Abstract
Some Bacillus thuringiensis strains have high toxicity to nematodes. Nematicidal activity has been found in several families of crystal proteins, such as Cry5, Cry6, and Cry55. The B. thuringiensis strain YBT-1518 has three cry genes that have high nematicidal activity. The whole genome sequence of this strain contains multiple potential virulence factors. To evaluate the pathogenic potential of virulence factors, we focused on a metalloproteinase called Bmp1. It encompasses a consecutive N-terminal signal peptide, an FTP superfamily domain, an M4 neutral protease GluZincin superfamily, two Big-3 superfamily motifs, and a Gram-positive anchor superfamily motif as a C-terminal domain. Here, we showed that purified Bmp1 protein showed metalloproteinase activity and toxicity against Caenorhabditis elegans (the 50% lethal concentration is 610 ± 9.37 μg/ml). In addition, mixing Cry5Ba with Bmp1 protein enhanced the toxicity 7.9-fold (the expected toxicity of the two proteins calculated from their separate toxicities) against C. elegans. Confocal microscopic observation revealed that Bmp1 protein was detected from around the mouth and esophagus to the intestine. Striking microscopic images revealed that Bmp1 degrades intestine tissues, and the Cry5Ba causes intestinal shrinkage from the body wall. Thus, the B. thuringiensis Bmp1 metalloproteinase is a nematicidal virulence factor. These findings give a new insight into the relationship between B. thuringiensis and its host nematodes.
INTRODUCTION
Bacillus thuringiensis is a rod-shaped, Gram-positive, spore-forming bacterium. It is the most successful insect pathogen used for insect control (1). The action to the insect pest relies on insecticidal toxin and array of virulence factors (2). Upon sporulation, it produces insecticidal crystal inclusion that is formed by a variety of insecticidal proteins called Cry or Cyt proteins. These insecticidal crystal proteins are toxic to insects in the orders Lepidoptera, Dipteran, Coleoptera, Hymenoptera, Homoptera, Orthoptera, and Mallophage (2), and they are also toxic to nematodes. Until now, there are several families of Cry proteins (Cry5, Cry6, Cry12, Cry13, Cry14, Cry21, and Cry55) known to be toxic to the larvae of a number of free-living or parasitic nematodes (3). Besides having crystal proteins that are toxic to insects, B. thuringiensis has many virulence factors that contribute to its pathogenic effects. These virulence factors contain exotoxins and extracellular proteases (2). During the stationary growth phase, some B. thuringiensis strains secrete exotoxins, which are heat-stable, water-soluble, and low-molecular-mass compounds (701 Da). These compounds are highly toxic to a wide range of insect species by the oral route (4, 5). In addition, extracellular proteases, e.g., serine protease, chitinase, and collagenase (6), have been reported to be insect pests virulence factors (2).
In the past few years, some insecticidal virulence factors have been isolated from B. thuringiensis. The action mode of virulence factors against insect concerns two respects. First, the virulence factor breaches the epithelial cells of the insect midgut, and an increase in the insecticidal activity of Cry protein occurs (7). For example, Bel protein can enhance the activity of Cry1Ac protein to Helicoverpa armigera due to degrading the peritrophic matrix and the insect intestinal mucin of Lepidopteran insects (8). Second, the virulence factor can protect B. thuringiensis from the innate immune system through cleavage of antimicrobial peptides, whereby the insecticidal activity of the Cry protein is enhanced (7). It is lethal to inject immune inhibitor A (InhA) into the Lepidopteran larvae hemocoel (9). Thus, virulence factors are used by B. thuringiensis to target insects. Many virulence factors showed toxicity to nematodes. The neutral serine protease of the nematophagous fungus Arthrobotrys oligospora, when secreted, is toxic to nematodes; the protein induces dramatic structural changes in nematode cuticle (10). The Brevibacillus laterosporus secretes the serine protease (11), while Bacillus nematocida secrets a neutral protease (designated Bae16) that target the nematodes' essential intestinal protein (12). These findings confirm the important role played by extracellular proteases in suppressing nematodes. Despite this, no reports of B. thuringiensis virulence factors that target nematodes have been reported in the literature. Because B. thuringiensis is known to be toxic to nematodes, we speculate that virulence factors against nematodes would exist in this bacterium. The aim of the present study was therefore to isolate novel virulence factors in B. thuringiensis.
After analyzing the characteristics of the reported nematicidal virulence factors described above, bioinformatics analysis showed that these factors shared conserved motifs, such as the M4 superfamily motif, which is a zinc metal ion-binding motif. The extracellular neutral protease secreted by the B. laterosporus strain G4 (11) and B. nematocida (12, 13) both have the M4 superfamily motif. Analysis of B. thuringiensis genomic sequences (YBT-1518) revealed that this strain had many potential virulence factors containing M4 motifs. We identified a novel protease, called Bmp1, which has a mosaic structure and a M4 superfamily motif. Interestingly, this motif exhibited low similarity to other published sequences. We found not only that Bmp1 was toxic to Caenorhabditis elegans but that its intestinal tissue degradation activity enhanced the toxicity to crystal protein Cry5Ba against C. elegans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. The B. thuringiensis YBT-1518 was isolated by our group (14, 15). All Escherichia coli and B. thuringiensis strains were maintained on Luria-Bertani (LB) agar plates (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 1.5% agar) and appended with appropriate antibiotics at 37 and 28°C, respectively.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristicsa | Origin or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | supE44 ΔlacU1169(ϕlacZΔ)hsdR17 recA1 endA1 gyrA96 Δthi relA1 | –b |
| BL21(DE3) | T7 promoter | – |
| EMB0717 | pEMB0717 (contains bmp; Kanr) | This study |
| Bacillus thuringiensis | ||
| YBT-1518 | Wide type, containing the cry55Aa, cry6Aa, and cry5Ba genes | 14 |
| BMB0215 | Derivative of YBT-1518 (containing cry5Ba; Ermr) | 14 |
| BMB31 | Derivative of BMB171 (containing cry1Ac10; Ermr) | 8 |
| Plasmids | ||
| pET28a | ori E. coli (Kanr), 5.4 kb | – |
| pEMB0717 | Derivative of pET28a (containing the bmp gene; Kanr) | This study |
Ermr, erythromycin resistance; Kanr, kanamycin resistance.
–, stored at State Key Laboratory of Agricultural Microbiology, College of Life Science and Technology, Huazhong Agricultural University, Wuhan, Hubei, China.
Isolation of the bmp1 gene.
The B. thuringiensis bmp1 gene was PCR amplified from YBT-1518 genomic DNA with the following pair of primers, bmpP1 (5′-GTAGGATCCATGGGAAATAAAAAAGAAAT-3′, where the BamHI recognition sequence is underlined) and bmpP2 (5′-GCGAAGCTTTTATTTTTGTATCTTTCTAA-3′, where the HindIII recognition sequence is underlined). The PCR was subjected to the following thermal cycles: initial denaturation at 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 52°C for 30 s, and extension at 72°C for 4 min, with a final extension at 72°C for 5 min.
Purification and labeling of Bmp1 protein.
The gene bmp1 was amplified from strain YBT-1518 and then inserted into BamHI and HindIII sites of the expression vector pET28a for expression as a His-tagged protein to create the recombinant expression vector pEMB0717. The recombinant plasmid was then transferred into E. coli BL21(DE3), yielding the recombinant EMB0717. The bacteria were cultured in 5 ml of LB medium with kanamycin, followed by vigorous shaking (220 rpm) overnight at 37°C, and transferred into 1 ml of stain liquid plus 100 ml of LB medium along with an appropriate amount of kanamycin shake cultured to an optical density at 600 nm of ∼0.6. For an efficiently soluble yield of Bmp1 protein, the induction scheme included the following: final IPTG (isopropyl-β-d-thiogalactopyranoside) concentration, 0.1 mM; induction temperature, 16°C; total induction time, 18 h; and shaking speed, 100 rpm. The soluble protein in the supernatant was collected by refrigerated centrifugation (12,000 rpm) after high-pressure shaking. Finally, the target protein was purified with His-Bind columns (Qiagen, Germany) according to the manufacturer's instructions. The purified protein was labeled with fluorescein isothiocyanate (FITC; Pierce, catalog no. 46425) according to the method of Griffitts et al. (16).
Preparation and labeling of crystal proteins.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to examine the Cry5B proteins from the BMB0215 strain (14) and the Cry1Ac10 protein from the BMB31 strain (8). For crystal protein purification, recombinant bacteria were cultivated in liquid ICPM medium (pH 7.0) containing peptone(0.6%), glucose (0.5%), CaCO3 (0.1%), MgSO4 (0.05%), and KH2PO4 (0.05%) (17) at 28°C with erythromycin (25 μg/ml) until the spore and crystals had separated. The crude spore lysate pellets were treated using the method of Griffitts et al. (16). The purified protein was labeled with Rho (rhodamine; Pierce, catalog no. 46102) according to the method of Griffitts et al. (16).
Nematode toxicity bioassay.
The purified Cry proteins were used for bioassay. C. elegans were harvested from a nematode growth plate. We conducted quantitative growth analyses, mortality tests, and brood size assays to test the toxicity of proteins against nematodes. The quantitative growth of proteins against nematodes was tested by photographing at least 30 worms for each condition at ×100 magnification using a compound microscope and then normalizing the average area for each protein concentration to the average area of the no-protein control. The mortality of proteins against nematodes was tested based on the observation of motility; a visibly moving nematode was marked as alive, and nematodes that failed to respond after several touches were marked as dead. The brood size assay of proteins against nematodes was tested by counting the number of the progeny. The entire bioassays were repeated a minimum of three times to calculate the activity for each dose-response of proteins. The bioassay procedure and 50% lethal concentration (LC50) evaluation were undertaken according to the method of Bischof et al. (18).
Protease assay.
The influence of temperature, pH, metal ions, and protease inhibitors on the activity of the purified protease was tested using a method described by Niu et al. (13). The temperature and pH ranges tested were 4 to 100°C (10 min) and pH 3 to 13. The metal ions tested included Zn2+, Ca2+, Co2+, Mg2+, and Mn2+, whereas the protease inhibitors included phenylmethylsulfonyl fluoride (PMSF), EDTA, and pepstatin A.
CLSM detection.
C. elegans samples were incubated in M9 medium contain proteins at 1 μg/ml and then sampled at different times and washed five times in M9 medium before being observed using confocal laser scanning microscopy (CLSM; Zeiss LSM 510) imaging. The images were captured using a ×40 objective lens. Green fluorescence was monitored at an excitation wavelength of 488 nm, with a main dichroic beam splitter (HFT488/543), whereas red fluorescence was monitored at an excitation wavelength of 543 nm, with a high-pass filter (LP505). The images were merged, and the data were stored.
Bioinformatics analysis.
All of the genome sequences of B. thuringiensis strains in GenBank, plus those of our own sequencing group, were used for analysis. The proteins with a metalloproteinase domain were identified by the hmm search command of the HMMER version 3.0 software (19). Other conserved domains in these proteins were predicted using the CDD database (20).
Nucleotide sequence accession number.
The nucleotide sequence published here has been submitted to GenBank under accession number JX898865 (bmp1).
RESULTS
Presence and comparison of the Bmp1 proteins in the genomes of sequenced B. thuringiensis strains.
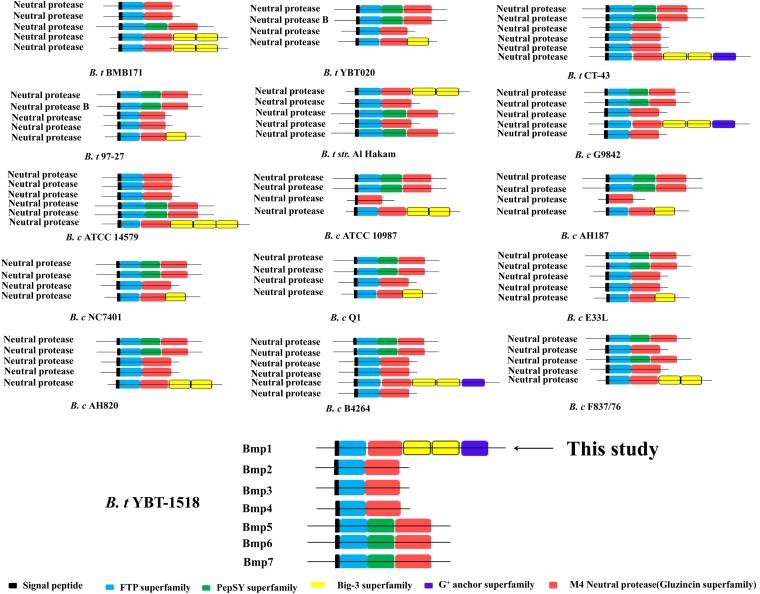
Previously, we demonstrated that B. thuringiensis YBT-1518 exhibited high activity against root-knot nematodes due to the presence of its three cry genes (14, 15). However, in subsequent tests on Cry protein toxicity, we found that the purified Cry proteins had lower toxicity than mixtures containing Cry proteins and spores (unpublished data). Hence, we speculated that there were other virulence factors besides the Cry proteins in this strain. The whole genome shotgun sequence for YBT-1518 (unpublished data) revealed that there were multiple potential virulence factors (Table 2), many of which were proteases. These include the following: (i) seven proteins containing the M4 superfamily motif, which is a metal ion (primarily a zinc metal)-binding motif seen in neutral proteases (Npr); (ii) five proteins containing the M9 superfamily motif, i.e., collagenase (ColE); (iii) five proteins containing the M6 superfamily motif, i.e., immune inhibitor A (InhA), and (iv) two proteins containing the M48 superfamily motif, i.e., zinc metalloproteinase and cell surface protein. Previous studies have reported that B. laterosporus (11) and B. nematocida (13) have virulence factors containing M4 superfamily motif that are active against nematodes. Here, we choose one of the proteins with the M4 superfamily, Bmp1, which contains two Big-3 superfamily motif and Gram-positive anchor superfamily motif, to test its nematicidal activity.
Table 2.
Potential virulence factors in B. thuringiensis YBT-1518
| Protein | Function | Major motif | No. of proteins | Reference |
|---|---|---|---|---|
| Crystal protein | 14 | |||
| Cry5Ba | Insecticidal crystal protein | Conserved blocks | 1 | |
| Cry6Aa | Insecticidal crystal protein | Conserved blocks | 1 | |
| Cry55Aa | Insecticidal crystal protein | Conserved blocks | 1 | |
| Proteases | 2 | |||
| Bacillolysin | Neutral protease | M4 neutral protease GluZincin superfamily | 7 | |
| ColB | Collagenase | Peptidase M9 superfamily | 5 | |
| InhA | Neutral protease | Peptidase M6 superfamily | 5 | |
| Sfp | Subtilase family protease | Peptidase_S8 superfamily | 15 | |
| Phospholipases | 2 | |||
| PI-PLC | Phosphatidylinositol-specific phospholipase | PI-PLCc_GDPD_SF superfamily | 4 | |
| PC-PLC | Phosphatidylcholine-specific phospholipase | ZnPC_S1P1 superfamily | 1 | |
| Enterotoxins | 2 | |||
| Enterotoxin C | Enterotoxin C | Bacillus_HBL superfamily | 4 | |
| HblB | Enterotoxin Hbl, binding component B | Bacillus_HBL superfamily | 3 | |
| HblC | Enterotoxin Hbl, lytic component L | Bacillus_HBL superfamily | 5 | |
| Hly | Hemolysin | Bacillus_HBL superfamily | 3 | |
| Nonspecific toxins | 21 | |||
| CytK | Hemolysin, cytotoxin K | Leukocidin superfamily/hemolysin toxin superfamily | 1 | |
| CerO | Hemolysin, cereolysin O | HlyIII superfamily | 2 | |
| Hypothetical protein | Hypothetical protein | Enhancin family | 6 | 8 |
Among the seven putative proteases with M4 superfamily motif in YBT-1518, Bmp1, named after Bacillus metalloproteinase, showed low amino acid sequence identity to that of other reported neutral proteases, while the other six proteins shared 75 to 98% sequence identity with Bmp1. Bmp1 shares 35% sequence identity with bacillolysin MA from B. megaterium (22) and has 28 identity with the neutral protease from B. anthracis (23). As shown in Fig. 1, the predicted (894-amino-acid residue) Bmp1 amino acid sequence encompasses a consecutive of an N-terminal signal peptide with signal peptidase cleavage site, a FTP superfamily domain (24) of ∼50 amino acids, an M4 neutral protease GluZincin superfamily motif of ∼280 amino acids, two Big-3 superfamily motifs (25), and a Gram-positive anchor superfamily motif (26) as a C-terminal domain. However, the other six proteins only have the M4 neutral protease GluZincin superfamily as the C-terminal domain (Fig. 1). Because Bmp1 exhibited mosaic structural features and low sequence identity with the M4 superfamily neutral proteases reported to date, we selected it for nematicidal activity testing.
Fig 1.
Genetic motif of Bmp1 proteins from B. cereus group strains.
Bmp1 is a metalloproteinase.
The purified Bmp1 protein was tested for its metalloproteinase activity at different temperatures and pH levels and with different metal ions and protease inhibitors (Table 3). The optimal catalytic conditions and enzyme stability were examined using casein as a substrate. Bmp1 protein exhibited protease activity at a wide range of temperatures from 20 to 60°C, with an optimal temperature of 40°C; pH levels from 5.0 to 10.0 were tested, and the maximum activity was recorded at pH 7.4. We examined the effect of five metal ions on Bmp enzyme activity. CoCl2 was found to increase the Bmp1 protease activity at concentrations between 4-8 mM. In contrast, MgCl2 and MnCl2 decreased its protease activity when the metal ions concentrations were low. At higher concentrations, ZnCl2 decreased Bmp1 protease activity. CaCl2 had a negligible effect on protease activity. Three inhibitors, EDTA, PMSF, and pepstatin A inhibited Bmp1 protease activity. Although EDTA decreased the Bmp1 activity, pepstatin A had little effect. When a concentration of 10 mM PMSF was tested, the protein activity was inhibited 5-fold compared to the control (the concentration of the protease inhibitors is 0 mM). These results confirm that Bmp1 is a metalloproteinase.
Table 3.
Effect of protease inhibitors on Bmp1 protein
| Inhibitor | Mean remaining activity (%) ± SDa |
||||
|---|---|---|---|---|---|
| 0 mM | 1 mM | 4 mM | 8 mM | 10 mM | |
| EDTA | 100 | 90.48 ± 1.86 | 53.57 ± 1.16 | 59.76 ± 1.13 | 49.29 ± 1.84 |
| PMSF | 100 | 64.97 ± 2.81 | 56.80 ± 2.33 | 51.20 ± 3.56 | 26.68 ± 1.15 |
| Pepstatin A | 100 | 86.31 ± 2.93 | 84.52 ± 5.93 | 96.43 ± 3.49 | 105.36 ± 7.19 |
| Zn2+ | 100 | 106.00 ± 4.60 | 72.00 ± 2.91 | 38.00 ± 2.52 | 43.00 ± 2.86 |
| Ca2+ | 100 | 80.00 ± 6.88 | 73.00 ± 3.86 | 63.00 ± 10.07 | 51.00 ± 3.12 |
| Co2+ | 100 | 87.00 ± 2.76 | 128.00 ± 3.14 | 129.00 ± 4.49 | 61.00 ± 2.18 |
| Mg2+ | 100 | 24.00 ± 9.50 | 37.00 ± 8.09 | 18.00 ± 1.43 | 16.00 ± 1.09 |
| Mn2+ | 100 | 32.00 ± 1.80 | 41.00 ± 1.04 | 34.00 ± 1.04 | 38.00 ± 1.33 |
The control protease activity corresponds to 100% activity in the absence of inhibitor. Values represent the means of three replicates.
Bmp1 protein showed toxicity and enhanced the activity of Cry5Ba against C. elegans.
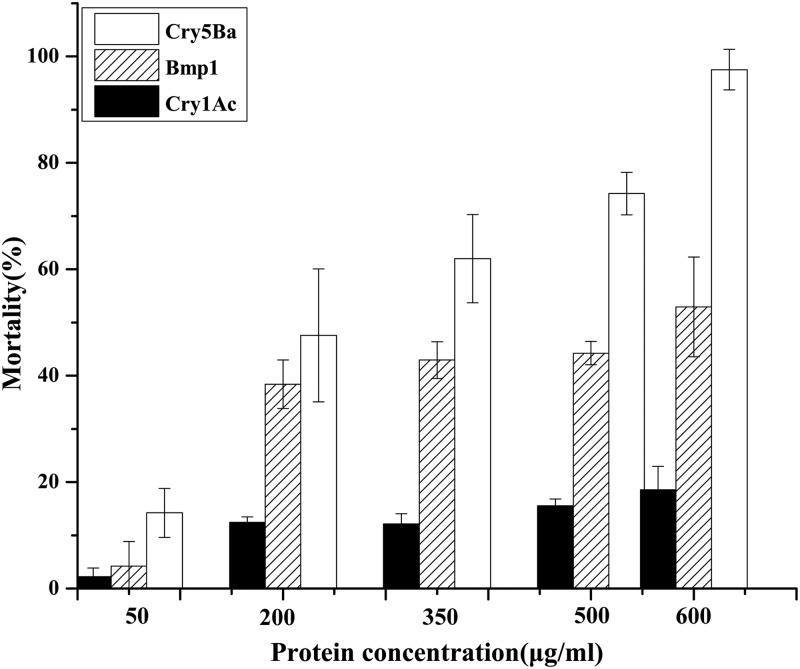
To understand the biochemical activity of the Bmp1 protein, we used purified recombinant Bmp1 to test its activity against C. elegans while comparing it to the nematicidal crystal protein Cry5Ba and the insecticidal crystal protein Cry1Ac. Bmp1 activity against C. elegans was evident (Fig. 2) and was slightly lower than that of crystal protein Cry5Ba. The LC50 was determined by using probit analysis (SAS 8.0) to be 610 ± 9.37 μg/ml for Bmp1 protein and 226 ± 12.49 μg/ml for Cry5Ba. In contrast, no activity was observed for Cry1Ac protein at a concentration of 700 μg/ml.
Fig 2.
Bmp1 protein showed toxicity to C. elegans. We used purified recombinant Bmp1 protein to test its activity against C. elegans, while comparing it with the nematicidal crystal protein Cry5Ba and the insecticidal crystal protein Cry1Ac. C. elegans was exposed to five doses of each protein. The data shown represent the percentage of animals that were intoxicated when fed each protein. Error bars represent the standard deviations from mean averages over three independent experiments. Each data point represents the averaged result from 60 animals.
Using enhance factors and/or a combination of toxins has become one of the most effective methods for increasing the toxicity of B. thuringiensis biopesticides (27). We previously found that the combination of nematicidal crystal proteins Cry6Aa and Cry55Aa showed significant synergistic toxicity against Meloidogyne incognita (27). With this in mind, we wanted to find out whether the Bmp1 protein could enhance the activity of Cry5Ba against C. elegans. To investigate this, we conducted quantitative growth analyses, mortality tests, and brood size assays to test whether the enhanced activity occurs (18).
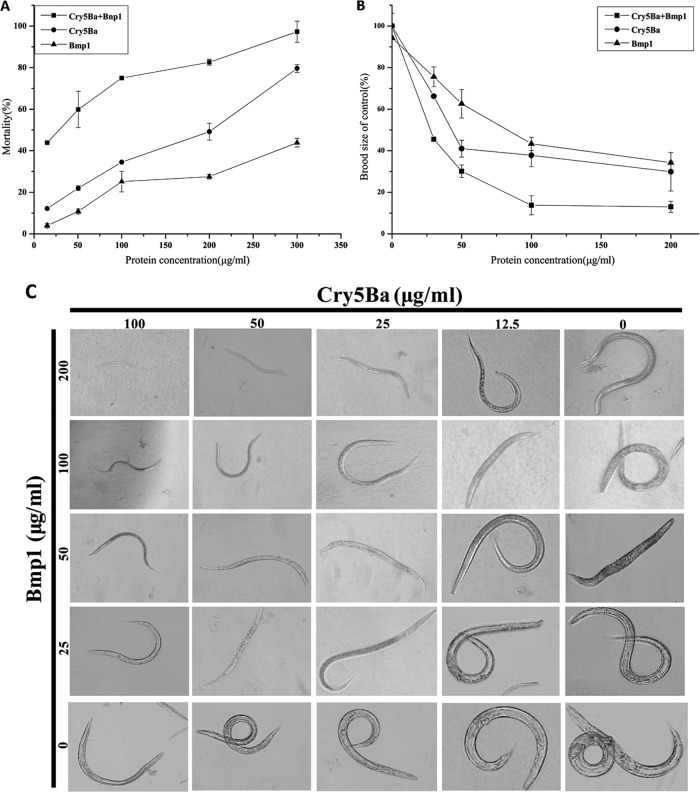
For the quantitative growth test, the bioassay data against C. elegans L1 larvae with Cry5Ba and Bmp1, alone or in combinations, are summarized in Fig. 3C. The nematodes in double-distilled H2O (ddH2O; cultured without Cry5Ba protein and Bmp1 protein) were healthy and vigorous. Nematodes cultured with Cry5Ba-Bmp1 mixture were smaller than when cultured with Cry5Ba protein or Bmp1 protein alone. The nematode size was decreased with the increased gradient of Cry5Ba protein and Bmp1 protein dosage. We also compared the mortality of single Cry5Ba protein and Cry5Ba-Bmp1 combination to C. elegans (Fig. 3A) and calculated the synergistic factor according to the formula of Tabashnik (28). The mortality associated with Cry5Ba protein alone (LC50 = 226 ± 4.09 μg/ml) is lower than that for the Cry5Ba-Bmp1 mixture (LC50 = 37.50 ± 8.72 μg/ml). When bioassay performed with the Cry5Ba-Bmp1 mixture (1:3) showed a significant synergistic effect of ∼7.9 times the reduction in LC50 compared to the expected value (we refer to this as a synergistic factor of 7.9). It shows that the Bmp1 protein has significant synergistic effect to crystal protein Cry5Ba against nematodes (Table 4). To extend these results, the Cry5Ba-Bmp1 combination was also subjected to a brood size assay on adult nematodes (Fig. 3B). When Bmp1 protein was added to Cry5Ba assay system, the nematodes displayed a smaller brood size than without the Bmp1 protein. These assay results indicated that the Bmp1 protein is a novel nematicidal virulence factor in B. thuringiensis.
Fig 3.
Bmp1 enhanced the activity of crystal protein Cry5Ba against C. elegans. We conducted quantitative growth, mortality tests, and brood size assays at the condition of 20°C and pH 7.0 to test the toxicity of crystal proteins against nematodes. (A) Mortality assay using different concentrations of each protein (Cry5Ba, Cry5Ba-Bmp1 mixtures, and Bmp1) against synchronized L4 nematodes. Error bars represent the standard deviations from the mean averages over three independent experiments. (B) Brood size assay using different concentrations of each protein (Cry5Ba, Cry5Ba-Bmp1 mixtures, and Bmp1) against synchronized L4 nematodes. Error bars represent the standard deviations from mean averages over three independent experiments. (C) Growth assay using different concentrations of each protein (Cry5Ba, Cry5Ba-Bmp1 mixtures, and Bmp1) against synchronized L4 nematodes. The quantitative growth of crystal proteins against nematodes was tested by photograph at least 30 worms for each condition at ×100 magnification on a compound microscope.
Table 4.
Effect of crystal protein Cry5Ba, Bmp1 protein, and Cry5Ba-Bmp1 on the mortality of C. elegans
| Toxin (ratio) | Toxicity |
|||
|---|---|---|---|---|
| Regression coefficient | LC50 (μg/ml)a |
SFb | ||
| Observed | Expected | |||
| Cry5Ba | 0.9517 | 226.67 (180.66–290.72) | ||
| Bmp1 | 0.9861 | 610.61 (579.99–893.44) | ||
| Cry5Ba-Bmp1 (1:1) | 0.9477 | 113.355 (90.82–213.36) | 384.30 | 3.39 |
| Cry5Ba-Bmp1 (1:2) | 0.9915 | 46.87 (58.63–73.34) | 333.62 | 7.12 |
| Cry5Ba-Bmp1 (1:3) | 0.9429 | 37.50 (23.46–42.88) | 297.94 | 7.95 |
| Cry5Ba-Bmp1 (1:4) | 0.9614 | 30.52 (25.18–36.98) | 210.05 | 6.88 |
| Cry5Ba-Bmp1 (1:8) | 0.9654 | 45.09 (35.99–56.51) | 133.26 | 2.96 |
The 95% confidence intervals determined by probit analysis are given in parentheses. Expected LC50 values were calculated using the equation of Tabashnik (28).
SF, synergistic factor.
Bmp1 protein destroyed the intestinal tissues of C. elegans.
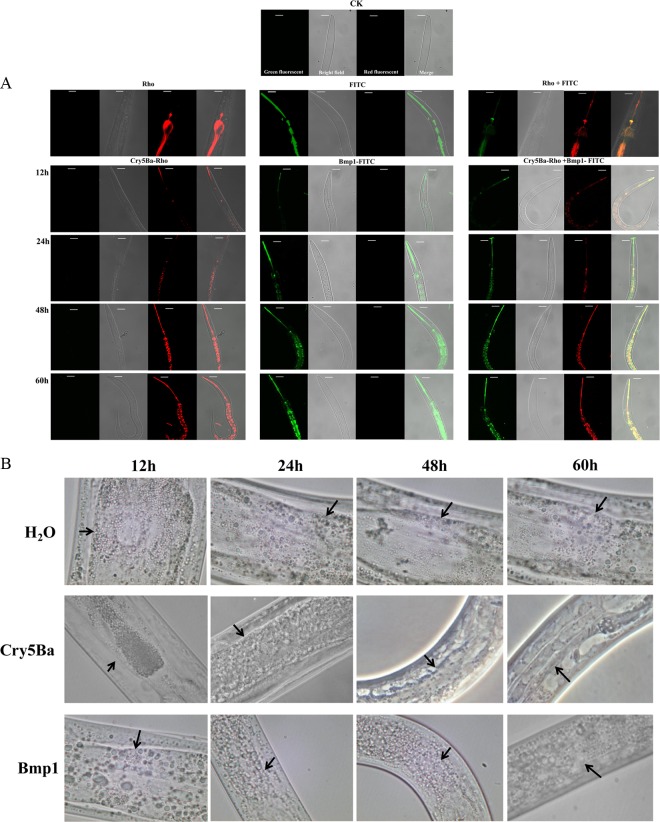
It has been reported that the neutral proteases Bace16 and Bae16 from B. nematocida B16 cause nematode death by damaging their intestines (12). The assays described above showed that the Bmp1 protein is a metalloproteinase and is toxic to nematodes. We next investigated whether Bmp1 protein had an effect on the C. elegans intestine. Previously, we found that the Cry5Ba protein enters the nematodes through the mouth and proceeds to the intestine but not via the cuticle (3). We fed nematodes with FITC-labeled Bmp1 protein and the Rho-labeled Cry5Ba protein separately. The fluorescence signals of Rho-labeled Cry5Ba protein and FITC-labeled Bmp1 protein emitted from nematodes were observed and recorded by CLSM (Fig. 4A). Rho alone and FITC alone were used as controls. Four types of images were obtained: green fluorescence illumination, bright field, red fluorescence illumination, and merge. FITC and Rho signals were observed only in the intestines. After we fed the nematodes the FITC-labeled Bmp1 protein, we observed that the fluorescence signals of Bmp1 protein were emitted from nematodes from around the mouth and esophagus to the intestine and then spread into the body. The images associated with the Bmp1 protein were same as that for the Cry5Ba protein (Fig. 4A). To extend the data, the nematodes were fed Bmp1 protein, and then the intestinal tissues of nematodes were observed under a light microscope (Fig. 4B). We found that the intestinal tissue became ambiguous after 24 h and disappeared after 60 h. However, shrinkage of the gut from the body wall was observed under crystal protein Cry5Ba treatment, whereas the intestinal tissues of the nematodes remained intact (ddH2O treatment). This shows that the Bmp1 protein is toxic to nematodes via its effect on the intestines.
Fig 4.
Bmp1 protein activity in the intestines of nematodes. (A) Localization of the Rho and FITC in C. elegans intestines. The confocal laser scanning microscope image showed the localization of the Rho, FITC, or Rho+FITC in C. elegans intestines. Nematodes were incubated with labeled protein four different times, and then the images were merged. (B) C. elegans intestinal tissues observed under a light microscope after feeding with Cry5Ba and Bmp1 at different times. Original magnification, ×1,000. The arrows point to the nematode intestines.
In order to confirm that the Bmp1 protein acts the intestinal tissue, we fed Cry5Ba, Bmp1, and the Cry5Ba-Bmp1 combination separately to nematodes and then isolated the intestinal proteins. The profiles of the intestinal protein were determined, and those associated with the Bmp1 and Cry5Ba-Bmp1 combination treatments showed that a protein with molecular mass of 45 kDa had disappeared (Fig. 5A). On the other hand, we isolated intestinal proteins from healthy nematodes and incubated them with the Bmp1 protein at different ratios in vitro at 20°C (the nematodes' normal growth temperature) and at 40°C (the optimal temperature for enzyme activity) for 40 min (Fig. 5B). We observed that when the ratio of Bmp1 protein to intestinal protein was 5:1, the intestinal proteins were degraded. When the ratio was 10:1, the differences became more obvious. These results suggest that the Bmp1 protein can degrade the intestinal tissue of C. elegans.
Fig 5.
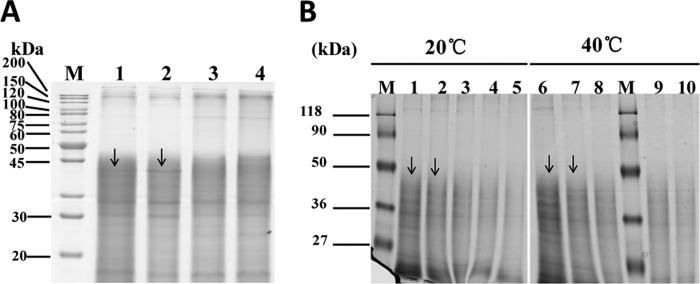
Bmp1 protein acts in the intestinal tissues. (A) SDS-PAGE analysis shows the protease degraded the intestinal tissue in vivo. We fed the Cry5Ba protein, Bmp1 protein, and Cry5Ba-Bmp1 combination separately to nematodes and then isolated the intestinal proteins. The profiles of the intestinal proteins were detected by SDS-PAGE analysis. Lanes: M, molecular mass standards; 1, intestinal protein of C. elegans observed by using the ddH2O test; 2, intestinal protein of C. elegans observed by using the crystal protein Cry5Ba test; 3, intestinal protein of C. elegans observed by using the Bmp1 protein (448.4 μg/ml) test; 4, intestinal protein of C. elegans observed by using the crystal protein Cry5Ba (221.7 μg/ml)/Bmp1 protein (224.4 μg/ml) mixture test. (B) SDS-PAGE analysis shows the protease degraded the intestinal tissue in vitro. We incubated the Bmp1 protein with the intestinal protein of normal C. elegans at 20 and 40°C in phosphate-buffered saline (pH 7.4) for 40 min. Lanes: M, molecular mass standards; 1 and 6, intestinal protein; 2 and 7, Bmp1 protein and intestinal protein at a ratio of 1:2 (wt/wt); 3 and 8, Bmp1 protein and intestinal protein at a ratio of 2:1 (wt/wt); 4 and 9, Bmp1 protein and intestinal protein at a ratio of 5:1 (wt/wt); 5 and 10, Bmp1 protein and intestinal protein at a ratio of 10:1 (wt/wt). The arrows point to the degradative proteins.
DISCUSSION
Some proteases from A. oligospora (10), B. laterosporus (11), and B. nematocida (12) have been reported to have nematicidal activities. However, in B. thuringiensis, there have been no reports of proteases with nematicidal activities. In the present study, we found that Bmp1 protein was toxic to nematodes and also enhances the toxicity of Cry5Ba against nematodes. Bmp1 protein shares some common characteristics with known metalloproteinases, such as temperature and pH tolerance and inhibition by EDTA, PMSF, and some metal ions. Furthermore, the Bmp1 protein has the mosaic structural feature and low identity to the reported neutral metalloproteinase, which suggests that the Bmp1 protein is a novel nematicidal virulence factor that exhibits toxicity to nematodes in the B. cereus group. In B. thuringiensis YBT-1518, there are six other metalloproteinases, and they lack the Big-3 superfamily and G+ anchor superfamily motifs. It would be interesting to study their activity against nematodes in future studies. In addition, there are six predicted InhA-like metalloproteinases in this strain. Based on the premises that InhA metalloproteinase has toxicity to Lepidoptera (9) and that strain YBT-1518 has high toxicity to nematodes, it would be interesting to test whether they have nematicidal activities and explore their mechanisms of action.
B. thuringiensis is one of the most successful insecticides due to producing insecticidal crystal inclusion. Many virulence factors other than Cry protein are toxic to insects. For example, InhA metalloproteinase can protect B. thuringiensis from the innate immune system through the cleavage of antimicrobial peptides or by facilitating its escape from hemocytes (7). We previously found that the Bel protein enhances Cry1Ac toxicity to H. armigera larvae by degrading the insect intestinal mucin (8). Nematicidal activity has been noted in several families of crystal proteins, such as Cry5, Cry6, Cry12, Cry13, Cry14, Cry21, and Cry55 (3). In comparison to insecticidal virulence factors, there are some reports that proteases were secreted by B. nematocida can degrade the cuticle and break down the host external to physical barriers (13) or the intestines of nematodes to help the bacteria invade and then break down the host immune system (12). However, there has been no report about nematicidal virulence factors in B. thuringiensis. In the present study, Bmp1 protein was shown to be a metalloproteinase and has toxicity to and enhances the toxicity of Cry5Ba to C. elegans. After histopathological detection, we found that the Bmp1 protein can destroy nematode intestines. Cry5Ba caused shrinkage of the gut from the body wall, whereas Bmp1 protein can degrade the intestinal tissues; hence, their damage effects differ from each other (Fig. 5B). Thus, we speculate that Bmp1 protein increases the osmolality of intestinal tissues and finally accelerates the Cry5Ba protein entry into the enteric cavity to exert toxicity. The Cry5Ba protein and Bmp1 proteinase cooperate act the nematodes, the Bmp1 as a novel virulence factor due to degrading intestine tissue of nematodes.
B. thuringiensis and B. cereus can be isolated from diverse environments, such as soil, rhizosphere, phylloplane, freshwater, grain dusts, invertebrates, and insectivorous mammals (7). Nematodes are the most abundant metazoans in soil, and most nematodes present in soil are bacterium feeders. Generally, 90 to 99% of nematodes inhabit in the area of high microbial activity (29). B. thuringiensis is an important species among microorganisms. The soil provides a survival space for B. thuringiensis, and nematodes must maintain a relationship between themselves and B. thuringiensis. B. cereus strains produce an abundance of proteolytic enzymes that can degrade different kinds of proteins, including many proteins involved in transporting and utilizing various peptides and amino acids. Compared to B. subtilis, the B. cereus and B. thuringiensis strains contain many more nitrogen metabolism-associated genes than carbon metabolism-associated genes. Thus, as speculated by Ivanova et al. (30), these organisms may be prone to survive in animal hosts. Nematode tissues consist of protein components that B. cereus group strains use as a source of nutrition. Based on analysis of the genomes of B. thuringiensis and B. cereus, we found many putative metalloproteinases that contain the M4 neutral protease GluZincin superfamily were among these two species (Fig. 1). Moreover, four to six of these types of proteins were found from every strain of B. cereus group when their genome sequences were analyzed (Table 5). In addition, there are many other protease class virulence factors found in B. cereus group (31). We speculate that one animal host of B. thuringiensis or B. cereus may be a nematode and that B. thuringiensis is a nematode pathogen.
Table 5.
B. cereus proteins with the M4 superfamily motif
| Strain | No.a | Identity (%)b |
|---|---|---|
| B. thuringiensis | ||
| YBT-1518 | 7 | 75–98 |
| BMB171 | 5 | 32–97 |
| CT-43 | 6 | 31–98 |
| YBT-020 | 4 | 34–80 |
| 97-27 | 5 | 33–81 |
| Al Hakam | 5 | 28–81 |
| B. cereus | ||
| ATCC 14579 | 6 | 32–97 |
| ATCC 10987 | 4 | 33–80 |
| AH187 | 4 | 34–80 |
| NC7401 | 4 | 34–80 |
| Q1 | 4 | 33–80 |
| E33L | 5 | 33–80 |
| AH820 | 5 | 33–81 |
| B4264 | 6 | 31–96 |
| F837/76 | 5 | 28–81 |
| G9842 | 5 | 32–95 |
No., the number of predicted metalloproteinases in B. cereus group strains.
The identity between Bmp1 proteins and predicted metalloproteinases in B. cereus group strains.
In conclusion, we isolated the novel nematicidal virulence factor Bmp1 protein in B. thuringiensis. Bmp1 is toxic to nematodes and also enhances the toxicity of Cry5Ba against nematodes due to its ability to degrade the intestinal tissues of nematodes. We believe that these findings shed new light on the relationship between B. thuringiensis and nematodes.
ACKNOWLEDGMENTS
This study was supported by grants from the National High Technology Research and Development Program (program 863) of China (grant 2011AA10A203), China 948 Program of Ministry of Agriculture (grant 2011-G25), the National Basic Research Program (program 973) of China (grants 2009CB118902 and 2013CB127504), and the National Natural Science Foundation of China (grants 31170047 and 31000020).
Footnotes
Published ahead of print 2 November 2012
REFERENCES
- 1. Bravo A, Likitvivatanavong S, Gill SS, Soberon M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bravo A, SM 2005. Bacillus thuringiensis: mechanisms and use. Comprehensive Mol. Insect Sci. 6:175–205 [Google Scholar]
- 3. Zhang F, Peng D, Ye X, Yu Z, Hu Z, Ruan L, Sun M. 2012. In vitro uptake of 140-kDa Bacillus thuringiensis nematicidal crystal proteins by the second stage juvenile of Meloidogyne hapla. PLoS One 7:e38534 doi:10.1371/journal.pone.0038534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu XY, Ruan LF, Hu ZF, Peng DH, Cao SY, Yu ZN, Liu Y, Zheng JS, Sun M. 2010. Genome-wide screening reveals the genetic determinants of an antibiotic insecticide in Bacillus thuringiensis. J. Biol. Chem. 285:39191–39200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perchat S, Buisson C, Chaufaux J, Sanchis V, Lereclus D, Gohar M. 2005. Bacillus cereus produces several nonproteinaceous insecticidal exotoxins. J. Invertebr Pathol. 90:131–133 [DOI] [PubMed] [Google Scholar]
- 6. Guttmann DM, Ellar DJ. 2000. Phenotypic and genotypic comparisons of 23 strains from the Bacillus cereus complex for a selection of known and putative B. thuringiensis virulence factors. FEMS Microbiol. Lett. 188:7–13 [DOI] [PubMed] [Google Scholar]
- 7. Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. 2010. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 18:189–194 [DOI] [PubMed] [Google Scholar]
- 8. Fang S, Wang L, Guo W, Zhang X, Peng D, Luo C, Yu Z, Sun M. 2009. Bacillus thuringiensis Bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl. Environ. Microbiol. 75:5237–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillemet E, Cadot C, Tran SL, Guinebretiere MH, Lereclus D, Ramarao N. 2010. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 192:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahman J, Johansson T, Olsson M, Punt PJ, van den Hondel CA, Tunlid A. 2002. Improving the pathogenicity of a nematode-trapping fungus by genetic engineering of a subtilisin with nematotoxic activity. Appl. Environ. Microbiol. 68:3408–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang X, Tian B, Niu Q, Yang J, Zhang L, Zhang K. 2005. An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res. Microbiol. 156:719–727 [DOI] [PubMed] [Google Scholar]
- 12. Niu Q, Huang X, Zhang L, Xu J, Yang D, Wei K, Niu X, An Z, Bennett JW, Zou C, Yang J, Zhang KQ. 2010. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc. Natl. Acad. Sci. U. S. A. 107:16631–16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niu Q, Huang X, Zhang L, Li Y, Li J, Yang J, Zhang K. 2006. A neutral protease from Bacillus nematocida, another potential virulence factor in the infection against nematodes. Arch. Microbiol. 185:439–448 [DOI] [PubMed] [Google Scholar]
- 14. Guo S, Liu M, Peng D, Ji S, Wang P, Yu Z, Sun M. 2008. New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl. Environ. Microbiol. 74:6997–7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Z, Bai P, Ye W, Zhang F, Ruan L, Sun M. 2008. A novel negative regulatory factor for nematicidal Cry protein gene expression in Bacillus thuringiensis. J. Microbiol. Biotechnol. 18:1033–1039 [PubMed] [Google Scholar]
- 16. Griffitts JS, Whitacre JL, Stevens DE, Aroian RV. 2001. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293:860–864 [DOI] [PubMed] [Google Scholar]
- 17. Shao Z, Liu Z, Yu Z. 2001. Effects of the 20-kilodalton helper protein on Cry1Ac production and spore formation in Bacillus thuringiensis. Appl. Environ. Microbiol. 67:5362–5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bischof LJ, Huffman DL, Aroian RV. 2006. Assays for toxicity studies in Caenorhabditis elegans with Bt crystal proteins. Methods Mol. Biol. 351:139–154 [DOI] [PubMed] [Google Scholar]
- 19. Finn RD, Clements J, Eddy SR. 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39:W29–W37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fagerlund A, Ween O, Lund T, Hardy SP, Granum PE. 2004. Genetic and functional analysis of the cytK family of genes in Bacillus cereus. Microbiology 150:2689–2697 [DOI] [PubMed] [Google Scholar]
- 22. Narasaki R, Kuribayashi H, Shimizu K, Imamura D, Sato T, Hasumi K. 2005. Bacillolysin MA, a novel bacterial metalloproteinase that produces angiostatin-like fragments from plasminogen and activates protease zymogens in the coagulation and fibrinolysis systems. J. Biol. Chem. 280:14278–14287 [DOI] [PubMed] [Google Scholar]
- 23. Chung MC, Popova TG, Millis BA, Mukherjee DV, Zhou W, Liotta LA, Petricoin EF, Chandhoke V, Bailey C, Popov SG. 2006. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J. Biol. Chem. 281:31408–31418 [DOI] [PubMed] [Google Scholar]
- 24. Tang B, Nirasawa S, Kitaoka M, Marie-Claire C, Hayashi K. 2003. General function of N-terminal propeptide on assisting protein folding and inhibiting catalytic activity based on observations with a chimeric thermolysin-like protease. Biochem. Biophys. Res. Commun. 301:1093–1098 [DOI] [PubMed] [Google Scholar]
- 25. Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 99:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novick RP. 2000. Sortase: the surface protein anchoring transpeptidase and the LPXTG motif. Trends Microbiol. 8:148–151 [DOI] [PubMed] [Google Scholar]
- 27. Peng D, Chai L, Wang F, Zhang F, Ruan L, Sun M. 2011. Synergistic activity between Bacillus thuringiensis Cry6Aa and Cry55Aa toxins against Meloidogyne incognita. Microb. Biotechnol. 4:794–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabashnik BE. 1992. Evaluation of synergism among Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 58:3343–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou J, Li X, Jiang Y, Wu Y, Chen J, Hu F, Li H. 2011. Combined effects of bacterial-feeding nematodes and prometryne on the soil microbial activity. J. Hazard Mater. 192:1243–1249 [DOI] [PubMed] [Google Scholar]
- 30. Ivanova N, Sorokin A, Anderson I, Galleron N, Candelon B, Kapatral V, Bhattacharyya A, Reznik G, Mikhailova N, Lapidus A, Chu L, Mazur M, Goltsman E, Larsen N, D'Souza M, Walunas T, Grechkin Y, Pusch G, Haselkorn R, Fonstein M, Ehrlich SD, Overbeek R, Kyrpides N. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87–91 [DOI] [PubMed] [Google Scholar]
- 31. Gohar M, Okstad OA, Gilois N, Sanchis V, Kolsto AB, Lereclus D. 2002. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784–791 [DOI] [PubMed] [Google Scholar]