advantage of reducing bacterial adaptability to the host environment, facilitating the host immune system to combat the infection and reducing the strong selective pressure exerted by conventional antibiotics (6), although this is not yet supported by direct clinical evidence.
In many bacteria, pathogenicity is controlled and coordinated by an intercellular communication process named quorum sensing (QS). QS is based on the synthesis and secretion of a signal molecule that binds to a cognate receptor. The signal-activated receptor controls the expression of target genes. Since the production of the signal molecule is proportional to bacterial growth, QS coordinates gene expression in response to a bacterial population density (7). So far, QS is considered one of the most promising targets for antivirulence therapies (6, 8, 9).
In this study, the SOSA approach has been applied to the identification of antivirulence drugs targeting bacterial QS, using Pseudomonas aeruginosa as the model organism. P. aeruginosa is one of the most dreaded Gram-negative pathogens in developed countries, being responsible for both community- and hospital-acquired infections. In addition, P. aeruginosa chronic lung infection is the major cause of death in cystic fibrosis (CF) patients, a genetic disease affecting about 1/3,000 newborns in the Caucasian population (10–13). Besides being intrinsically resistant to several antibiotics, P. aeruginosa can easily acquire new resistance determinants, and indeed the emergence of pan-resistant strains has already been documented (14). For these reasons, P. aeruginosa infections are generally characterized by high morbidity and mortality rates (13, 15).
The pathogenic potential of P. aeruginosa relies on the coordinated expression of a large array of virulence factors (16), the majority of which are positively controlled by QS (17). The P. aeruginosa QS network consists of three different QS systems, based on the production of specific signal molecules: N-3-oxododecanoyl-homoserine lactone (3OC12-HSL), N-butanoyl-homoserine lactone (C4-HSL), and 2-heptyl-3-hydroxy-4-quinolone (PQS). P. aeruginosa QS is hierarchically organized, since 3OC12-HSL is required for optimal production of the other QS signals (17).
QS controls the expression of nearly 10% of the P. aeruginosa genome, including genes for biofilm formation, secreted virulence factors, and immune-modulatory and proinflammatory agents (17). QS signal molecules can be detected in clinical samples, proof that QS is active during P. aeruginosa infections. Moreover, QS-defective mutants show strongly impaired virulence in several animal models of infection, corroborating the importance of QS for P. aeruginosa pathogenicity and its suitability as a target for the development of anti-Pseudomonas drugs (18, 19).
We recently developed a convenient system for the identification of compounds affecting the P. aeruginosa 3OC12-HSL-based QS system at multiple levels: (i) expression/activity of the signal receptor, (ii) expression/activity of the signal synthase, and (iii) activity/availability of the signal molecule (20). Here, the screening of a library of about 1,000 compounds with known pharmacological activities has validated this system.
Seven hit compounds were identified. Among these, we focused our investigation on the anthelmintic drug niclosamide, which showed high inhibitory activity against P. aeruginosa QS and virulence both in vitro and in vivo. To the best of our knowledge, this is the first demonstration that the SOSA approach can be successfully applied to the search for anti-QS drugs.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemicals.
Wild-type P. aeruginosa PA14 (21), the PA14 lasI mutant (22), and the 3OC12-HSL reporter strain PA14-R3 (20) were routinely grown in Luria-Bertani (LB) broth (23) supplemented with 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS; pH 7.0), unless otherwise stated. AB minimal medium supplemented with 0.02% (wt/vol) glucose as carbon source was used in the biofilm assay (24). Synthetic acyl-HSLs were purchased from the University of Nottingham, United Kingdom. Stock solutions (25 mM) were prepared in ethyl acetate acidified with 0.1% (vol/vol) acetic acid. Niclosamide was purchased from Sigma-Aldrich and resuspended in dimethyl sulfoxide (DMSO) at a 10 mM final concentration. Niclosamide ethanolamine salt was purchased from Chemos and resuspended in DMSO at 1 M and then diluted in water to a 10 mM final concentration.
QSI screening assay.
P. aeruginosa PA14 and the 3OC12-HSL reporter strain PA14-R3 were grown overnight at 37°C on LB agar plates. Bacteria were scraped from plate surfaces and diluted in LB to an absorbance at 600 nm (A600) of 0.045 and 0.015 for PA14-R3 and PA14, respectively (3/1 reporter/wild type ratio) (20). Aliquots of 200 μl of the coculture were grown at 37°C in 96-well microtiter plates in the presence of 10-fold dilutions of each compound from the Prestwick Chemical Library (final concentrations of 100, 10, and 1 μg/ml). The A600 and light counts per second (LCPS) were measured at 4 h of growth in a Wallac 1420 Victor3V multilabel plate reader (PerkinElmer). Six wells containing the same coculture grown in the absence of any added compound were used as controls in each microtiter plate.
Quantification of QS signal molecules.
Levels of QS signal molecules in P. aeruginosa culture supernatants were determined at different times during bacterial growth by using the previously described reporter strains specific for 3OC12-HSL (20), C4-HSL (25), and PQS (26), according to a recently developed protocol (20). Briefly, 10 μl of culture supernatant (or appropriate dilutions) was added to 190 μl of LB inoculated with each reporter strain (final A600, 0.045) in 96-well microtiter plates. Microtiter plates were incubated at 37°C with gentle shaking, and the A600 and LCPS were measured after 4 h of growth. Dedicated calibration curves were generated by growing each reporter strain in the presence of increasing concentrations of the corresponding synthetic signal molecule, and these curves were used to calculate the concentration of the different QS signal molecules in each culture supernatant.
Transcriptome analysis.
P. aeruginosa PA14 was inoculated at an A600 of 0.01 into 20 ml of LB supplemented with 50 mM MOPS (pH 7.0), with or without 20 μM niclosamide. The cultures were grown at 37°C with vigorous shaking until they reached an A600 of 2.5, and then 1 ml of cells was harvested by centrifugation and resuspended in 2 ml of RNAProtect bacteria reagent (Qiagen). Transcriptome analysis was performed by using high-density oligonucleotide microarrays as previously described (27), with minor modifications. Briefly, total RNA was purified by using RNeasy minicolumns (Qiagen), including the on-column DNase I digestion described by the manufacturer. In addition, the eluted RNA samples were incubated for 1 h at 37°C with Turbo DNase (0.2 U per μg of RNA; Ambion) and SUPERase-In (0.4 U per μg of RNA; Ambion). DNase I was removed by using the RNeasy MinElute cleanup kit (Qiagen) according to the manufacturer's instructions. RNA integrity was monitored by agarose gel electrophoresis of glyoxylated samples and use of an RNA 6000 Nano LabChip in an Agilent 2100 bioanalyzer (Agilent Technologies); the RNA integrity number (RIN) was ≥9.7. Next, 10 μg of total RNA was used with random primers and Superscript II reverse transcriptase (Life Technologies) to perform cDNA synthesis. cDNA fragmentation, labeling, hybridization, staining, and washing steps were performed according to the manufacturer's protocol for the Affymetrix P. aeruginosa GeneChip arrays. Finally, the arrays were scanned with the Affymetrix GeneChip scanner 3000. Processing of the P. aeruginosa GeneChip (Affymetrix) was performed at the Genopolis Consortium for Functional Genomics (University of Milan-Bicocca, Milan, Italy). Under each condition, cultures were grown in triplicate, and RNAs from these cultures were pooled before proceeding to cDNA synthesis. In addition, biological replicates for each condition were performed on a separate day and run on a different microarray chip. The P value threshold was <0.05, and the cutoff for fold changes in gene expression was >2.
Assays for production of secreted virulence factors.
Pyocyanin was extracted with 3 ml of chloroform from 5-ml cell-free supernatants of P. aeruginosa cultures grown at 37°C for 10 h in LB supplemented with different concentrations of niclosamide and then reextracted into 1 ml of 0.2 N HCl. The A520 of the resulting solution was measured to determine the amount of extracted pyocyanin (28). Elastase activity was determined in 100 μl of the same cell-free supernatants by the elastin-Congo red method as described previously (29).
Rhamnolipids in cell-free supernatants of P. aeruginosa cultures grown for 24 h in LB at 37°C were determined by the orcinol method as described previously (30), using defined concentrations of rhamnose as a standard. The rhamnolipid concentration was calculated based on the assumption that 1 μg of rhamnose corresponds to 2.5 μg of rhamnolipids (30).
Bacterial motility and biofilm assays.
Swimming, swarming, and twitching motilities were assessed as described previously (31). PA14 cultures grown in LB for 14 h were either directly transferred to swimming (0.1% [wt/vol] tryptone, 0.05% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl, 0.3% [wt/vol] bacteriological agar) and twitching (1.0% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl, 1.0% [wt/vol] bacteriological agar) plates by using a sterile toothpick or diluted in fresh LB medium to an A600 of 0.1 and then spotted (2 μl) onto swarming plates (0.8% [wt/vol] nutrient broth N.2, 0.5% [wt/vol] glucose, 0.5% [wt/vol] bacteriological agar). Plates were supplemented or not with increasing concentrations of niclosamide. After 16 h of growth at 37°C, swimming and swarming motilities were directly observed at the air-agar interface, while twitching motility was measured at the agar-plastic interface after removal of the agar layer and staining with crystal violet (31).
Biofilm formation was assessed using the microtiter plate biofilm assay (32). Bacterial cells were grown in LB for 14 h, washed twice with AB medium, and resuspended in AB medium supplemented with 0.02% (wt/vol) glucose at an A600 of 0.1 in the presence or absence of increasing niclosamide concentrations. Aliquots of 100 μl were transferred to a sterile 96-well polystyrene microtiter plate (3 wells per sample) and incubated at 30°C for 24 h. Planktonic cells (80 μl) were transferred to a sterile microtiter plate for A600 measurements in a Wallac 1420 Victor3V multilabel plate reader, while the attached cells were gently washed three times with sterile phosphate-buffered saline, air dried, and stained with 1% (wt/vol) crystal violet. After washing the wells four times with distilled water, the surface-associated dye was solubilized with 200 μl of ethanol. The A600 of the dye solutions was measured in a Wallac 1420 Victor3V multilabel plate reader.
Galleria mellonella killing assay.
The G. mellonella killing assay was performed as previously described (33), with minor modifications. Briefly, G. mellonella caterpillars in the final instar larval stage (average weight, 490 ± 90 mg) were infected with a lethal inoculum of P. aeruginosa (adjusted to about 10 bacterial cells in 10 μl of saline) containing or not niclosamide ethanolamine salt at 750 μM. Although P. aeruginosa cells were incubated in the presence of niclosamide for less than 5 min before injection, preliminary assays showed that 750 μM niclosamide treatment in vitro (for up to 90 min) does not significantly affect P. aeruginosa PA14 cell viability (data not shown). G. mellonella larvae were incubated at 28°C in petri dishes (five larvae per dish) and monitored for a week. Larvae were considered dead when they did not respond to gentle prodding. At least 30 larvae were inoculated per condition in three independent experiments. To rule out any growth-inhibitory effect of niclosamide due to conversion into a toxic compound(s) in the larval hemolymph, additional larvae were inoculated with 10 μl of 750 μM niclosamide or saline as a control. Five-microliter aliquots of the larval hemolymph recovered 2, 4, or 6 h after treatment were spotted on Pseudomonas isolation agar plates previously inoculated with P. aeruginosa PA14 cells to produce a lawn of confluent growth. The appearance of growth inhibition halos was checked after 14 h of incubation at 37°C. In this experiment, no inhibition halos were detected.
Statistical analysis.
Statistical analysis was performed with the software GraphPad Instat, using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests. Survival curves for the G. mellonella killing assay were generated by the Kaplan-Meier method and analyzed by the log-rank test. Differences having a P value of <0.05 were considered statistically significant.
RESULTS
Identification of FDA-approved compounds that inhibit P. aeruginosa QS.
We recently developed a novel screening system for the identification of P. aeruginosa QSI. This system is based on the cocultivation of a biosensor strain for 3OC12-HSL detection, PA14-R3, and a wild-type P. aeruginosa PA14 strain. The 3OC12-HSL signal synthesized by the wild-type PA14 induces bioluminescence emission by the biosensor (20). The addition of a molecule with inhibitory activity toward any process related to the 3OC12-HSL-dependent QS system, including 3OC12-HSL synthesis, transport, and perception, reduces the luminescence emitted by the biosensor with respect to a control coculture without any compound added (20).
The PA14/PA14-R3 cocultivation system was used to screen a commercial library of marketed drugs from Prestwick Chemicals (www.prestwickchemical.fr). This library contained 1,120 chemical compounds with known biological activities, selected for their high chemical and pharmacological diversities, as well as for known bioavailability and safety information for humans. Each drug was tested at three different concentrations (100, 10, and 1 μg/ml) in duplicate. Criteria used for the selection of hit compounds were (i) ≥50% inhibition of bioluminescence emission and (ii) ≤20% reduction of growth with respect to the untreated controls. The latter criterion was aimed at avoiding any unspecific effect of impaired growth on the QS response.
The screening assay allowed the identification of seven putative QSIs that reproducibly inhibited the QS response of the PA14/PA14-R3 cocultivation system, without affecting bacterial growth at the highest concentration tested. The seven hits were further tested in triplicate at 100, 80, 60, 40, 20, 10, 5, and 2.5 μg/ml final concentrations, showing a half-maximal inhibitory concentration (IC50) in the range of 3 to 77 μg/ml (corresponding to 10 to 150 μM) (Table 1). Four of the identified compounds were antibiotics, in agreement with the well-known negative effect of subinhibitory concentrations of antibiotics on the P. aeruginosa QS response (34, 35). The remaining three compounds corresponded to a quaternary ammonium salt, an anticancer drug, and a teniacide for the treatment of tapeworm infections (Table 1). Among nonantibiotic drugs, the teniacide niclosamide showed the highest anti-QS activity (lowest IC50) (Table 1) and was therefore selected for further investigations.
Table 1.
Hit compounds identified by screening the Prestwick Chemical Library with the PA14/PA14-R3 QSI screening system
| Prestwick code | Compound name | IC50 (μM)a | Property |
|---|---|---|---|
| 01D11 | Niclosamide | 10 | Anthelmintic |
| 02H11 | Gentamicin | 20 | Aminoglycoside antibiotic |
| 05G06 | Mitoxantrone dihydrochloride | 150 | Antineoplastic agent |
| 07E06 | Rifampin | 50 | Antibiotic of the rifamycin group |
| 07H08 | Dirithromycin | 50 | Macrolide glycopeptide antibiotic |
| 13C08 | Sanguinarine | 60 | Quaternary ammonium salt of the benzylisoquinoline alkaloids group |
| 14G10 | Rifabutin | 10 | Antibiotic of the rifamycin group |
The IC50 values were determined using the PA14/PA14-R3 coculture grown for 4 h at 37°C in the presence of 100, 80, 60, 40, 20, 10, 5 and 2.5 μg/ml of each compound.
Niclosamide inhibits the 3OC12-HSL-dependent QS system of P. aeruginosa.
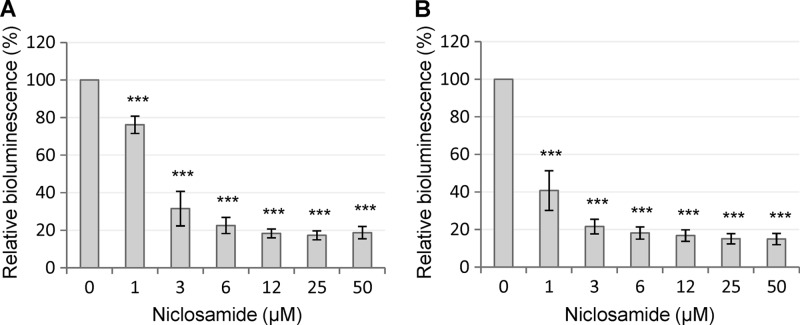
To verify the result of the screening assay, niclosamide was purchased from an alternative supplier (Sigma-Aldrich) and retested in the PA14/PA14-R3 cocultivation system. As expected, a strong inhibition of the 3OC12-HSL-dependent QS response was observed, with an IC50 even lower than that calculated for the compound from the Prestwick library (Fig. 1A). Notably, niclosamide was also able to inhibit luminescence emission by the PA14-R3 reporter strain grown in the presence of exogenously added synthetic 3OC12-HSL (3 μM final concentration) (Fig. 1B). This result suggests that the QS-inhibitory activity of niclosamide relies on its ability to hamper the response of P. aeruginosa to the signal molecule rather than to inhibit its synthesis. The possibility that the observed QS-inhibitory activity of niclosamide was due to unspecific inhibition of either bioluminescence-generating enzymes or bacterial transcription was ruled out by the observation that niclosamide had no effect on the bioluminescence emitted by a P. aeruginosa strain in which bioluminescence genes were under the control of the promoter region of the QS-independent cysB gene (data not shown), which is involved in cysteine metabolism and iron uptake (36).
Fig 1.
Effect of niclosamide on the 3OC12-HSL-dependent QS of P. aeruginosa. Response to increasing concentrations of niclosamide (0 to 50 μM) of the PA14/PA14-R3 cocultivation system (A) and the PA14-R3 biosensor (B) in the presence of 3 μM exogenously provided 3OC12-HSL. Bioluminescence emission was normalized to the cell density of the bacterial culture (relative bioluminescence, LCPS/A600) and expressed as a percentage relative to untreated controls. Values are the means (± standard deviations) of at least three independent experiments. ***, P < 0.001 (ANOVA).
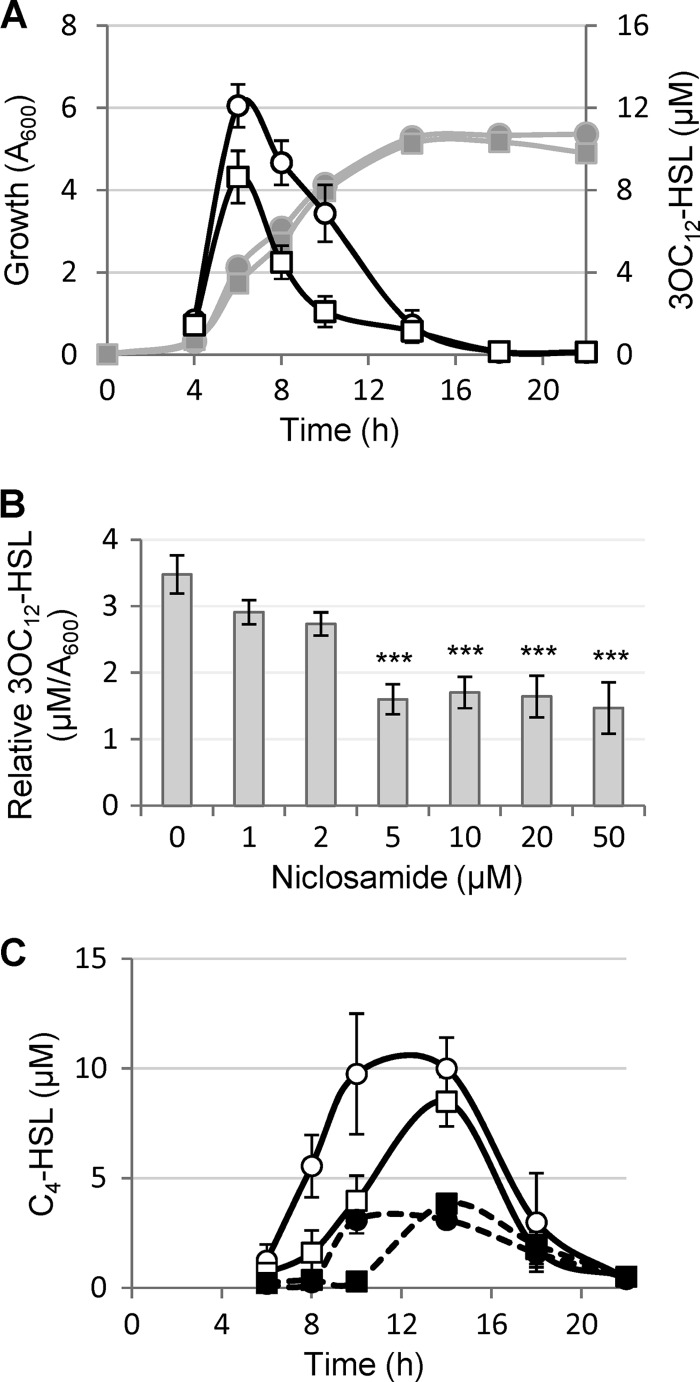
The effect of niclosamide on the production of 3OC12-HSL was then assessed. P. aeruginosa PA14 was grown in the absence or in the presence of 20 μM niclosamide, and 3OC12-HSL levels in culture supernatants were quantitatively determined during the whole growth curve. Niclosamide caused a significant reduction (30 to 60%) of 3OC12-HSL production from late exponential growth to the entry into the stationary phase, after which the 3OC12-HSL concentration fell to almost-undetectable levels in both niclosamide-treated and -untreated cultures (Fig. 2A). When 3OC12-HSL levels were determined in P. aeruginosa PA14 cultures grown in the presence of different niclosamide concentrations (0 to 50 μM), the maximum inhibitory effect on 3OC12-HSL production (about 60% reduction) was observed at 5 μM, and higher concentrations did not further reduce 3OC12-HSL production (Fig. 2B).
Fig 2.
Effect of niclosamide on acyl-HSL production. (A) Growth curve and 3OC12-HSL production by P. aeruginosa PA14 treated (squares) or untreated (circles) with 20 μM niclosamide. Symbols: bacterial growth (left vertical axis), gray lines and filled symbols; 3OC12-HSL levels in culture supernatants (right vertical axis), black lines and open symbols. (B) Relative 3OC12-HSL levels (in μM/A600) in culture supernatants of P. aeruginosa PA14 grown for 8 h in the presence of increasing concentrations of niclosamide (0 to 50 μM). (C) C4-HSL levels in culture supernatants of P. aeruginosa PA14 (solid lines, white symbols) and PA14 lasI mutant (dashed lines, black symbols) grown in the presence (squares) or in the absence (circles) of 20 μM niclosamide. Growth curves of bacterial cultures were comparable to those reported in panel A. Values are the means (± standard deviations) of at least three independent experiments. ***, P < 0.001 (ANOVA).
Since 3OC12-HSL influences the expression of other QS systems, the effects of niclosamide on the production of C4-HSL and PQS were tested. To this aim, C4-HSL and PQS levels in P. aeruginosa PA14 cultures treated or not with 20 μM niclosamide were determined along the growth curve. While niclosamide did not significantly affect PQS levels (data not shown), it considerably delayed the production of C4-HSL (Fig. 2C). By comparison with untreated cultures, C4-HSL levels were significantly lower in niclosamide-treated cultures during exponential growth, while levels were comparable between treated and untreated cultures in the stationary phase (Fig. 2C). This could be due, at least in part, to the positive effect exerted by 3OC12-HSL on C4-HSL production (17). However, the niclosamide-induced delay in C4-HSL production was also evident in a 3OC12-HSL-defective mutant strain inactivated for the lasI gene, encoding 3OC12-HSL synthase (Fig. 2C), indicating that niclosamide also affects C4-HSL production independently of its inhibitory activity on 3OC12-HSL production.
Niclosamide represses QS-activated genes.
To investigate the global effect of niclosamide on the P. aeruginosa transcriptome, the transcriptional profiles of PA14 grown to an A600 of 2.5 in the presence or in the absence of 20 μM niclosamide were compared by Affymetrix high-density oligonucleotides microarray analysis. Niclosamide affected the transcription of 258 genes, 73.2% of which were repressed by this drug, including genes involved in the production of important virulence factors, such as phospholipase C, LasA protease, pyocyanin, chitinase, rhamnolipids, and LasB elastase (Table 2; see also Table S1 in the supplemental material). Moreover, niclosamide repressed the transcription of genes involved in adhesion and biofilm formation, such as those coding for adhesins (PA0852-cbpD and PA2570-pa1L) and for cyclic di-GMP turnover or response proteins (PA1120-tpbB, PA2572, and PA4781). The transcription of the mexGHI-opmD genes, encoding an efflux pump required for full virulence in rat and plant infection models (37), was also strongly decreased in the presence of niclosamide (Table 2). Notably, among the 189 genes repressed by niclosamide, 96 have been identified as genes activated by 3OC12-HSL and/or C4-HSL in the main reference studies (38–40) (see Table S1), and an additional 25 genes have been suggested to be part of the QS network (27, 31, 41–44). Among the core components of the P. aeruginosa QS network (i.e., signal synthases and signal receptor genes), only the C4-HSL receptor gene rhlR was significantly repressed by niclosamide (Table 2). Similar results have been reported for other QSIs, such as furanone C-30 (38), iberin (45), and ajoene (46). In total, 121 out of the 189 genes repressed by niclosamide (64%) can be classified as QS regulated.
Table 2.
List of selected genes whose transcription is affected by niclosamidea
| Gene role and PA no. | Gene name | Fold changeb | Gene product |
|---|---|---|---|
| Virulence factors | |||
| PA0026 | plcB | −4.15 | Phospholipase C, PlcB |
| PA0070 | tagQ1 | 2.73 | Protein secretion apparatus, type VI secretion system |
| PA0085 | hcp1 | 2.99 | Type VI protein secretion system component Hcp |
| PA1871 | lasA | −10.05 | LasA protease precursor |
| PA1901 | phzC1, phzC2 | −2.22 | Phenazine biosynthesis protein PhzC |
| PA1905 | phzG2 | −2.19 | Probable pyridoxamine 5′-phosphate oxidase |
| PA2300 | chiC | −7.24 | Chitinase |
| PA3478 | rhlB | −5.99 | Rhamnosyltransferase chain B |
| PA3479 | rhlA | −5.65 | Rhamnosyltransferase chain A |
| PA3724 | lasB | −2.90 | Elastase LasB |
| PA4210 | phzA1, phzA2 | −3.06 | Probable phenazine biosynthesis protein |
| PA4211 | phzB1, phzB2 | −2.42 | Probable phenazine biosynthesis protein |
| Adhesion and biofilm formation | |||
| PA0852 | cbpD | −3.92 | Chitin-binding protein CbpD precursor |
| PA1120 | tpbB | −2.19 | Diguanylate cyclase |
| PA2570 | pa1L | −4.08 | PA-I galactophilic lectin |
| PA2572 | −3.17 | Probable two-component response regulator | |
| PA4781 | −2.50 | Cyclic di-GMP phosphodiesterase | |
| Gene regulation | |||
| PA2931 | cifR | 2.10 | Transcriptional regulator |
| PA3477 | rhlR | −2.56 | Transcriptional regulator RhlR |
| PA4296 | pprB | −3.21 | Two-component response regulator |
| Drug efflux | |||
| PA4205 | mexG | −29.17 | Hypothetical protein |
| PA4206 | mexH | −16.94 | Probable RND efflux membrane fusion protein precursor |
| PA4207 | mexI | −13.87 | Probable RND efflux transporter |
| PA4208 | opmD | −9.12 | Probable outer membrane protein precursor |
The PA number, gene name, and gene product are from the Pseudomonas Genome Database. Genes previously reported to be activated by the 3OC12-HSL and/or C4-HSL QS systems are shown in bold characters (38–40). RND, resistance-nodulation-cell division.
Fold change in gene expression of P. aeruginosa PA14 grown in LB supplemented with 20 μM niclosamide compared to the same strain grown in LB.
Niclosamide displayed a positive effect on the transcription of 69 genes, including two genes involved in type VI secretion pathways (PA0070-tagQ1 and PA0085-hcp1) (Table 2). These genes are the only virulence-related determinants whose transcription is induced by niclosamide. Only four of the niclosamide-activated genes were previously reported to be repressed by 3OC12-HSL and/or C4-HSL (38–40) (see Table S2 in the supplemental material), suggesting that the majority of the genes induced by niclosamide are affected via a QS-independent pathway(s). This observation, together with the finding that 36% of the niclosamide-repressed genes have never been reported to be QS controlled, suggests that this drug may have additional cellular targets besides the QS network. Notably, a total of 16 putative or confirmed transcriptional regulators were identified among the genes repressed or activated by niclosamide (see Tables S1 and S2). Besides rhlR, niclosamide decreased the transcription of pprB, which encodes a transcriptional activator associated with biofilm formation (47). Conversely, it positively affected the transcription of cifR (Table 2), which encodes the transcriptional repressor of the Cif toxin, responsible for apical membrane downregulation of the cystic fibrosis transmembrane conductance regulator (CFTR) in epithelial cells (48). The niclosamide-affected transcriptional factors may act as ancillary regulators, increasing the number of genes whose expression is altered by this drug beyond the QS regulon. However, a complete understanding of the niclosamide impact on P. aeruginosa physiology is partially hampered by the high percentage of niclosamide-controlled genes (∼41%) coding for proteins still classified as hypothetical (see Tables S1 and S2 in the supplemental material).
Niclosamide strongly reduces the virulence potential of P. aeruginosa in vitro.
In order to validate the transcriptomic data at the phenotypic level, we assessed the effect of niclosamide on the production of a set of QS-regulated virulence traits. In particular, we focused on (i) the LasB elastase, which is directly regulated by the 3OC12-HSL receptor LasR at the transcriptional level (49), (ii) pyocyanin and rhamnolipids, which are regulated by a number of different regulatory pathways and extracellular signals (50, 51), and (iii) multifactorial phenotypes, such as motility and biofilm, which are crucial for the establishment and persistence of P. aeruginosa infections (52–54).
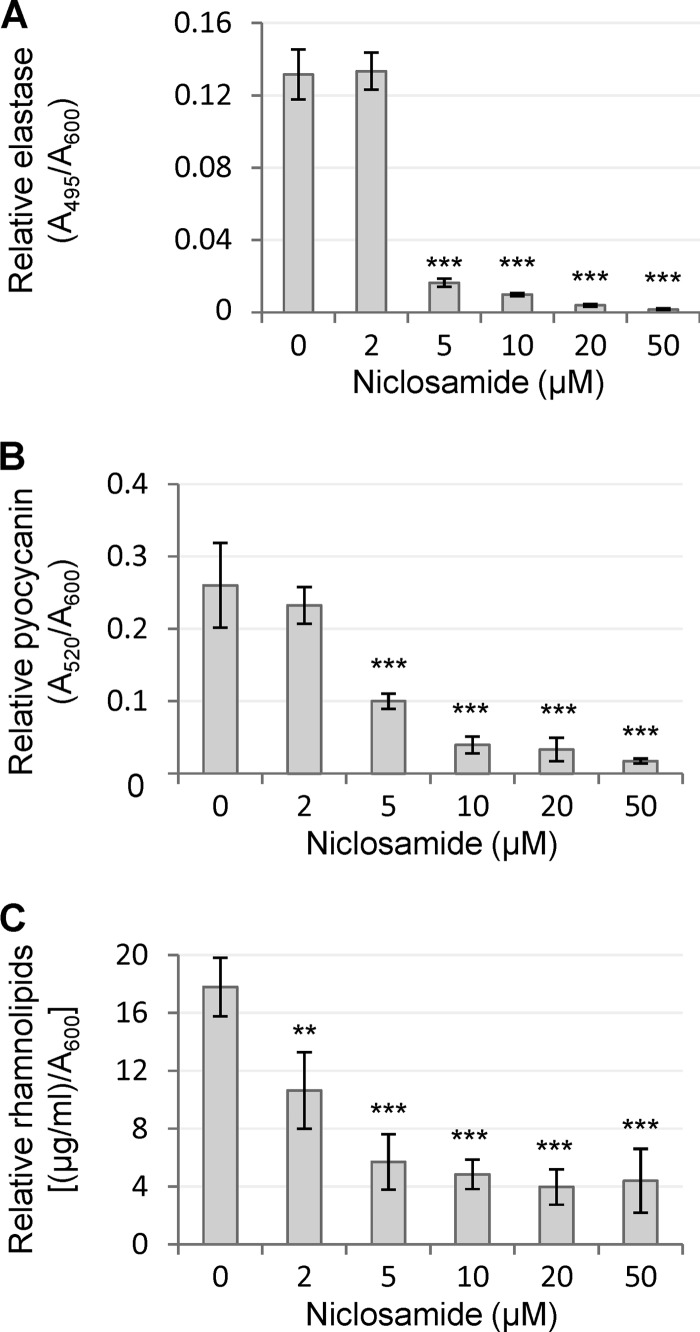
In accordance with microarray analysis, niclosamide had a marked inhibitory effect on the levels of QS-regulated secreted virulence factors of P. aeruginosa PA14 (Fig. 3). Production levels of both pyocyanin and elastase were dramatically reduced (85 to 90%) by 5 to 10 μM niclosamide. Likewise, the amount of rhamnolipids in supernatants of niclosamide-treated cultures was about 25% of the niclosamide-untreated control level (Fig. 3).
Fig 3.
Effects of niclosamide on the production of QS-regulated extracellular virulence factors. LasB elastase (A), pyocyanin (B), and rhamnolipids (C) levels in culture supernatants of P. aeruginosa PA14 grown for 10 h (A and B) or for 24 h (C) in the presence of increasing concentrations of niclosamide (0 to 50 μM). Values were normalized to the cell density of the bacterial culture (relative values) and are the means (± standard deviations) of four independent experiments. **, P < 0.01; ***, P < 0.001 (ANOVA).
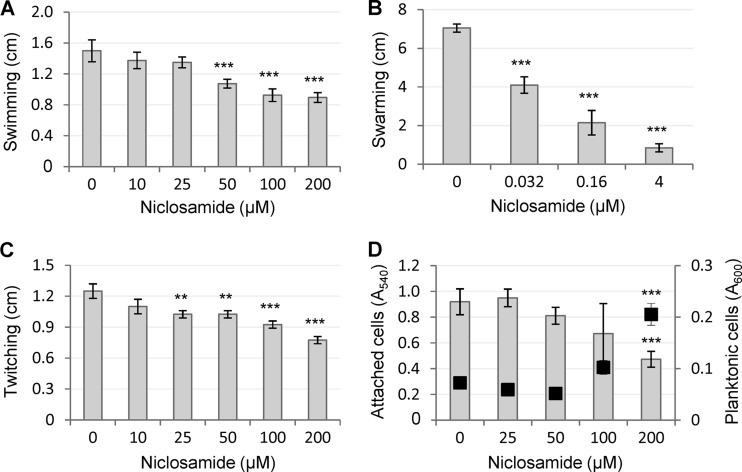
Regarding bacterial motility, niclosamide only slightly reduced swimming and twitching motilities of P. aeruginosa PA14 at high concentrations (≥50 to 100 μM), while it exerted a dramatic inhibitory effect on swarming motility (Fig. 4A to C). Swarming was completely prevented at 4 μM niclosamide, although a significant reduction was also observed at lower concentrations (Fig. 4B) which, however, had no effect on the 3OC12-HSL-dependent QS system (Fig. 1 and 2).
Fig 4.
Effects of niclosamide on P. aeruginosa motility and biofilm formation. (A to C) Swimming (A), swarming (B), and twitching (C) by P. aeruginosa PA14 in the presence of increasing concentrations of niclosamide. (D) Biofilm formation by P. aeruginosa PA14 in the presence of increasing concentrations of niclosamide, assessed as the amount of attached cells (gray histograms, left axis) versus planktonic cells (black squares, right axis). Values are the means (± standard deviations) of at least three independent experiments. **, P < 0.01; ***, P < 0.001 (ANOVA).
Niclosamide was also tested for its effect on P. aeruginosa biofilm formation in a standard crystal violet binding assay (32). Niclosamide showed a significant biofilm-inhibitory activity, resulting in a 2-fold reduction and 3-fold increase in the number of attached and planktonic cells, respectively (Fig. 4D). However, such inhibitory activity was only observed at ≥200 μM niclosamide, i.e., at concentrations that are exceedingly higher than those active against the QS response and virulence factor production (Fig. 1 to 3), suggesting that the effect of niclosamide on biofilm formation is independent of its anti-QS activity.
Niclosamide protects G. mellonella from P. aeruginosa infection.
In order to explore the suitability of niclosamide as an antivirulence drug against P. aeruginosa infections, we assessed the ability of this compound to inhibit the pathogenicity of P. aeruginosa in the G. mellonella insect model of infection (33).
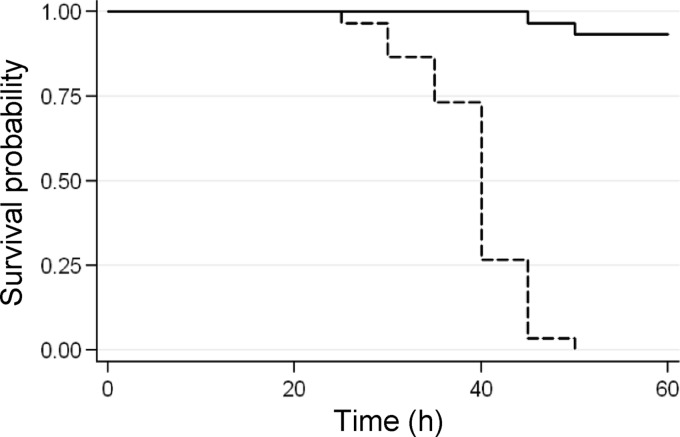
Larvae of the wax moth G. mellonella are extremely sensitive to P. aeruginosa injected into the hemolymph, and the PA14 strain was found to be highly virulent in this model, with a 50% lethal dose of about one bacterial cell (33). In our study, G. mellonella larvae were inoculated with 10 μl of saline solution containing a lethal dose of P. aeruginosa PA14 (10 ± 4 cells from exponential cultures) and containing or not 750 μM niclosamide ethanolamine salt and then incubated at 28°C for up to 1 week. Niclosamide ethanolamine salt was used because of its higher solubility in aqueous solutions compared with niclosamide (55). Notably, the two niclosamide formulations displayed comparable inhibitory effects on virulence factor production, motility, and QS signal molecule production (data not shown). Considering that the average weight of the G. mellonella larvae was about 500 mg (see Materials and Methods), and arbitrarily assuming 500 μl as the hemolymph volume of a larva, the final concentration of niclosamide in each larva was estimated to be approximately 15 μM. We showed above that such niclosamide concentration inhibits 3OC12-HSL production and expression of 3OC12-HSL-dependent virulence factors (Fig. 2 and 3), without affecting bacterial growth (Fig. 2A). While 100% of the larvae not treated with niclosamide died within 60 h postinfection, niclosamide almost completely protected G. mellonella larvae from the lethal challenge with P. aeruginosa (Fig. 5), even if incubation was prolonged for a week (data not shown). To monitor the presence of PA14 in niclosamide-treated and -untreated larvae, at 60 h postinfection five larvae per group were homogenated in saline solution, and serial dilutions of the resulting homogenates were plated on Pseudomonas isolation agar. Dead larvae contained about 7 (±5) × 108 P. aeruginosa cells per larva, while no bacterial cells were detectable in niclosamide-treated larvae. Notably, niclosamide-treated larval hemolymph had no effect on P. aeruginosa PA14 growth in vitro (see details in Materials and Methods), further confirming that the observed effect of niclosamide on P. aeruginosa pathogenicity is due to virulence inhibition rather than to a growth-inhibitory effect. Overall, these findings indicate that niclosamide allowed the innate immune response of G. mellonella to efficiently counteract the infection.
Fig 5.
Efficacy of niclosamide in protecting G. mellonella larvae from P. aeruginosa killing. The Kaplan-Meier plot shows the survival of G. mellonella larvae inoculated with a lethal dose of P. aeruginosa PA14 (10 ± 4 exponentially growing cells) in 10 μl of saline supplemented or not with 750 μM niclosamide ethanolamine salt and then incubated at 28°C. All untreated larvae died within 60 h postinfection, while G. mellonella killing was almost completely prevented upon treatment with the niclosamide ethanolamine salt. χ2(1) = 61.07; P = 0.0000 (log-rank).
DISCUSSION
The need for new anti-infective strategies based on nonantibiotic compounds, together with the growing awareness of QS importance in bacterial infections, has raised interest toward the identification of QSIs endowed with antivirulence properties (reviewed in reference 56).
The relationships among QS, virulence regulation, and biofilm formation have most extensively been studied in P. aeruginosa. Therefore, it is not surprising that most of the research on QS inhibition has been centered on this bacterium as a model system (56).
Research on QS inhibition in Gram-negative bacteria has largely been focused on structural homologues of QS signal molecules, targeting the site of the signal receptor protein that is occupied by the natural ligand (56). Alternatively, promising QSIs belonging to different chemical classes have been discovered by screening random libraries of synthetic and natural compounds, and some of them have proved to be effective in preventing P. aeruginosa infection in animal models (38, 56, 57). Probably due to the high toxicity of the majority of the QSIs identified to date, garlic extract is the only QSI that has been tested in humans. Although the results were not statistically significant, a trend toward improvement of the clinical outcome of P. aeruginosa-infected CF patients after oral garlic extract administration was observed (58). A recent study identified ajoene as the most active QSI compound in garlic extract. However, synthesized ajoene was less active than the crude garlic extract in vitro, and it was very poorly effective in an in vivo murine model of infection (46). Thus, despite the huge efforts made to date in the field of anti-QS research, clinical applications remain far away (reviewed in reference 56).
The main aim of this work is to validate a new strategy for the identification of QSIs rapidly deliverable for clinical use, by proving that a lateral anti-QS activity can be identified in drugs already used in humans.
By screening a library of FDA-approved chemicals, we identified some hit compounds disclosing relevant QSI activity at concentrations that did not cause substantial inhibition of P. aeruginosa growth. The salicylanilide compound niclosamide, a cestocide already approved for use in humans (55), was characterized in detail for its anti-QS activity. At micromolar concentrations, niclosamide strongly inhibited both 3OC12-HSL and C4-HSL production, as well as production of several secreted virulence factors, such as pyocyanin, elastase, and rhamnolipids (Fig. 2 and 3). As a comparative example, the previously described QSI ajoene only inhibits C4-HSL production at very high concentrations (>300 μM), while it has no effect on 3OC12-HSL, the major QS signal produced by P. aeruginosa (46).
The large percentage of QS-regulated genes repressed by niclosamide (64%) highlights a high degree of target specificity toward C4-HSL- and 3OC12-HSL-dependent regulons and is comparable to or even higher than that disclosed for other QSIs identified so far (38, 45, 46, 59). Overall, niclosamide strongly decreased the transcription of multiple genes involved in P. aeruginosa pathogenicity, corroborating its potential as an antivirulence drug.
Consistent with the strong antivirulence activity in vitro, niclosamide suppressed P. aeruginosa pathogenicity in an acute infection model based on G. mellonella larvae (Fig. 5). P. aeruginosa can also cause chronic infections characterized by a biofilm mode of growth. Several studies using cell flow chambers for biofilm formation, coupled with confocal scanner microscopy observations, have shown that a proficient QS system is required for optimal biofilm shaping and development (60). Accordingly, biofilms treated with QSIs show specific structural features and decreased resistance to antibiotics (19). In this work, we performed a pilot experiment using a simple biofilm model, showing that niclosamide is able to reduce cell attachment to a plastic surface, while increasing the number of planktonic cells (Fig. 4D). Although this effect was only observed at high niclosamide concentrations, this preliminary result should encourage further characterization of the effect of niclosamide on biofilm development and resistance to antibiotics by using advanced biofilm models.
Concerning future developments of niclosamide as an anti-P. aeruginosa drug, there are some issues that need to be addressed. First of all, the effect of niclosamide on a wide panel of clinical P. aeruginosa strains isolated from different infection sites should be assessed, including CF patient chronically infected lungs. Moreover, even if niclosamide is currently used as an anthelmintic drug to treat intestinal infections and displays overall low toxicity (55), it is poorly soluble in water, shows low intestinal absorption, and once in the bloodstream, it is quickly cleared via the urinary tract or by enzymatic modification in the liver (55). Although these features could represent drawbacks to the systemic administration of niclosamide, they could be advantageous in the local treatment of wound infections, burns, otitis, gastrointestinal infections, and other external P. aeruginosa infections. It is also worth mentioning that the toxicity of inhaled niclosamide powder is quite low for mammals (55), opening new perspectives for aerosol treatment of P. aeruginosa lung infections. Additional studies in different mammalian models of both acute and chronic infections are required to assess the suitability of niclosamide as an anti-P. aeruginosa drug, prior to the move into clinical trials. However, in accordance with the SOSA approach (2), niclosamide could also be used as a promising scaffold for the design of structural analogues endowed with improved activity and pharmacokinetic properties. The hypothesis that some niclosamide derivatives may retain anti-QS activity is strengthened by our preliminary observation that other compounds belonging to the same structural class of niclosamides, i.e., the salicylanilides rafoxanide and oxyclozanide, showed in vitro the same anti-QS activity as niclosamide (data not shown).
The development of niclosamide-based QSIs could be pursued either by screening random chemical modifications introduced within the salicylanilide structure or by rational drug design. The latter approach requires detailed information about the anti-QS mechanism of action of niclosamide. Unfortunately, despite niclosamide's use since the 1960s, its mechanism of action remains elusive. The anthelmintic activity of niclosamide seems to rely on its ability to uncouple mitochondrial oxidative phosphorylation (61). More recently, it has been found to inhibit proliferation of some tumor cells by hampering different regulatory pathways, without relevant effects on normal nontumor cells (62–64). Notably, niclosamide has also been reported to act as an antimycobacterial agent (64), plausibly disrupting Mycobacterium membrane potential and pH homeostasis (65). Although the characterization of the molecular targets of niclosamide was not the aim of this study, some of our observations might be the first steps toward the comprehension of the niclosamide mechanism of action in P. aeruginosa. First, niclosamide is likely to target the 3OC12-HSL reception process rather than signal biosynthesis (Fig. 1). Second, the maximum inhibitory effect disclosed for niclosamide on 3OC12-HSL production (60%) is reached at 5 μM, and increases in concentration did not reduce 3OC12-HSL production (Fig. 2), ruling out the possibility that niclosamide competes with 3OC12-HSL for receptor binding. Third, microarray analysis showed that niclosamide also affects the expression of genes not controlled by QS, including some putative or confirmed transcriptional regulators. Fourth, niclosamide represses C4-HSL production both dependently and independently from its action on 3OC12-HSL (Fig. 2). Finally, the repressive effect disclosed by niclosamide is higher on swarming motility and on the production of virulence factors than on 3OC12-HSL production (Fig. 2, 3, and 4). Although this could be due to the combined effect of this QSI on 3OC12-HSL and C4-HSL production, it cannot be excluded that niclosamide also influences some QS-independent cellular process involved in virulence gene regulation.
Putting together our observations and data from the available literature on the cellular processes affected by niclosamide in other organisms, a very preliminary hypothesis about the mechanism of action of niclosamide in P. aeruginosa is that this molecule targets some regulatory pathway(s) responsive to the energetic/metabolic status of the cell and that is required for full activity of the QS signaling network. Studies on the mechanism of action of niclosamide in P. aeruginosa are therefore in progress in our laboratory.
In conclusion, the major outcome of this study was the identification of a strong anti-QS activity in a compound already approved for use in humans. Our findings provide a new promising drug candidate against P. aeruginosa and a proof of concept that FDA-approved drugs may be endowed with antivirulence properties that are worthy of exploration.
ACKNOWLEDGMENTS
We heartily thank Paolo Landini (University of Milan, Milan, Italy) for encouraging this work, for providing us with the Prestwick library, and for precious advice.
This work was supported by a grant from the Ministry of University and Research of Italy PRIN-2008 (232P4H_003) and by grants from the Italian Cystic Fibrosis Research Foundation (Projects FFC 14/2010 and FFC 13/2011).
Footnotes
Published ahead of print 17 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01952-12.
REFERENCES
- 1. Gilbert N. 2010. Universities shun Europe's drug initiative. Nature 466:306–307 [DOI] [PubMed] [Google Scholar]
- 2. Wermuth CG. 2006. Selective optimization of side activities: the SOSA approach. Drug Discov. Today 11:160–164 [DOI] [PubMed] [Google Scholar]
- 3. Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, Tyers M, Brown ED, Wright GD. 2011. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 7:348–350 [DOI] [PubMed] [Google Scholar]
- 4. Antoniani D, Bocci P, Maciag A, Raffaelli N, Landini P. 2010. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl. Microbiol. Biotechnol. 85:1095–1104 [DOI] [PubMed] [Google Scholar]
- 5. Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. 2008. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9:117–128 [DOI] [PubMed] [Google Scholar]
- 7. Atkinson S, Williams P. 2009. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface 6:959–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Njoroge J, Sperandio V. 2009. Jamming bacterial communication: new approaches for the treatment of infectious diseases. EMBO Mol. Med. 1:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amara N, Krom BP, Kaufmann GF, Meijler MM. 2011. Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem. Rev. 111:195–208 [DOI] [PubMed] [Google Scholar]
- 10. Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG; Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668 [DOI] [PubMed] [Google Scholar]
- 11. Orsi GB, Raponi M, Franchi C, Rocco M, Mancini C, Venditti M. 2005. Surveillance and infection control in an intensive care unit. Infect. Control Hosp. Epidemiol. 26:321–325 [DOI] [PubMed] [Google Scholar]
- 12. Driscoll JA, Brody SL, Kollef MH. 2007. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67:351–368 [DOI] [PubMed] [Google Scholar]
- 13. Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, Leblebicioglu H, Fisher D, Aacute;lvarez-Moreno C, Khader IA, Del RocíO González Martínez M, Cuellar le, Navoa-Ng JA, Abouqal R, Guanche Garcell H, Mitrev Z, Pirez GarcíA MC, Hamdi A, Dueñas L, Cancel E, Gurskis V, Rasslan O, Ahmed A, Kanj SS, Ugalde OC, Mapp T, Raka L, Yuet Meng C, Thu le, Ghazal TAS, Gikas A, Narváez LP, MejíA N, Hadjieva N, Gamar Elanbya MO, Guzmán Siritt ME, Jayatilleke K; INICC members 2012. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am. J. Infect. Control 40:396–407 [DOI] [PubMed] [Google Scholar]
- 14. Page MG, Heim J. 2009. Prospects for the next anti-Pseudomonas drug. Curr. Opin. Pharmacol. 9:558–565 [DOI] [PubMed] [Google Scholar]
- 15. Breidenstein EB, de la Fuente-Núñez C, Hancock RE. 2011. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19:419–426 [DOI] [PubMed] [Google Scholar]
- 16. Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL, Miyata S, Diggins LT, He J, Saucier M, Déziel E, Friedman L, Li L, Grills G, Montgomery K, Kucherlapati R, Rahme LG, Ausubel FM. 2006. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 7:R90 doi:10.1186/gb-2006-7-10-r90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams P, Cámara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 12:182–191 [DOI] [PubMed] [Google Scholar]
- 18. Winstanley C, Fothergill JL. 2009. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiol. Lett. 290:1–9 [DOI] [PubMed] [Google Scholar]
- 19. Bjarnsholt T, Tolker-Nielsen T, Høiby N, Givskov M. 2010. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 12:e11. [DOI] [PubMed] [Google Scholar]
- 20. Massai F, Imperi F, Quattrucci S, Zennaro E, Visca P, Leoni L. 2011. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens. Bioelectron. 26:3444–3449 [DOI] [PubMed] [Google Scholar]
- 21. Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902 [DOI] [PubMed] [Google Scholar]
- 22. Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 24. Clark JMB, Clark DJ, Maaløe O. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99–112 [Google Scholar]
- 25. Duan K, Surette MG. 2007. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 189:4827–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fletcher MP, Diggle SP, Crusz SA, Chhabra SR, Cámara M, Williams P. 2007. A dual biosensor for 2-alkyl-4-quinolone quorum-sensing signal molecules. Environ. Microbiol. 9:2683–2693 [DOI] [PubMed] [Google Scholar]
- 27. Rampioni G, Schuster M, Greenberg EP, Bertani I, Grasso M, Venturi V, Zennaro E, Leoni L. 2007. RsaL provides quorum sensing homeostasis and functions as a global regulator of gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 66:1557–1565 [DOI] [PubMed] [Google Scholar]
- 28. Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohman DE, Burns RP, Iglewski BH. 1980. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J. Infect. Dis. 142:547–555 [DOI] [PubMed] [Google Scholar]
- 30. Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE. 2007. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 189:6695–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rampioni G, Schuster M, Greenberg EP, Zennaro E, Leoni L. 2009. Contribution of the RsaL global regulator to Pseudomonas aeruginosa virulence and biofilm formation. FEMS Microbiol. Lett. 301:210–217 [DOI] [PubMed] [Google Scholar]
- 32. Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1:Unit 1B.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jander G, Rahme LG, Ausubel FM. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 51:3677–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babić F, Venturi V, Maravić-Vlahovicek G. 2010. Tobramycin at subinhibitory concentration inhibits the RhlI/R quorum sensing system in a Pseudomonas aeruginosa environmental isolate. BMC Infect. Dis. 10:148 doi:10.1186/1471-2334-10-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imperi F, Tiburzi F, Fimia GM, Visca P. 2010. Transcriptional control of the pvdS iron starvation sigma factor gene by the master regulator of sulfur metabolism CysB in Pseudomonas aeruginosa. Environ. Microbiol. 12:1630–1642 [DOI] [PubMed] [Google Scholar]
- 37. Aendekerk S, Diggle SP, Song Z, Høiby N, Cornelis P, Williams P, Cámara M. 2005. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 151:1113–1125 [DOI] [PubMed] [Google Scholar]
- 38. Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuster M, Lohstroh CP, Ogi T, Greemberg EP. 2003. Identification, timing and signal specifity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewsky BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, Xiao G, Rahme LG. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 55:998–1014 [DOI] [PubMed] [Google Scholar]
- 42. Bredenbruch F, Geffers R, Nimtz M, Buer J, Häussler S. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 8:1318–1329 [DOI] [PubMed] [Google Scholar]
- 43. Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. 2006. A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J. Bacteriol. 188:3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rampioni G, Pustelny C, Fletcher MP, Wright VJ, Bruce M, Rumbaugh KP, Heeb S, Cámara M, Williams P. 2010. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ. Microbiol. 12:1659–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Høiby N, Bjarnsholt T, Givskov M. 2012. Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78:2410–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen TB, Friedrich K, Uthe F, Jensen PØ Moser C, Nielsen KF, Eberl L, Larsen TO, Tanner D, Høiby N, Bjarnsholt T, Givskov M. 2012. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 56:2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giraud C, Bernard CS, Calderon V, Yang L, Filloux A, Molin S, Fichant G, Bordi C, de Bentzmann S. 2011. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 13:666–683 [DOI] [PubMed] [Google Scholar]
- 48. MacEachran DP, Stanton BA, O'Toole GA. 2008. Cif is negatively regulated by the TetR family repressor CifR. Infect. Immun. 76:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson RM, Zimprich CA, Rust L. 1999. A second operator is involved in Pseudomonas aeruginosa elastase (lasB) activation. J. Bacteriol. 181:6264–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lau GW, Hassett DJ, Ran H, Kong F. 2004. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 10:599–606 [DOI] [PubMed] [Google Scholar]
- 51. Reis RS, Pereira AG, Neves BC, Freire DM. 2011. Gene regulation of rhamnolipid production in Pseudomonas aeruginosa: a review. Bioresour. Technol. 102:6377–6384 [DOI] [PubMed] [Google Scholar]
- 52. Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677–701 [DOI] [PubMed] [Google Scholar]
- 53. Zolfaghar I, Evans DJ, Fleiszig SM. 2003. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infect. Immun. 71:5389–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arora SK, Neely AN, Blair B, Lory S, Ramphal R. 2005. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 73:4395–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andrews P, Thyssen J, Lorke D. 1982. The biology and toxicology of molluscicides, Bayluscide. Pharmacol. Ther. 19:245–295 [DOI] [PubMed] [Google Scholar]
- 56. Galloway WR, Hodgkinson JT, Bowden S, Welch M, Spring DR. 2012. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 20:449–458 [DOI] [PubMed] [Google Scholar]
- 57. Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Köte M, Nielsen J, Eberl L, Givskov M. 2005. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187:1799–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Cámara M, Barrett DA, Knox A. 2010. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis: a pilot randomized controlled trial. Pediatr. Pulmonol. 45:356–362 [DOI] [PubMed] [Google Scholar]
- 59. Müh U, Hare BJ, Duerkop BA, Schuster M, Hanzelka BL, Heim R, Olson ER, Greenberg EP. 2006. A structurally unrelated mimic of a Pseudomonas aeruginosa acyl-homoserine lactone quorum-sensing signal. Proc. Natl. Acad. Sci. U. S. A. 103:16948–16952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kirisits MJ, Parsek MR. 2006. Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell. Microbiol. 8:1841–1849 [DOI] [PubMed] [Google Scholar]
- 61. Weinbach EC, Garbus J. 1969. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 221:1016–1018 [DOI] [PubMed] [Google Scholar]
- 62. Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J. 2010. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-κB pathway and generation of reactive oxygen species. Cancer Res. 70:2516–2527 [DOI] [PubMed] [Google Scholar]
- 63. Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA, Lyerly HK. 2011. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 71:4172–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun Z, Zhang Y. 1999. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber. Lung. Dis. 79:319–320 [DOI] [PubMed] [Google Scholar]
- 65. de Carvalho LP, Darby CM, Rhee KY, Nathan C. 2011. Nitazoxanide disrupts membrane potential and intrabacterial pH homeostasis of Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2:849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]