Abstract
We previously designed the consensus signal peptide (CSP) and demonstrated that it can be used to strongly stimulate heterologous protein production in Escherichia coli. A comparative study using CSP and two bacterial signal sequences, pelB and ompA, showed that the effect of signal sequences on both expression level and translocation efficiency can be highly protein specific. We report here the generation of CSP mutant libraries by a combinatorial mutagenesis approach. Degenerated CSP oligonucleotides were cloned in frame with the 5′ end of the bla gene, encoding the mature periplasmic β-lactamase released from its native signal sequence. This novel design allows for a direct selection of improved signal sequences that positively affect the expression level and/or translocation efficiency of β-lactamase, based on the ampicillin tolerance level of the E. coli host cells. By using this strategy, 61 different CSP mutants with up to 8-fold-increased ampicillin tolerance level and up to 5.5-fold-increased β-lactamase expression level were isolated and characterized genetically. A subset of the CSP mutants was then tested with the alternative reporter gene phoA, encoding periplasmic alkaline phosphatase (AP), resulting in an up to 8-fold-increased production level of active AP protein in E. coli. Moreover, it was demonstrated that the CSP mutants can improve the production of the medically important human interferon α2b under high-cell-density cultivations. Our results show that there is a clear potential for improving bacterial signal sequences by using combinatorial mutagenesis, and bioinformatics analyses indicated that the beneficial mutations could not be rationally predicted.
INTRODUCTION
Signal sequences target proteins to their site of function, and this has been exploited for the production of a number of recombinant proteins in Escherichia coli (1–4). The periplasmic accumulation of a recombinant protein is known to provide several advantages, including a simplified downstream purification scheme, enhanced biological activity, correct folding, higher product stability and solubility, and the authentic N termini of the mature proteins (5). Inclusion bodies also form in the periplasm (6), and in general there seem to be no strict correlation between the conformational quality of a protein and its solubility state (7). Several proteins have been shown to retain biological activity within periplasmic inclusion bodies (7–10), which could be described as dynamic reservoirs consisting of both active and inactive protein with a variety of conformations (11).
There seems to be a rather flexible consensus, both at the DNA level and at the protein level, for signal sequences targeted to the Sec translocation pathway in E. coli (for reviews, see references 12, 13, and 14) and the choice of signal sequence to ensure effective secretion should therefore be individually tested for each new protein (15–17). Typically, signal sequences are composed of a short, positively charged amino-terminal domain with 1 to 3 basic amino acid residues, followed by a hydrophobic core of 7 to 15 hydrophobic and neutral amino acids (13, 18). A more polar segment is found immediately preceding the cleavage site, which includes small amino acids at positions −3 and −1, forming a so-called Ala-X-Ala box that is recognized and cleaved by signal peptidase I (13, 18).
Recent studies have shown that signal sequences may play a more intricate role in protein expression than previously anticipated. A whole-genome study of the E. coli open reading frames revealed that the highest frequencies of rare codons are found in the signal sequences of secretory proteins (19). An analysis of the amino acid distribution in the E. coli secretory proteins pointed out a bias for the AAA codon (encoding lysine) at the +2 position (20), possibly due to its high rate of translation initiation. The purpose of rare codons in signal sequences is unclear; however, their exchange for more frequent codons has been shown to reduce the mature protein yield of the β-lactamase and the maltose-binding protein (21, 22). In accordance with these reports is a study of the adaptation between the transcript sequence and the tRNA pool, where it was suggested that the order of frequent and rare codons is important and can be utilized by evolution to design the best possible schedule for the ribosomal flow on the transcripts (23). We previously designed the consensus signal peptide (CSP), based on sequence alignments of available bacterial signal sequences, and CSP was demonstrated to function efficiently for translocation of two different heterologous proteins produced in E. coli (17, 24). A functional comparison of CSP with the two frequently used bacterial signal sequences pelB and ompA showed that the choice of signal sequence for effective expression and translocation can be highly protein specific and apparently unpredictable in E. coli.
Directed evolution using random mutagenesis, followed by screening and selection, was originally invented to improve enzymes (25, 26). Among the many techniques used to create random mutant libraries the most commonly used are error-prone PCR (for a review, see reference 27), DNA shuffling (28), and chemical mutagenesis (29, 30). We have previously applied a combinatorial mutagenesis approach involving the use of degenerated oligonucleotides to improve the bacterial expression system Pm-xylS (31–33). By using synthetic oligonucleotide mixtures, the mutagenesis can be focused to specific genetic regions (20 to 100 bp in length), a method that allows for multiple point mutations to be introduced simultaneously. A different approach was applied by Caspers et al. (16) on the amyE signal peptide. By using saturation mutagenesis on the sequence encoding the N domain (amino acids 2 to 7) of amyE, they were able to increase the production yield of their reporter cutinase ∼3-fold (16). We report here the application of combinatorial mutagenesis and the selection of CSP to develop new and improved signal sequences useful for high expression and translocation of heterologous proteins in E. coli. Libraries were constructed by using degenerated oligonucleotide mixtures encoding CSP sequence variants to substitute for the native signal sequence of the periplasmic β-lactamase. By screening these libraries with respect to increased ampicillin tolerance of the E. coli host, a number of improved CSP mutants could be easily selected. The results demonstrate that signal sequences can be improved and optimized for efficient production and translocation of heterologous proteins in E. coli, and the genetic tools and technology developed here should be directly useful to improve any bacterial signal sequence.
MATERIALS AND METHODS
Biological materials, DNA manipulations, and growth conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. E. coli strains were generally grown at 37°C in lysogeny broth (LB) (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl liter−1) or on LA (LB with 15 g of agar liter−1) supplemented with ampicillin (0.2 g liter−1 [unless stated differently]) or kanamycin (0.05 g liter−1) when appropriate. For expression experiments, recombinant cells were grown at 30°C, and induction of the Pm-xylS system was done by adding m-toluic acid as indicated. Standard recombinant DNA procedures were performed as described by Sambrook and Russell (34), and transformations were done according to the RbCl method (New England BioLabs). Plasmids were isolated by using a Qiagen midi kit (Qiagen) or a Wizard Plus SV miniprep kit (Promega). DNA was extracted from agarose gel slabs or PCR mixtures by the QIAquick gel extraction or PCR purification kit (Qiagen). PCRs were run using an Expand high-fidelity PCR system kit (Roche). Custom PCR primers were supplied by Eurofins MWG operon, while spiked oligonucleotide mixes were supplied by Medprobe AS. Site-specific mutagenesis was done by using a QuikChange site-directed mutagenesis kit (Stratagene). DNA sequencing was performed by Eurofins MWG operon.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid(s) | Descriptiona | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Bethesda Research Laboratories |
| RV308 | K-12 derivative used for industrial protein production; lacIq su ΔlacX74 gal IS II::OP308 | ATCC 31608 |
| Plasmids | ||
| pIB11 | RK2-based expression vector containing the Pm-xylS promoter system and bla with is native signal sequence as a reporter gene for Pm; Kmr; 8.1 kb | 32 |
| pGM29CSP | RK2-based vector expressing the CSP-GM-CSF-c-myc-His6 fusion protein from the Pm-xylS promoter system; Apr; 8.8 kb | 17 |
| pTA13 | RK2-based expression vector containing the Pm-xylS promoter system and bla as a reporter gene for Pm; Kmr; 8.1 kb | 31 |
| pLITMUS28 | General cloning vector; Apr; 2.8 kb | New England Biolabs |
| pHOGscFv141-AP-His | A pBluescript II SK(+) derivative expressing the scFv141-AP-His6 fusion protein from the Plac promoter; Apr; 5.1 kb | Affitech AS (unpublished data) |
| pIFN30SpelB | RK2-based vector expressing the codon-optimized pelB-IFN-α2b-c-myc-His6 fusion protein from the Pm/xylS promoter system; Apr; 8.9 kb | 17 |
| pTMB4 | Derivative of pIB11 in which a 0.6-kb BglII-BseRI fragment in the xylS gene was replaced by the corresponding fragment from pTA13, removing an endogenic NcoI site; Kmr; 8.1 kb | This study |
| pTMB5 | Derivative of pTMB4 in which the 1.1-kb XbaI-KpnI fragment was replaced by a 2.1-kb fragment from pGM29CSP; Kmr; 9.2 kb | This study |
| pTMB6 | Derivative of pLITMUS28 in which a 1.4-kb XbaI-SbfI fragment from pGM29CSP was inserted; Apr; 4.2kb. | This study |
| pTMB7 | Derivative of pTMB6 in which a 0.8kb PCR-fragment containing the bla gene generated from pLITMUS28 (see the text) was subcloned into the NcoI-HindIII sites, replacing a 0.5-kb fragment; Apr; 4.5 kb | This study |
| pCSP1bla | RK2-based expression vector containing the Pm-xylS promoter system and bla with the CSP signal sequence as a reporter gene for Pm; Kmr; 9.5 kb | This study |
| pCSP1S1bla–pCSP1S61bla | Derivatives of pCSP1bla in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the S1–S61 mutant sequences; Kmr; 9.5 kb | This study |
| pCSP1Si1bla–pCSP1Si15bla | Derivatives of pCSP1bla in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the Si1–Si15 mutant sequences; Kmr; 9.5 kb | This study |
| pBSP1bla | Derivative of pCSP1bla in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the native β-lactamase signal peptide; Kmr; 9.5 kb | This study |
| pTMB14 | Derivative of pCSP1bla where the NdeI-NcoI CSP fragment was removed, the DNA ends filled by Klenow polymerase and the plasmid circularized; Kmr; 9.4 kb | This study |
| pTMB15 | Derivative of pLITMUS28 in which a 3.2-kb BglII-EcoRI fragment from pTMB14 was inserted; Apr; 5.9 kb | This study |
| pTMB16 | Derivative of pTMB15 where 2 bp in the transition between Pm and bla was removed by site-directed mutagenesis, generating an NdeI site; Apr; 5.9 kb | This study |
| pNSP1bla | Derivative of pCSP1bla encoding the bla gene without its native signal sequence; Kmr; 9.4 kb | This study |
| pCSP1phoA | Derivative of pCSP1bla in which the NcoI-BstAPI fragment encoding the bla reporter gene was replaced by a PCR fragment encoding the mature phoA gene (see the text); Kmr; 10.0 kb | This study |
| pCSP1S2phoA–pCSP1S61phoA | Derivatives of pCSP1phoA in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the S2, S38, S48, S60, and S61mutant sequences; Kmr; 10.0 kb | This study |
| pASP1phoA | Derivative of pCSP1phoA in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the native phoA signal sequence; Kmr; 10.0 kb | This study |
| pCSP2ifn | RK2-based vector expressing CSP-IFNα-2b-c-myc-His6 fusion protein from the Pm-xylS promoter system; trfAcop271; Apr; 8.9 kb | This study |
| pCSP2S2ifn–pCSP2S61ifn | Derivatives of pCSP2ifn in which the NdeI-NcoI fragment encoding CSP was replaced by oligonucleotides encoding the S2 and S61 mutant sequences; Apr; 8.9 kb | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance.
Vector constructions. (i) Vectors with the bla reporter gene.
The plasmid pCSP1bla, used for construction of mutant libraries (Fig. 1A), is based on the vector pIB11 (32) in which the endogenous secretion signal sequence of the bla gene was replaced by the CSP signal sequence from pGM29CSP (17). A series of plasmids were made as intermediates of the final vectors pCSP1bla and pNSP1bla (expressing the bla gene without any signal sequence). Plasmid pTMB4 is a pIB11 derivative in which the endogenous NcoI restriction site in xylS has been removed. It was constructed by replacing the 0.6-kb BglII-BseRI fragment by the corresponding fragment from pTA13 (31). The Pm-CSP-bla expression cassette was then generated in pTMB4 through several intermediates. Plasmid pTMB5 was created by exchanging the 1.1-kb Pm-bla-encoding XbaI-KpnI fragment in pTMB4 by the 2.1-kb Pm-CSP-gm-csf-cmyc-his6 fusion fragment from pGM29CSP. The 1.4-kb XbaI-SbfI fragment from pGM29CSP, containing the Pm-CSP-gm-csf-cmyc-his6 fusion, was inserted into pLITMUS28 (New England BioLabs), creating pTMB6. Plasmid pTMB7 was generated by subcloning the mature part of the bla gene, PCR amplified from pLITMUS28 (primer pair, 5′-ATCCATGGCTCACCCAGAAACGCTG-3′ and 5′-CTCAAGCTTACCAATGCTTAATCAGTGAGGCAC--3′) into the NcoI-HindIII sites of pTMB6, in frame with the CSP sequence, replacing a 0.5-kb fragment (fusion coding for GM-CSF-cMyc-His6). Finally, pCSP1bla was made by replacing the 1.4-kb XbaI-SbfI Pm-CSP-gm-csf-cmyc-his6 fusion fragment of pTMB5 by the 1.8-kb Pm-CSP-bla fragment from pTMB7. pCSP1S1bla to pCSP1S61bla and pCSP1Si1bla to pCSP1Si15bla are pCSP1bla derivatives in which the CSP encoding sequence (NdeI-NcoI) was replaced by annealed, synthetic oligonucleotides corresponding to the various mutant CSP sequences. The pBSP1bla vector was made from pCSP1bla, by replacing the NdeI-NcoI fragment containing the CSP sequence, with annealed oligonucleotides corresponding the native bla signal sequence (5′-TATGAGTATTCAACATTTCCGTGTCGCCCTTATTCCCTTTTTTGCGGCATTTTGCCTTCCTGTTTTCGC-3′ and 5′-CATGGCGAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCA-3′). pTMB14 was constructed by removing the NdeI-NcoI-encoding CSP fragment in pCSP1bla. The DNA ends were filled by Klenow polymerase before the plasmid was circularized. The 3.2-kb BglII-EcoRI fragment from pTMB14 was inserted into pLITMUS28, generating pTMB15, and the 2 bp in the transition between Pm and bla in pTMB15 were replaced by an NdeI site via site-directed mutagenesis using the primers 5′- CAATAATAATGGAGTCATGAACATATGGCTCACCCAGAAAC-3′ and 5′-GTTTCTGGGTGAGCCATATGTTCATGACTCCATTATTATTG-3′, generating pTMB16. Finally, pNSP1bla was constructed by replacing the 3.2-kb BglII-EcoRI fragment in pCSP1bla with the pTMB16 counterpart. All expression vectors constructed were verified by DNA sequencing and established in E. coli DH5α.
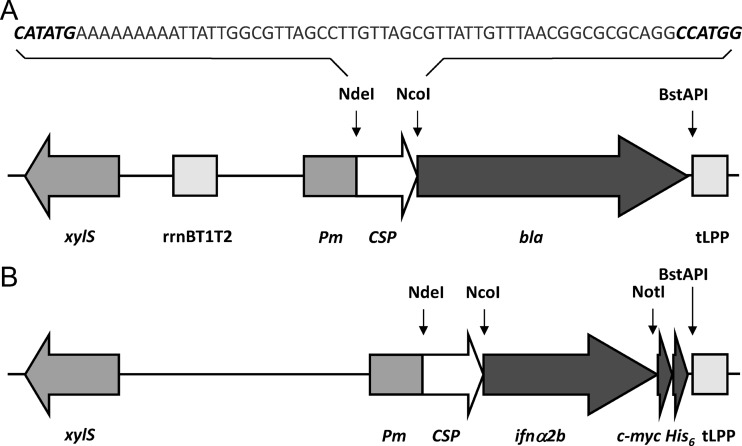
Fig 1.
Schematic representation of the expression related features of the pCSP1bla screening vector (A) and the pCSP2ifn protein expression vector (B). In these vectors, the signal peptide and target genes are easily exchanged using the NdeI/NcoI and NcoI/BstAPI (removing the c-myc-His6 tag) or the NcoI/NotI (preserving the c-myc-His6 tag) restriction sites, respectively. Pm, positively regulated promoter; xylS, gene encoding the Pm activator; bla, ampicillin resistance gene encoding β-lactamase; tLPP, transcriptional terminator; rrnBT1T2, bidirectional transcriptional terminator; ifnα2b, codon optimized gene encoding the human cytokine IFN-α2b. The DNA sequence corresponding to the CSP region is displayed at the top with the unique NdeI and NcoI restriction sites in bold. The vector backbones of pCSP1bla and pCSP2ifn are generally conserved but differ with respect to the selection marker (kan in pCSP1bla and bla in pCSP2ifn), and pCSP2ifn harbors the cop271C mutant of the trfA replication gene, leading to a 3- to 4-fold-increased plasmid copy number (50).
(ii) Vectors with the phoA reporter gene and with the codon-optimized ifn-α2bS gene.
The NcoI-BstAPI fragment in pCSP1bla, encoding the bla gene, was substituted by the phoA gene, giving rise to pCSP1phoA. The enhanced phoA gene used described by Mandecki et al. (35) was PCR amplified from pHOGscFv141-AP-His (unpublished data, kindly provided by Affitech AS, Oslo) with the primers 5′-TTTCCATGGCACGGGCACCAGAAATGCCTGTTC-3′ and 5′-TTTGCACAATGTGCTTATTTCAGCCCCAGAGCGGCTTTC-3′. In the vectors pCSP1S2phoA, pCSP1S38phoA, pCSP1S48phoA, pCSP1S60phoA, and pCSP1S61phoA, the CSP encoding sequence (NdeI-NcoI) was replaced by annealed, synthetic oligonucleotides corresponding to the various mutant CSP sequences. The pASP1phoA control plasmid was constructed by exchanging the NdeI-NcoI fragment encoding the CSP signal sequence in pCSP1phoA by oligonucleotides corresponding to the endogenous phoA signal sequence (36): 5′-TATGAAACAAAGCACTATTGCACTGGCACTCTTACCGTTACTGTTTACCCCTGTGACAAAAGC-3′ and 5′-CATGGCTTTTGTCACAGGGGTAAACAGTAACGGTAAGAGTGCCAGTGCAATAGTGCTTTGTTTCA-3′. This resulted in a construct with two additional amino acids in the fusion site (due to the NcoI site used for cloning). A vector with the CSP sequence fused in frame with the gene encoding the codon-optimized IFN-α2b was constructed by indirectly replacing the pelB signal sequence in pIFN30SpelB (17) by the CSP signal sequence from pGM29CSP generating pCSP2ifn (Fig. 1B). These plasmids have the same vector backbone and only differ with respect to the choice of signal sequence and target gene. For technical reasons, pCSP2ifn was cloned by replacing the NcoI-NotI fragment containing the gm-csf gene in pGM29CSP with the ifn-α2bS gene from pIFN30SpelB and not by interchanging the short signal sequence encoding fragments (NdeI-NcoI) directly. Once established, selected CSP mutant signal sequences were introduced as annealed, synthetic oligonucleotides, replacing the corresponding NdeI-NcoI fragment containing the CSP sequence, generating the pCSP2S2ifn and pCSP2S61ifn plasmids. All plasmids were verified by DNA sequencing and established in E. coli DH5α. The IFN-α2b-expressing vectors were, in addition to DH5α, also established in the E. coli production host strain RV308 to be used for production analysis in high-cell-density cultivations.
Combinatorial mutagenesis for the construction of signal sequence mutant libraries.
To introduce mutations in the CSP coding sequence, a strategy involving degenerated, synthetic oligonucleotides, similar to the protocol previously described for the Pm promoter and the untranslated region, was used (32, 33). Synthetic oligonucleotides were designed to constitute a double-stranded DNA fragment with the CSP sequence and NdeI and NcoI compatible ends when annealed for subsequent easy cloning into the pCSP1bla vector. Eight different nucleotide mixtures, indicated in the oligonucleotide sequences by the numbers 1 to 8, were used to synthesize degenerated oligonucleotide mixtures (MedProbe AS). The probability of keeping the original base in each position was set to 79% (80% for some bases in the silent library), and the other accepted bases were introduced at equal frequencies. The mixtures used were as follows: 1, 79% A + 7% C + 7% G + 7% T; 2, 7% A + 79% C + 7% G + 7% T; 3, 7% A + 7% C + 79% G + 7% T; 4, 7% A + 7% C + 7% G + 79% T; 5, 80% A + 20% G; 6, 80% C + 20% T; 7, 20% A + 80% G; and 8, 20% C + 80% T. A completely randomized CSP library was generated by replacing the original CSP sequence by an oligonucleotide mixture defined by 5′-TATG1111111114414433234413224434413234414434441123323232133C-3′, while a silent CSP library (Si library) was generated from the oligonucleotide mixture defined by 5′-TATGAA5AA5AA58T18T3GC38T1GC28T38T1GC38T18T3TT8AA6GG2GC3CA7GC-3′. The noncoding strands in both libraries were kept complementary to the original CSP sequence (5′-CATGGCCTGCGCGCCGTTAAACAATAACGCTAACAAGGCTAACGCCAATAATTTTTTTTTCA-3′). Wild-type and mutated oligonucleotides were mixed (100 μM each) and phosphorylated using polynucleotide kinase. NaCl was added to a final concentration of 200 mM, and the phosphorylated oligonucleotides were annealed by gradual cooling from 95 to 20°C for 20 min in a thermocycler. Dilutions of the resulting DNA fragments were ligated into the NdeI-NcoI-digested plasmid pCSP1bla, which had been dephosphorylated by calf intestinal phosphatase and purified by using a QIAquick PCR purification kit. The ligated plasmids were transferred into E. coli DH5α using kanamycin as selection. Approximately 137,000 transformants containing random CSP mutants were mixed to constitute the CSP library, while the Si library was a mixture of ∼150,000 transformants.
Selection of CSP mutant libraries in E. coli cells based on increased ampicillin tolerance level.
Mutant strains with increased expression and/or translocation of β-lactamase were selected essentially as described for the Pm high-level expression mutants by Winther-Larsen et al. (37). In brief, 9 ml of LB with kanamycin (0.05 g liter−1) was inoculated with 90 μl of library cell mixture and grown at 37°C for ∼4 h. Then, 10−3 to 10−8 dilutions were plated onto LA containing m-toluic acid (50 to 500 μM) and ampicillin (0.2 to 12 g liter−1) or LA containing kanamycin only. The plates were incubated at 30°C for 2 days. The ampicillin concentration levels tolerated by the candidates identified in the screen were then determined as follow. Individual colonies were inoculated into 100 μl of LB containing kanamycin in 96-well microtiter plates (Nunc), followed by incubation at 30°C overnight. The strain harboring pCSP1bla was included as a wild-type control in a few wells. The cells were diluted twice by a 96-pin replicator into microtiter plates with 100 μl of LB in each well and subsequently spotted onto LA with m-toluic acid (0 to 100 μM) and various ampicillin concentrations (0 to 10 g liter−1). The plates were incubated at 30°C and inspected for growth after 1 and 2 days. The DNA sequence of the signal sequence region was determined for selected candidates, and all of the mutants were reproduced by annealing synthetic oligonucleotides with the different mutant signal sequences and ligating them into the NdeI-NcoI sites of pCSP1bla. Finally, the mutants were again verified by DNA sequencing.
Preparation of crude extracts for enzyme assays and protein analyses.
Recombinant E. coli cells were diluted 100-fold from an overnight culture and grown in selective medium at 37°C. At an optical density at 600 nm (OD600) of 0.1, the cells were induced by 0.1 mM m-toluic acid. The cells were further grown at 30°C for 5 h. Aliquots of cells were harvested by centrifugation (5,000 × g, 10 min, 4°C) and washed once in an assay buffer or 0.9% NaCl, and then the pellets were either kept at −20°C or further processed without storage. Cell extracts for enzyme assays were obtained by resuspending the cell pellets in specific assay buffer, and the cells were lysed by sonication (Branson Sonifier; DSM tip, 3 min on ice, duty cycle, 30%; output control, 2.5). Cell debris was removed by centrifugation at 10,000 × g for 45 min at 4°C.
The β-lactamase assay was performed as previously described (37). Alkaline phosphatase activities were measured by determining the production of p-nitrophenol from p-nitrophenyl phosphate, which was monitored spectrophotometrically at 405 nm. Total volumes of 160 μl in 96-well plates were used, and the assay mixture consisted of 100 mM Tris (pH 9.5), 5 mM MgCl2, 0.1% Tween 20, and 2.7 mM p-nitrophenyl phosphate. Enzymatic assays were performed using enzyme amounts in the linear range. All enzyme assays were carried out in triplicates, and all enzyme activity analyses were repeated at least twice with enzyme extracts obtained from independently grown cultures.
SDS-PAGE and Western blotting was performed essentially as described elsewhere (24), using the primary antibodies Mouse Anti-TetraHis IgG1 BSA-free (Qiagen, catalog no. 34670) diluted 1:7,500 (for His6-tagged proteins) or mouse monoclonal β-lactamase antibody (Abcam) diluted 1:5,000 (for β-lactamase), and the secondary antibody rabbit anti-mouse IgG conjugated to horseradish peroxidase (Dako, catalog no. PO260) diluted 1:1,500. Signals were developed by using 3,3′,5,5′-tetramethylbenzidine. For β-lactamase, the same cell-free protein extracts as in the enzymatic activity assays were applied, while total cell extracts were used in high-cell-density cultivation (HCDC) analyses (see below). Before loading, the HCDC cells were resuspended in SDS-PAGE running buffer and lysed by sonication (Branson sonifier, DSM tip, four × 90 s, with 30-s cooling periods, 30% duty control, and output control at 3.0). Protein concentrations were determined in samples diluted 50- to 200-fold in water by using a Bio-Rad DC protein assay (Bio-Rad Laboratories) as described by the manufacturer, using bovine serum albumin as the reference protein.
In silico analysis of mutant signal sequences.
A selection of available bioinformatic tools was applied to analyze the subset of 12 CSP mutants in a bla context. The 20 amino acids of the signal peptide (CSP or its mutant variant), together with the first 30 amino acids of the β-lactamase protein, were submitted to the SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/ [38]), which predicts the presence and location of signal peptide cleavage sites in amino acid sequences. The net charge of the N domain was found by counting the number of charged amino acids within the first five residues of each signal peptide sequence, with amino acids D and E defined as −1, R and K defined as +1, and any other amino acid defined as 0. The net hydrophobicity was calculated as the sum of hydrophobicities for the amino acids in the hydrophobic core (residues 6 to 17), based on the normalized consensus hydrophobicity scale (47). Translation initiation rates were predicted for the mRNA region −32/+99 (base +1 is the adenine of the translation initiation codon) of the original CSP sequence and the subset of 12 CSP mutant variants using the reverse engineering tool of the RBS calculator website (https://salis.psu.edu/software/doReverseRBS [39]), with input settings selected for the model organism E. coli K-12 substrain DH10B. The CSP wild-type mRNA sequence was (the translation initiation codon is underlined): 5′-AACAGAAACAATAATAATGGAGTCATGAACATATGAAAAAAAAATTATTGGCGTTAGCCTTGTTAGCGTTATTGTTTAACGGCGCGCAGGCCATGGCTCACCCAGAAACGCTGGTGAAAGTAAAAGATGCT-3′. The direct output of the RBS calculator is a list of start codons in the mRNA transcript, and their predicted translation initiation rates on a relative scale from <1 to 100,000+. In the present study, the values ranged from 522.82 (S46) to 9316.36 (S7), and the best score was generally associated with the position 32 start codon, which correlates to the true translation initiation site. To simplify data comparison, relative translation initiation rates were calculated, and the arbitrary number was set to 1 for the CSP sequence result. For all of the sequences investigated, the calculations were reported to not be at equilibrium, meaning that the mRNA was predicted to contain long-range nucleotide base pairings that are unlikely to form within the relevant time scale. Extending the coding region sequence beyond the length described here did not have any effect on the predictions of the translation initiation rate. The same RNA sequences were submitted to the RNAfold web server of the Vienna RNA package (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi [40]) for calculations of the minimum free energy (kcal mol−1) of the secondary structure of single-stranded RNA.
HCDC.
HCDC of E. coli RV308 cells harboring pIFN30SpelB, pCSP2ifn, pCSP2S2ifn, or pCSP2S61ifn were performed by automatically controlled fed-batch cultivation in DASGIP_1L fermentors. The defined preculture medium and the main culture medium used were composed as described elsewhere (41), whereas feeding solution 1 (FS1) was slightly modified and contained glucose (500 g kg−1 solution) and MgSO4·7H2O (15.8 g kg−1 solution). All media were supplemented with ampicillin (0.1 g liter−1). Inducer solution (0.5 M) was prepared by dissolving m-toluic acid in ethanol.
The fermentation precultures were prepared as described previously (24). From these cultures, cells were used to inoculate 0.35 liter of prewarmed main culture medium in the fermentor to an initial OD600 of 0.05. All fermentations were performed at 30°C, and the pH was maintained at 6.8 by the addition of 12.5% (vol/vol) NH3. Antifoam (Adecanol LG-109; Asahi Denka Kogyo, Japan) was added from the start (50 μl liter−1) and thereafter when needed. The airflow rate was 0.5 liter per liter of medium per min. The dissolved oxygen was maintained at 20% saturation by automatic adjustments of the stirrer speed (up to 1,200 rpm) and gradual oxygen enrichment at a maximum stirring rate (up to 50%). Inducer (m-toluic acid, 1 mM) was added to the fermentors when the cell growth reached an OD600 of 100.
The fermentations were divided into three different phases as follows. (i) In the batch phase, glucose (25 g liter−1) was included in the freshly inoculated main culture medium, and cell growth continued until all of the sugar had been consumed. (ii) In the exponential feeding phase, growth was controlled to a specific growth rate (μ) of 0.25 h−1 by feeding with FS1. The feed rate of FS1 was initially set to 18.7 g liter−1 h−1 and thereafter was increased gradually to 45 g liter−1 h−1. (iii) In the induction phase, bacterial growth was controlled by automatic adjustments of the airflow oxygen concentration at a constant feed rate of FS1 of 45 g liter−1 h−1 so that the dissolved oxygen and stirring speed were maintained at 20% saturation and 1,200 rpm, respectively. Throughout the fermentations, pH, dissolved oxygen, feeding rate, airflow, and the molar fraction of CO2 in the exhausted gas were monitored and recorded.
RESULTS AND DISCUSSION
Construction of the pCSP1bla vector, which can be used as an effective selection tool for improved signal sequences.
We previously demonstrated that the bla gene, encoding periplasmic β-lactamase, can be used as a valuable reporter in E. coli enabling direct selection based on the ampicillin tolerance levels of recombinant cells in response to different bla expression levels (31–33). In pCSP1bla the native endogenous signal sequence of the bla gene is replaced by CSP in such a way that this new sequence can easily be replaced by any CSP variant in a one-step cloning procedure (Fig. 1). Accordingly, pCSP1bla should allow for direct selection of CSP mutants conferring an increased expression level of the bla gene and/or increased translocation efficiency of the β-lactamase protein. As controls, we constructed the analogous vectors pBSP1bla (containing the bla gene with its native signal sequence) and pNSP1bla (containing the bla gene without a signal sequence).
E. coli DH5α (pCSP1bla) was able to grow on solid media with ampicillin concentrations up to 1.0 g liter−1 under induced conditions (Fig. 2), while the corresponding control strains containing pBSP1bla or pNSP1bla were able to grow in the presence of ampicillin concentrations up to 0.2 g liter−1 and 5 mg liter−1, respectively. Thus, substitution of the native signal secretion sequence with CSP in itself leads to a 5-fold-higher ampicillin tolerance of the host, and the results also confirmed that the bla gene needs a signal sequence to be efficiently produced and translocated to the periplasm, in agreement with previous reports (42–44).
Fig 2.
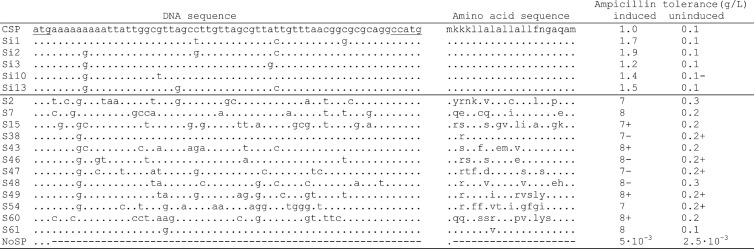
DNA and amino acid sequences of the original CSP and selected mutant variants. The corresponding ampicillin tolerance levels were obtained from spotting E. coli DH5α-strains harboring pCSP1bla and its derivatives with the 5 best silent CSP mutants and the 12 best random CSP mutants onto LA containing either ampicillin and m-toluic acid (20 μM, induced) or ampicillin only (uninduced). Ampicillin tolerance of DH5α (pNSP1bla) is included as a control (no signal sequence [NoSP]). Periods (.) indicate that the base or amino acid is the same as in the unmodified CSP sequence, while “–” indicates gaps in the sequence (in pNSP1bla). “+” behind the ampicillin concentration given indicates that poor growth was observed at even higher concentrations, while a “–” shows that the growth was reduced at the concentration indicated.
CSP variants selected from a completely randomized CSP mutant library give rise to up to 8-fold-increased ampicillin tolerance level of the host cells.
A completely randomized CSP library was constructed in pCSP1bla, using degenerated oligonucleotides, and all nucleotides between the NdeI and NcoI restriction sites were allowed to vary. The resulting library from ∼137,000 colonies, growing on LA-Km plates, was screened for mutants able to grow in the presence of elevated ampicillin concentrations (6 to 12 g liter−1) under Pm-XylS-induced conditions (20 to 500 μM m-toluic acid). Candidates with 6- to 8-fold-increased ampicillin tolerance compared to the pCSP1bla-carrying cells were easily identified and, in order to make sure that the phenotypes were actually caused by mutations in pCSP1bla, plasmid DNA was isolated from promising candidates and retransferred into DH5α cells and functionally confirmed. In this way, a total of 61 different CSP mutants causing up to 8-fold-increased ampicillin tolerance levels of the cells were isolated. DNA sequencing revealed that the average number of point mutations was 8, and they were typically randomly distributed throughout the CSP coding region. Among these, the 12 most promising CSP mutants were selected based on their overall characteristics using different combinations of inducer and ampicillin concentrations (data not shown), and the plasmids were then resynthesized to ensure that the observed phenotypes are solely caused by the identified CSP mutations. The DNA and amino acid sequences, as well as the ampicillin tolerance profiles of these mutants, are summarized in Fig. 2.
An alternative codon optimization strategy of CSP resulted in an only 2-fold-increased ampicillin tolerance level of the host cells.
Recent studies have highlighted the role of local mRNA folding (45) and a codon ramp (23) for optimizing heterologous gene expression. Consequently, the introduction of alternative codons at the 5′ coding sequence of a recombinant gene may potentially have a great influence on its expression level. Therefore, we next chose to generate an alternative library in pCSP1bla (designated the Si library) that contained only silent mutations in the CSP coding sequence and thus should not affect the function of CSP in translocation. The resulting recombinant cells were tested for ampicillin tolerance on solid medium under Pm-XylS-induced conditions (20 μM m-toluic acid) such as for the CSP random library. The Si library contained ∼150,000 clones, and 14 promising candidates were isolated that could tolerate an up to ∼2-fold-higher ampicillin concentration (1.9 g liter−1) compared to those containing the original CSP sequence (see Fig. 2 for the five best Si mutants). Based on these results, it seemed clear that silent mutations in CSP can, to a certain degree, contribute to increased expression levels of the reporter gene. However, these improvements were moderate compared to when completely randomizing CSP (see above), and we therefore chose not to characterize these Si mutants any further.
The production level of active β-lactamase protein is up to 5.5-fold higher in the CSP mutants compared to when using the original CSP.
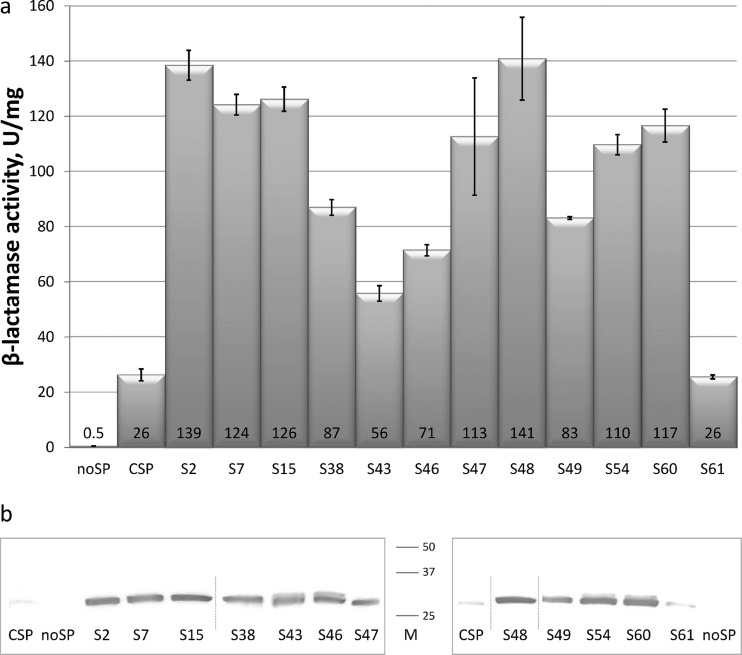
To investigate whether the observed improved ampicillin tolerance levels were truly accompanied by a concomitant increase in the β-lactamase production levels in the cells, the β-lactamase activities were measured spectrophotometrically in cell extracts from the 12 best CSP mutants. The strains DH5α (pCSP1bla) and DH5α (pNSP1bla) were included as controls. The results showed that the mutants displayed high and different β-lactamase activity profiles (Fig. 3a). For most of them, the β-lactamase activity and host ampicillin tolerance level correlated well, but mutant S61 displayed the same β-lactamase activity as when the original CSP sequence was used. One possible way of explaining this would be to assume that this mutant affects the translocation rate without affecting the total expression level of the β-lactamase, which would also have to imply that such a change affects the host tolerance to ampicillin under the conditions tested. It has previously been reported that increasing the hydrophobicity of the signal peptide can lead to reduced processing and the release of soluble β-lactamase in E. coli (46). Although we cannot rule out that this could be the case for S61, it seems unlikely since this particular mutant (like S38) contains only a single point mutation, leading to an alanine-to-valine substitution (Table 2), which only results in an insignificant increase in the net hydrophobicity compared to CSP. The net hydrophobicity of mutant S61 is also considerably lower than for the mutants S54 and S48 (see below), which were among the mutants displaying the highest β-lactamase activities in the assay. We also analyzed the extracts for soluble protein by Western blots to unravel any potential variation between the β-lactamase production level and the corresponding enzymatic activity (Fig. 3b). The results showed that several of the CSP mutants caused an at least 8-fold-increased β-lactamase production level compared to CSP, and there was generally a good correlation between the enzyme amount and the corresponding activity level. The data also confirmed that CSP mutant S61 gives rise to a similar amount of β-lactamase produced as the original CSP sequence, in agreement with the corresponding activity data. For some CSP mutants (e.g., S43 and S46), we noticed the appearance of faint bands presumably representing uncleaved cytoplasmic products (see also below). However, it cannot be ruled out that they could be inner membrane-bound precursors that were released into the soluble fraction due to cell lysis. Whether these are active proteins remained unknown.
Fig 3.
β-Lactamase production in E. coli DH5α strains harboring the plasmids pNSP1bla (no signal peptide [noSP]), pCSP1bla (CSP), and the 12 different plasmids pCSP1S2bla-pCSP1S61bla (CSP mutants S2 to S61). Gene expression was induced by 0.1 mM m-toluic acid. (a) Specific β-lactamase activities measured from cell extracts. 1 U = the amount of enzyme that catalyzes the transformation of 1 μmol of substrate min−1. The results are the averages of two biological replicas performed in triplicates, and the standard errors are indicated. (b) Western blot detection of soluble β-lactamase in the same extracts as in panel a. The same total protein amount was applied in all wells. The sizes of the mature and the uncleaved β-lactamase proteins are approximately 29 and 32 kDa, respectively. The two boxes show independent blots; vertical lines within the boxes indicate that one or more samples have been removed from the data set. The M column represents the migration of ladder proteins of sizes 25, 37, and 50 kDa, respectively.
Table 2.
Bioinformatics analysis of selected CSP random mutants: predicted signal sequence properties and translational attributes
| Mutant | Amino acid sequencea | D scoreb | Cleavage siteb | Net charge of N domainc | Net hydrophobicity of H domaind | Relative translation rate prediction (AU)e | Minimum free energy (kcal mol−1)f |
|---|---|---|---|---|---|---|---|
| CSP | mkkkllalallallfngaqam | 0.875 | AQA-MA | +3 | 9.11 | 1.00 | –29.84 |
| S2 | .yrnk.v...c...l..p... | 0.804 | AMA-HP | +2 | 8.67 | 0.53 | –31.40 |
| S7 | .qe..cq...i.......e.. | 0.520 | AMA-HP* | 0 | 7.19 | 2.57 | –28.80 |
| S15 | .rs...s.gv.li.a..gk.. | 0.682 | AMA-HP | +2 | 8.38 | 1.09 | –28.60 |
| S38 | ..r.................. | 0.877 | AQA-MA | +3 | 9.11 | 1.00 | –29.84 |
| S43 | ..s..f..em.v......... | 0.678 | AMA-HP | +2 | 7.92 | 0.99 | –24.24 |
| S46 | ..rs..s....e......... | 0.671 | AMA-HP | +2 | 6.95 | 0.14 | –26.50 |
| S47 | ..rtf.d.....s..s..... | 0.689 | AMA-HP | +2 | 6.95 | 0.58 | –27.20 |
| S48 | ..r...v.....v....eh.. | 0.725 | AMA-HP | +3 | 9.59 | 0.33 | –27.94 |
| S49 | ..r....i...rvsly..... | 0.707 | AMA-HP | +3 | 5.97 | 0.33 | –28.64 |
| S54 | ..r.ff.vt.i.gfgi..... | 0.615 | AMA-HP | +3 | 9.91 | 1.64 | –25.80 |
| S60 | .qq..ssr...pv.lys.... | 0.666 | AMA-HP | +1 | 4.18 | 0.87 | –26.10 |
| S61 | .......v............. | 0.884 | AQA-MA | +3 | 9.13 | 1.00 | –29.74 |
Periods (.) indicate that the amino acid is identical to the one in CSP at this position.
Calculated by SignalP 4.0 (38). S7 did not meet the cutoff criteria. *, the reported cleavage site gave the best score.
Calculated for the first five residues, with amino acids D and E defined as −1, R and K defined as +1 and any other amino acid as 0.
Calculated as the sum of hydrophobicity for the hydrophobic region (residues 6 to 17), based on the normalized consensus hydrophobicity scale (47).
Calculated by the RBS calculator v1.1, reverse engineering mode (39). AU, arbitrary unit(s).
Minimum free energy predicted by RNAfold (40) for the optimal secondary structure of the region surrounding the translation initiation site. The analyzed sequence included 32 bp of the nontranslated region, the signal sequence, and 36 bp of the mature bla coding sequence.
Bioinformatics analyses of the improved CSP mutants indicate that they could not be rationally predicted.
We next analyzed our 12 selected CSP mutants by using available bioinformatics methods (see Materials and Methods) and the results are summarized in Table 2. With the exception of S7, CSP and the remaining CSP mutants were predicted by the SignalP 4.0 server (38) to be signal sequences that are cleaved off between amino acids 20 and 21 (AQA-MA; CSP, S38, and S61) or between amino acids 22 and 23 (AMA-HP; S2, [S7], S15, S46 to S49, S54, and S60). The net charge of the N domain varied among the signal sequences, with a maximum of +3 (seen for several mutants and the original CSP) and a minimum of 0 (S7). The net hydrophobicity of the hydrophobic core also showed a high variation within the data set and was in particularly low for S60 (4.18) and S49 (5.97) and high for the mutants S54 (9.91), S48 (9.59), S61 (9.13), and S38 (9.11) and the original CSP (9.11). The translational initiation rates for the −32/+99 mRNA region, predicted by the RBS calculator (39), and the minimum free energy scores of the mRNA secondary structures determined by the RNAfold web server (40) emphasize that our collection of signal sequences varies considerably. In general, there seems to be no apparent correlation between the predicted translation rates and the minimum free energy scores of these signal sequences, and these data should imply that rational methods would not be a feasible strategy to generate improved CSP mutants.
CSP mutants can also be used for efficient expression and translocation of the alternative model protein AP.
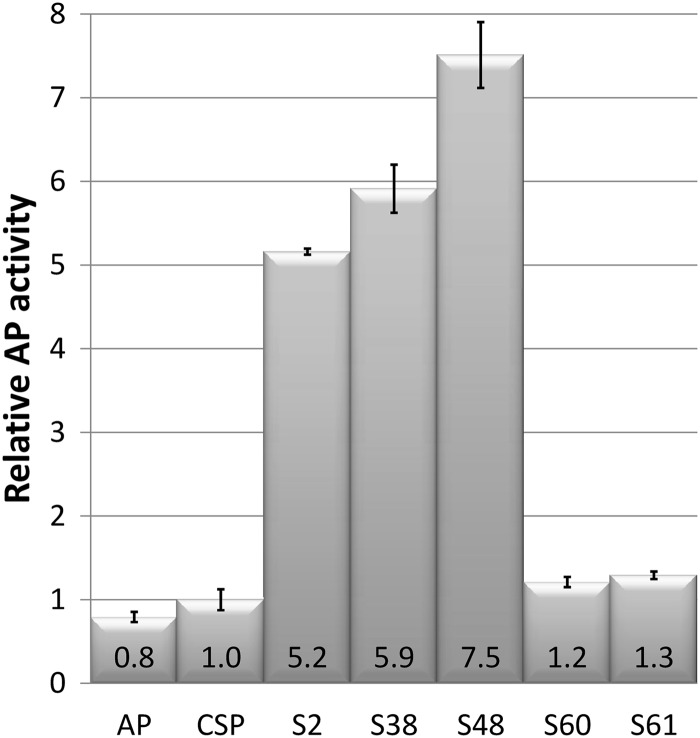
It was of interest to investigate whether the CSP mutants could also be useful for efficient expression and translocation of alternative heterologous proteins in E. coli. As a first model in these studies, we used alkaline phosphatase (AP), encoded by the phoA gene, partly because AP must be translocated to the periplasm to be active (48, 49). The pCSP1phoA plasmid was constructed containing the phoA gene under the control of Pm-XylS, and with the phoA native signal sequence replaced by CSP. As a control, we constructed pASP1phoA in which phoA is expressed with its native secretion signal sequence. E. coli DH5α (pCSP1phoA) and E. coli DH5α (pASP1phoA) cells were grown in shake flask cultures, harvested, and assayed for AP activity, and the results clearly showed that CSP could substitute for the native phoA signal sequence by leading to a similar production level of active AP protein (Fig. 4). Next, the CSP region of plasmid pCSP1phoA was replaced by a subset of the 12 CSP variants, S2, S38, S48, S60, and S61, yielding plasmids pCSP1S2phoA, pCSP1S38phoA, pCSP1S48phoA, pCSP1S60phoA, and pCSP1S61phoA, respectively. All plasmids were established in E. coli DH5α, and the resulting recombinant strains were subjected to AP production analyses as described above. The mutants S2, S38, and S48 displayed 5- to 8-fold-increased AP activities compared to CSP, which were in good agreement with the analogous data obtained when β-lactamase was used as the reporter protein (see Fig. 3). The CSP mutants S60 and S61 generated AP activities in the same range as when the original CSP was used (Fig. 4). For S61, this correlated well with the analogous β-lactamase production results (Fig. 3), while it was not the case for S60, which caused an ∼5-fold-increased β-lactamase production level compared to CSP. This may indicate some context dependency between the mutant CSP signal sequence and the recombinant gene with respect to efficient expression and concomitant product translocation, in agreement with previous results (17). Mutant S38 contains only one amino acid substitution (Lys to Arg) compared to the original CSP (Fig. 2), and this particular substitution is also present in CSP mutants S2 and S48. CSP mutants S60 and S61 do not harbor this substitution, which presumably has a positive effect on the production of active β-lactamase and AP proteins under the conditions tested. Summarized, these results demonstrated that CSP mutants can also be highly useful for the effective production of alternative translocated proteins in E. coli.
Fig 4.
Relative AP activity of E. coli DH5α strains harboring relevant expression constructs. All values are relative to the CSP result, which was arbitrarily set to 1. AP, endogenous AP signal sequence (pASP1phoA construct); CSP, original sequence (pCSP1phoA construct), while S2, S38, S48, S60, and S61 indicate the variant CSP sequences in pCSP1S2phoA, pCSP1S38phoA, pCSP1S48phoA, pCSP1S60phoA, and pCSP1S61phoA, respectively. Cultures were induced by 0.1 mM m-toluic acid. All results are the averages of two biological replicas with at least three samples, and the standard errors are indicated.
CSP mutants S2 and S61 can be used for efficient recombinant production of the human cytokine IFN-α2b under high-cell-density cultivation conditions.
We previously demonstrated that recombinant production under HCDC of the human cytokine α2b (IFN-α2b), encoded by a synthetic and codon-optimized gene (ifn-α2bS), was dramatically stimulated when fused to the pelB signal sequence, yielding ∼0.6 g liter−1 (17). Most of the product was insoluble protein, and in the absence of a pelB signal, no detectable IFN-α2b protein was produced. It was here of interest to test whether CSP mutants could be useful for the efficient production of IFN-α2b under HCDC, and both the original CSP and its mutants S2 and S61 were used to substitute for pelB in plasmid pIFN30SpelB (17), yielding plasmids pCSP2ifn (Fig. 1B), pCSP2S2ifn, and pCSP2S61ifn, respectively. S2 was chosen based on the general good production results obtained with both Bla and PhoA (see above), while S61 was chosen based on the assumption that it might specifically confer efficient protein translocation (see above). The plasmids were transferred into the production host E. coli RV308, and the resulting recombinant strains were analyzed for IFN-α2b production under HCDC by Western blotting. Initial analyses of crude extracts showed that the IFN-α2b protein was mainly detected in the insoluble fraction (data not shown) in agreement with previous observations (34), and total cell extracts were therefore used here to compare the total production levels in the different strains. Based on visual inspection of the Western blot (Fig. 5), it appeared that CSP gave production levels approximately similar to those with pelB, while CSP mutants S2 and S61 caused at least 2-fold-higher IFN-α2b production levels. We noticed that the protein products appeared in two slightly different sizes among the strains, presumably reflecting the cleavage status of the different signal peptides. The proteins expressed with pelB and mutant S2 seemed dominantly to yield an uncleaved version of the protein, while the original CSP gave a mixture of cleaved and uncleaved product. When mutant S61, on the other hand, was used, the protein product was predominantly cleaved off, and the biological reasons for these discrepancies remained unknown. These results indicated that IFN-α2b formed inclusion bodies also when being translocated to the periplasm. In any case, since all IFN-α2b protein produced by any recombinant strain tested here remained as insoluble protein, we chose to not investigate this any further. Summarized, these results show that CSP mutants have a potential for increasing the expression of difficult-to-express proteins, such as IFN-α2b, which even in a codon-optimized variant seems to be highly dependent on a signal sequence to be recombinantly expressed in E. coli (17).
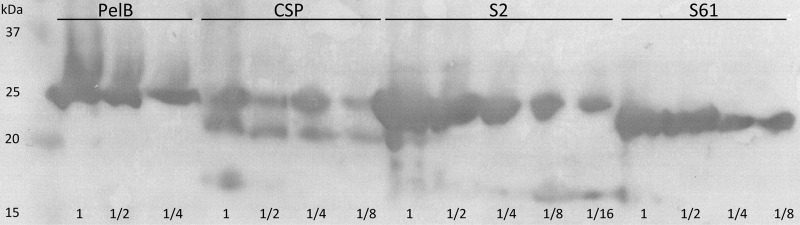
Fig 5.
Western blot analysis of E. coli RV308 strains (pIFN30SpelB, pCSP2ifn, pCSP2S2ifn, or pCSP2S61ifn) producing IFN-α2b fusion proteins with different secretion signal peptides in high-cell-density cultivations. Cell samples were withdrawn from each culture 6.3 h after induction. The cells were lysed by sonication, and the total crude cell extracts were subjected to analysis. Dilution series of decreasing total protein amount were loaded for each sample and are shown relative to the highest total protein concentration loaded (set arbitrarily to 1). Signal peptide–IFN-α2b–cMyc–His6 is 24.4 kDa, while the protein complex in which a signal peptide has been cleaved off is 22.4 kDa.
Concluding remarks.
We presented here an approach enabling the effective selection of improved signal sequences from large randomized mutant libraries by directly scoring for increased antibiotic tolerance levels of the E. coli host cells. Our previously designed signal sequence CSP was used as a model, and we show that this method enables selection of CSP mutants causing increased expression level and improved translocation of presumably any recombinant protein in E. coli. Our results support and extend previous reports demonstrating that signal sequences can be highly useful in promoting high-level recombinant production of heterologous proteins that are generally difficult to express in E. coli. Bioinformatics analyses of the CSP mutants indicated that they could not have been predicted by rational methods, further emphasizing the advantage of this combinatorial mutagenesis and selection approach for the generation of superior signal sequences useful for recombinant expression in bacteria.
ACKNOWLEDGMENTS
This study was supported by a grant from the Research council of Norway.
We thank Lihua Yu, Randi Aune, and Tone Haugen for valuable technical assistance.
Footnotes
Published ahead of print 9 November 2012
REFERENCES
- 1. Choi JH, Lee SY. 2004. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl. Microbiol. Biotechnol. 64:625–635 [DOI] [PubMed] [Google Scholar]
- 2. Esposito D, Chatterjee DK. 2006. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 17:353–358 [DOI] [PubMed] [Google Scholar]
- 3. Jonet MA, Mahadi NM, Murad AMA, Rabu A, Bakar FDA, Rahim RA, Low KO, Illias RM. 2012. Optimization of a heterologous signal peptide by site-directed mutagenesis for improved secretion of recombinant proteins in Escherichia coli. J. Mol. Microbiol. Biotechnol. 22:48–58 [DOI] [PubMed] [Google Scholar]
- 4. Mergulhão FJM, Summers DK, Monteiro GA. 2005. Recombinant protein secretion in Escherichia coli. Biotechnol. Adv. 23:177–202 [DOI] [PubMed] [Google Scholar]
- 5. Makrides SC. 1996. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Rev. 60:512–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baneyx F, Mujacic M. 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22:1399–1408 [DOI] [PubMed] [Google Scholar]
- 7. González-Montalbán N, García-Fruitós E, Villaverde A. 2007. Recombinant protein solubility: does more mean better? Nat. Biotechnol. 25:718–720 [DOI] [PubMed] [Google Scholar]
- 8. Arié JP, Miot M, Sassoon N, Betton JM. 2006. Formation of active inclusion bodies in the periplasm of Escherichia coli. Mol. Microbiol. 62:427–437 [DOI] [PubMed] [Google Scholar]
- 9. García-Fruitós E, González-Montalbán N, Morell M, Vera A, Ferraz RM, Arís A, Ventura S, Villaverde A. 2005. Aggregation as bacterial inclusion bodies does not imply inactivation of enzymes and fluorescent proteins. Microb. Cell Fact. 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stampolidis P, Kaderbhai NN, Kaderbhai MA. 2009. Periplasmically exported lupanine hydroxylase undergoes transition from soluble to functional inclusion bodies in Escherichia coli. Arch. Biochem. Biophys. 484:8–15 [DOI] [PubMed] [Google Scholar]
- 11. Ventura S, Villaverde A. 2006. Protein quality in bacterial inclusion bodies. Trends Biotechnol. 24:179–185 [DOI] [PubMed] [Google Scholar]
- 12. Dalbey RE, Wang P, van Dijl JM. 2012. Membrane proteases in the bacterial protein secretion and quality control pathway. Microbiol. Mol. Biol. Rev. 76:311–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Natale P, Brüser T, Driessen AJ. 2008. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane: distinct translocases and mechanisms. Biochim. Biophys. Acta 1778:1735–1756 [DOI] [PubMed] [Google Scholar]
- 14. Rusch SL, Kendall DA. 2007. Interactions that drive Sec-dependent bacterial protein transport. Biochemistry 46:9665–9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. 2006. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J. Mol. Biol. 362:393–402 [DOI] [PubMed] [Google Scholar]
- 16. Caspers M, Brockmeier U, Degering C, Eggert T, Freudl R. 2010. Improvement of Sec-dependent secretion of a heterologous model protein in Bacillus subtilis by saturation mutagenesis of the N-domain of the AmyE signal peptide. Appl. Microbiol. Biotechnol. 86:1877–1885 [DOI] [PubMed] [Google Scholar]
- 17. Sletta H, Tøndervik A, Hakvåg S, Aune TEV, Nedal A, Aune R, Evensen G, Valla S, Ellingsen TE, Brautaset T. 2007. The presence of N-terminal secretion signal sequences leads to strong stimulation of the total expression levels of three tested medically important proteins during high-cell-density cultivations of Escherichia coli. Appl. Environ. Microbiol. 73:906–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Auclair SM, Bhanu MK, Kendall DA. 2012. Signal peptidase I: cleaving the way to mature proteins. Protein Sci. 21:13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Power PM, Jones RA, Beacham IR, Bucholtz C, Jennings MP. 2004. Whole genome analysis reveals a high incidence of non-optimal codons in secretory signal sequences of Escherichia coli. Biochem. Biophys. Res. Commun. 322:7. [DOI] [PubMed] [Google Scholar]
- 20. Zalucki YM, Power PM, Jennings MP. 2007. Selection for efficient translation initiation biases codon usage at second amino acid position in secretory proteins. Nucleic Acids Res. 35:5748–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zalucki YM, Gittins KL, Jennings MP. 2008. Secretory signal sequence non-optimal codons are required for expression and export of β-lactamase. Biochem. Biophys. Res. Commun. 366:135–141 [DOI] [PubMed] [Google Scholar]
- 22. Zalucki YM, Jones CE, Ng PSK, Schulz BL, Jennings MP. 2010. Signal sequence non-optimal codons are required for the correct folding of mature maltose binding protein. Biochim. Biophys. Acta 1798:1244–1249 [DOI] [PubMed] [Google Scholar]
- 23. Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y. 2010. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141:344–354 [DOI] [PubMed] [Google Scholar]
- 24. Sletta H, Nedal A, Aune TEV, Hellebust H, Hakvåg S, Aune R, Ellingsen TE, Valla S, Brautaset T. 2004. Broad-host-range plasmid pJB658 can be used for industrial-level production of a secreted host-toxic single-chain antibody fragment in Escherichia coli. Appl. Environ. Microbiol. 70:7033–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hibbert E, Dalby P. 2005. Directed evolution strategies for improved enzymatic performance. Microb. Cell Fact. 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan L, Kurek I, English J, Keenan R. 2005. Laboratory-directed protein evolution. Microbiol. Mol. Biol. Rev. 69:373–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pritchard L, Corne D, Kell D, Rowland J, Winson M. 2005. A general model of error-prone PCR. J. Theor. Biol. 234:497–509 [DOI] [PubMed] [Google Scholar]
- 28. Stemmer WPC. 1994. Rapid evolution of a protein in vitro by DNA shuffling. Nature 370:389–391 [DOI] [PubMed] [Google Scholar]
- 29. Encell LP, Coates MM, Loeb LA. 1998. Engineering human DNA alkyltransferases for gene therapy using random sequence mutagenesis. Cancer Res. 58:1013–1020 [PubMed] [Google Scholar]
- 30. Lai Y-P, Huang J, Wang L-F, Li J, Wu Z-R. 2004. A new approach to random mutagenesis in vitro. Biotechnol. Bioeng. 86:622–627 [DOI] [PubMed] [Google Scholar]
- 31. Aune TEV, Bakke I, Drabløs F, Lale R, Brautaset T, Valla S. 2010. Directed evolution of the transcription factor XylS for development of improved expression systems. Microb. Biotechnol. 3:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakke I, Berg L, Aune TE, Brautaset T, Sletta H, Tøndervik A, Valla S. 2009. Random mutagenesis of the PM promoter as a powerful strategy for improvement of recombinant-gene expression. Appl. Environ. Microbiol. 75:2002–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berg L, Lale R, Bakke I, Burroughs N, Valla S. 2009. The expression of recombinant genes in Escherichia coli can be strongly stimulated at the transcript production level by mutating the DNA-region corresponding to the 5′-untranslated part of mRNA. Microb. Biotechnol. 2:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35. Mandecki W, Shallcross MA, Sowadski J, Tomazic-Allen S. 1991. Mutagenesis of conserved residues within the active site of Escherichia coli alkaline phosphatase yields enzymes with increased kcat. Protein Eng. 4:801–804 [DOI] [PubMed] [Google Scholar]
- 36. Kikuchi Y, Yoda K, Yamasaki M, Tamura G. 1981. The nucleotide sequence of the promoter and the amino-terminal region of alkaline phosphatase structural gene (phoA) of Escherichia coli. Nucleic Acids Res. 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winther-Larsen HC, Blatny JM, Valand B, Brautaset T, Valla S. 2000. Pm promoter expression mutants and their use in broad-host-range RK2 plasmid vectors. Metab. Eng. 2:92–103 [DOI] [PubMed] [Google Scholar]
- 38. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786 [DOI] [PubMed] [Google Scholar]
- 39. Salis HM, Mirsky EA, Voigt CA. 2009. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27:946–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. 2008. The Vienna RNA Websuite. Nucleic Acids Res. 36:W70–W74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horn U, Strittmatter W, Krebber A, Knüpfer U, Kujau M, Wenderoth R, Müller K, Matzku S, Plückthun A, Riesenberg D. 1996. High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl. Microbiol. Biotechnol. 46:524–532 [DOI] [PubMed] [Google Scholar]
- 42. Kadonaga JT, Gautier AE, Straus DR, Charles AD, Edge MD, Knowles JR. 1984. The role of the beta-lactamase signal sequence in the secretion of proteins by Escherichia coli. J. Biol. Chem. 259:2149–2154 [PubMed] [Google Scholar]
- 43. Smith H, Bron S, van Ee J, Venema G. 1987. Construction and use of signal sequence selection vectors in Escherichia coli and Bacillus subtilis. J. Bacteriol. 169:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith H, de Jong A, Bron S, Venema G. 1988. Characterization of signal-sequence-coding regions selected from the Bacillus subtilis chromosome. Gene 70:351–361 [DOI] [PubMed] [Google Scholar]
- 45. Kudla G, Murray AW, Tollervey D, Plotkin JB. 2009. Coding-sequence determinants of gene expression in Escherichia coli. Science 324:255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Dijl JM, de Jong A, Vehmaanpera J, Venema G, Bron S. 1992. Signal peptidase I of Bacillus subtilis: patterns of conserved amino acids in prokaryotic and eukaryotic type I signal peptidases. EMBO J. 11:2819–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eisenberg D, Schwarz E, Komaromy M, Wall R. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125–142 [DOI] [PubMed] [Google Scholar]
- 48. Hoffman CS, Wright A. 1985. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc. Natl. Acad. Sci. U. S. A. 82:5107–5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Manoil C, Beckwith J. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. U. S. A. 82:8129–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blatny JM, Brautaset T, Winther-Larsen HC, Karunakaran P, Valla S. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35–51 [DOI] [PubMed] [Google Scholar]