Abstract
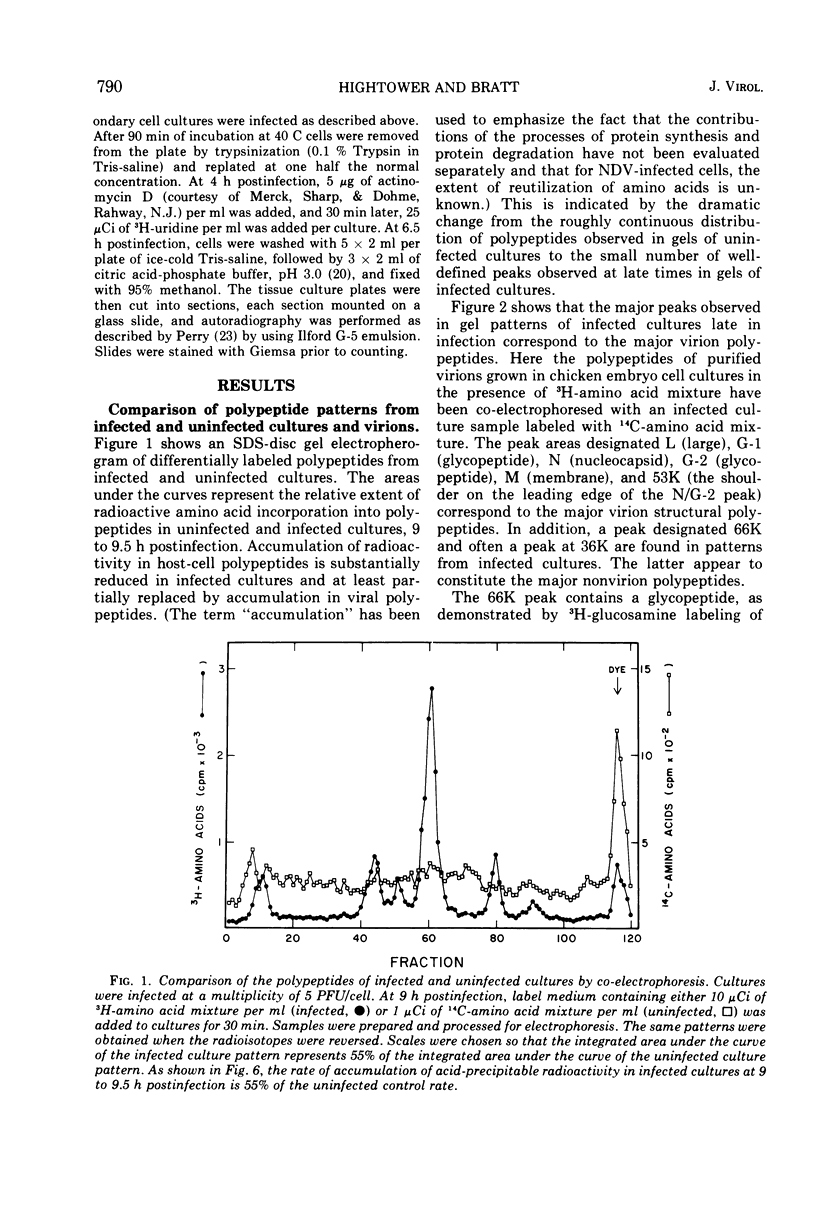
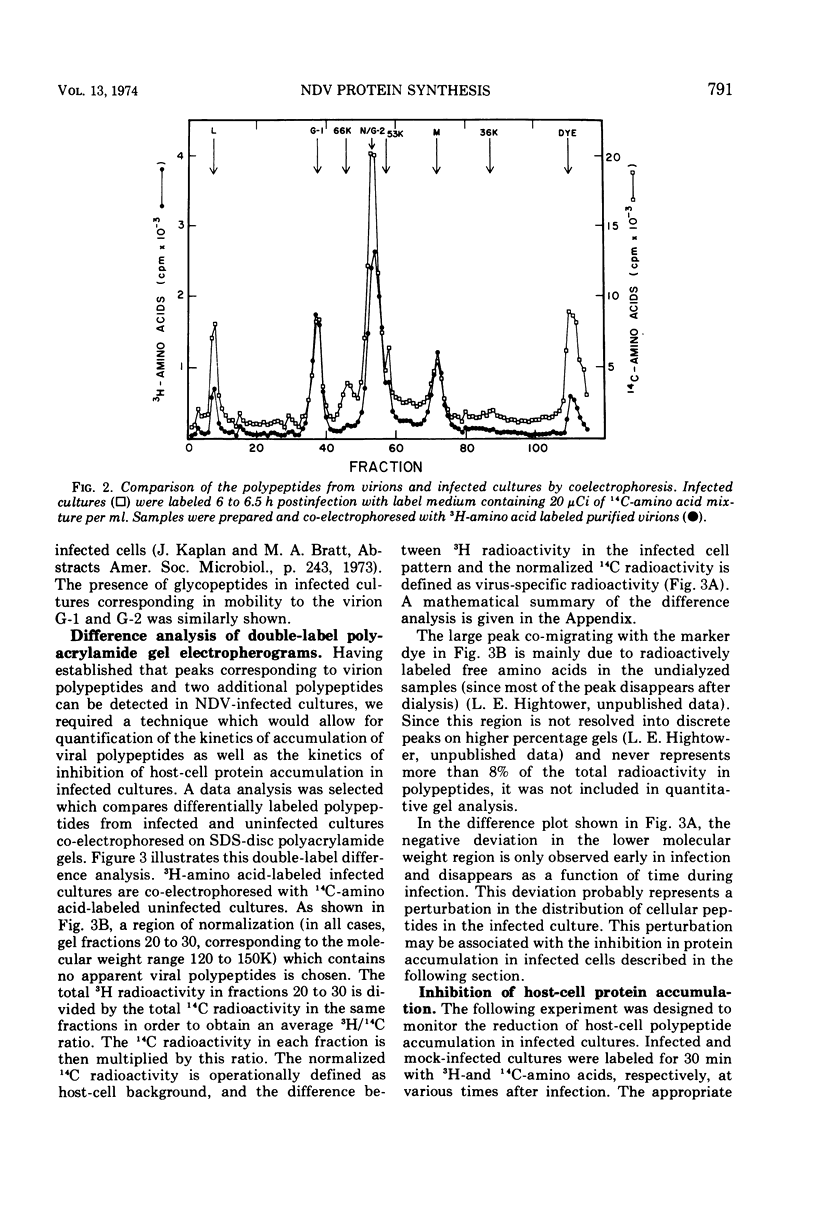
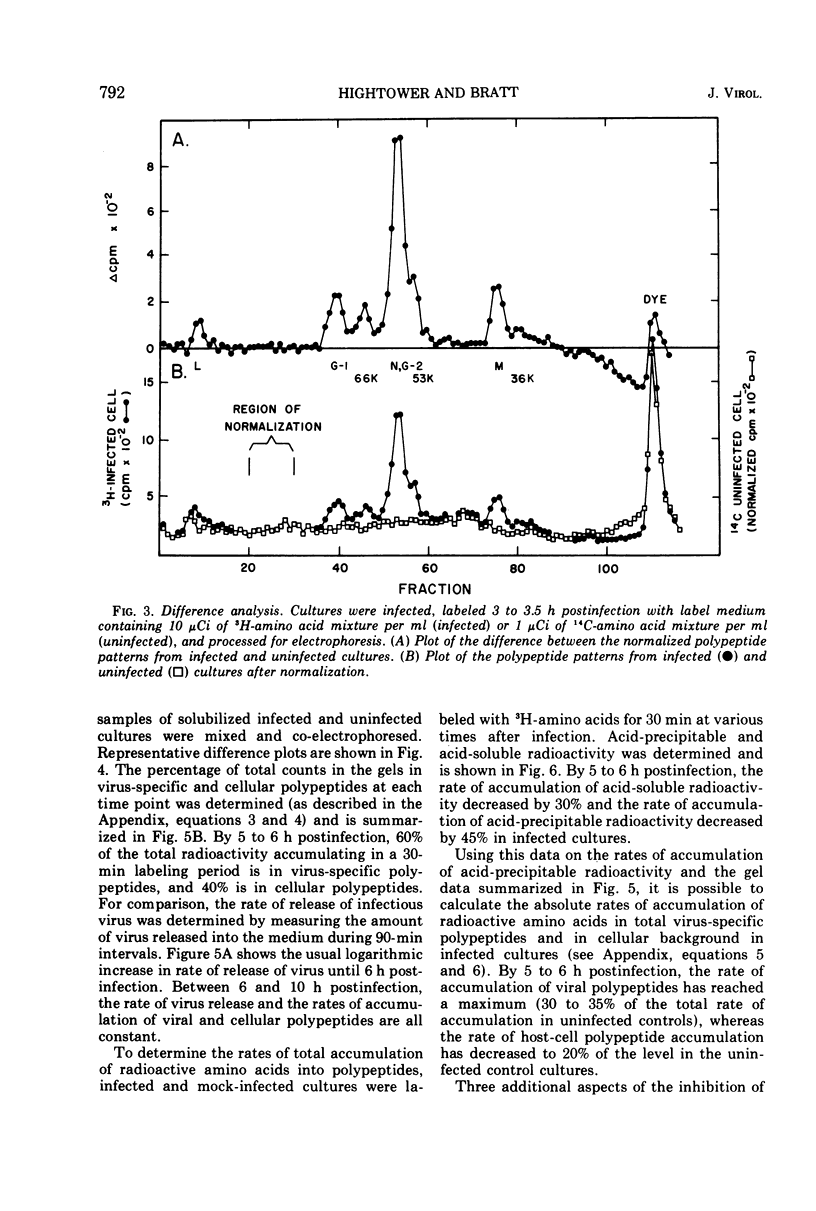
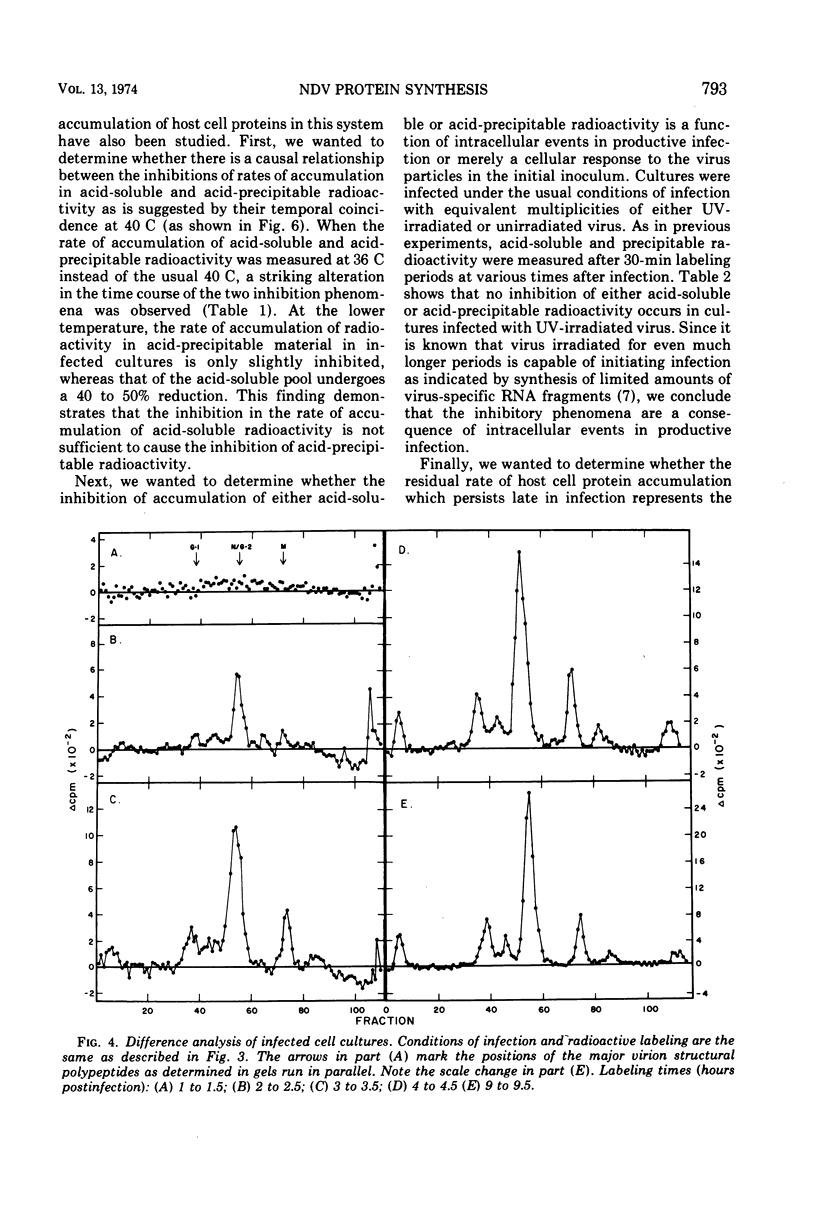
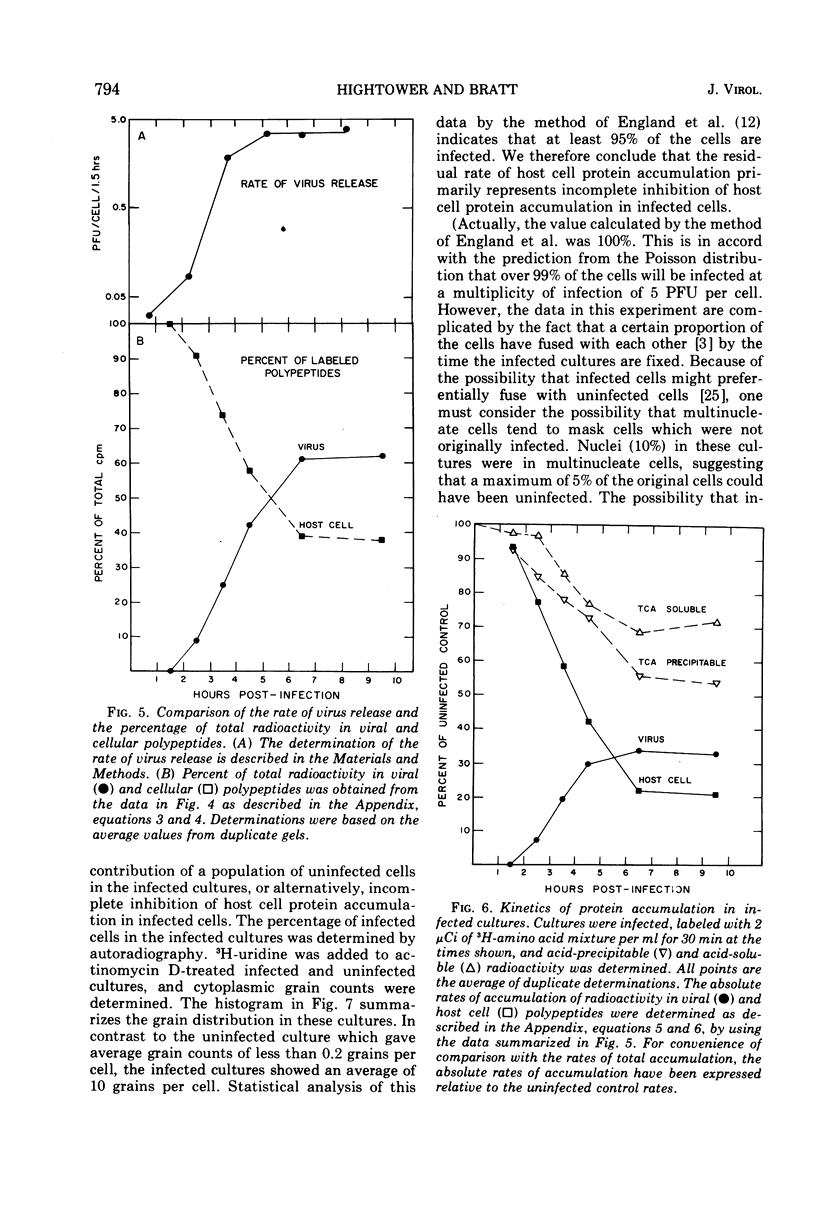
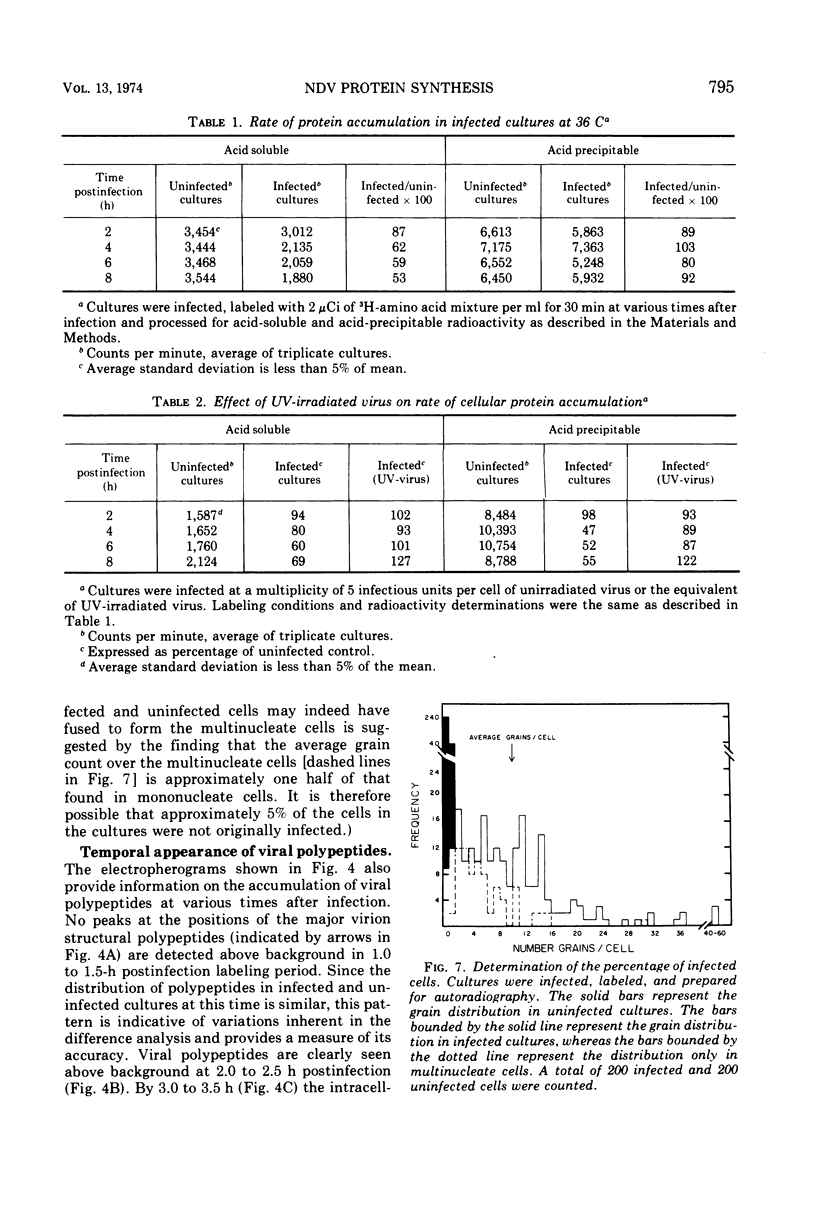
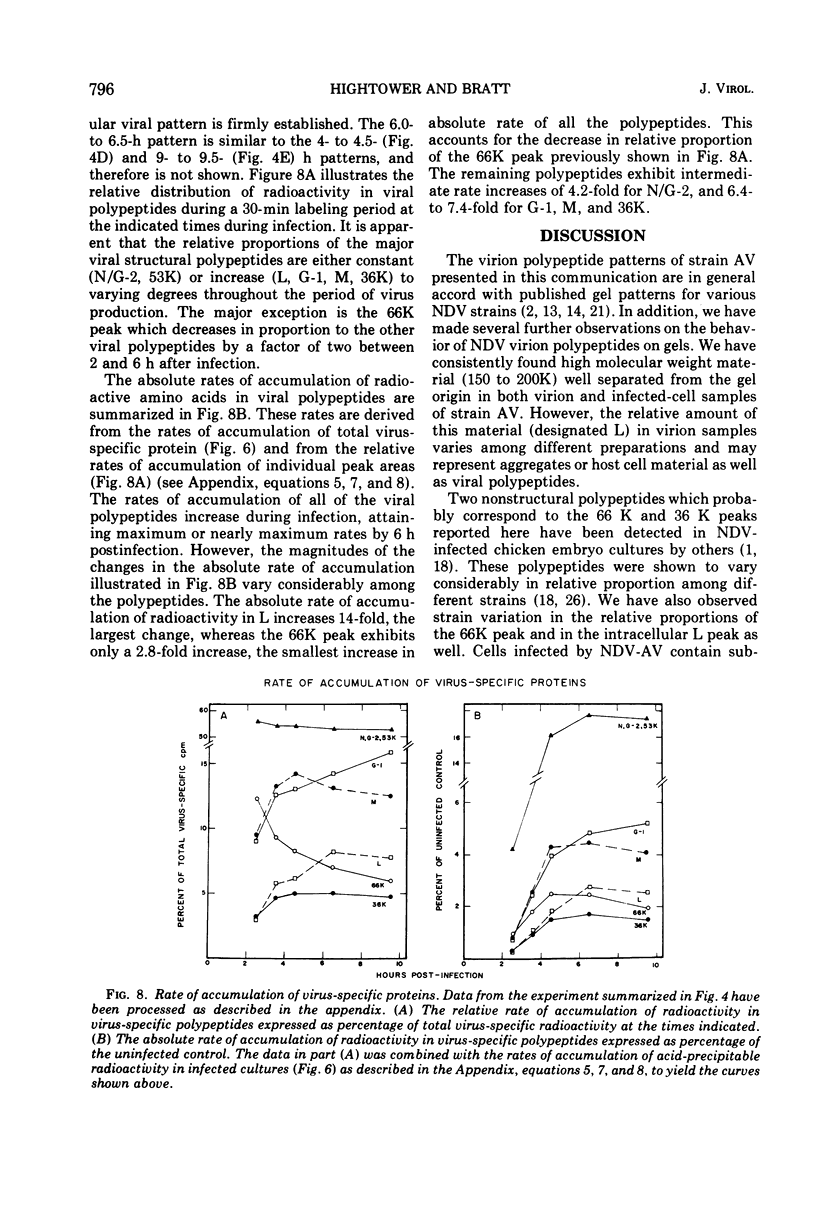
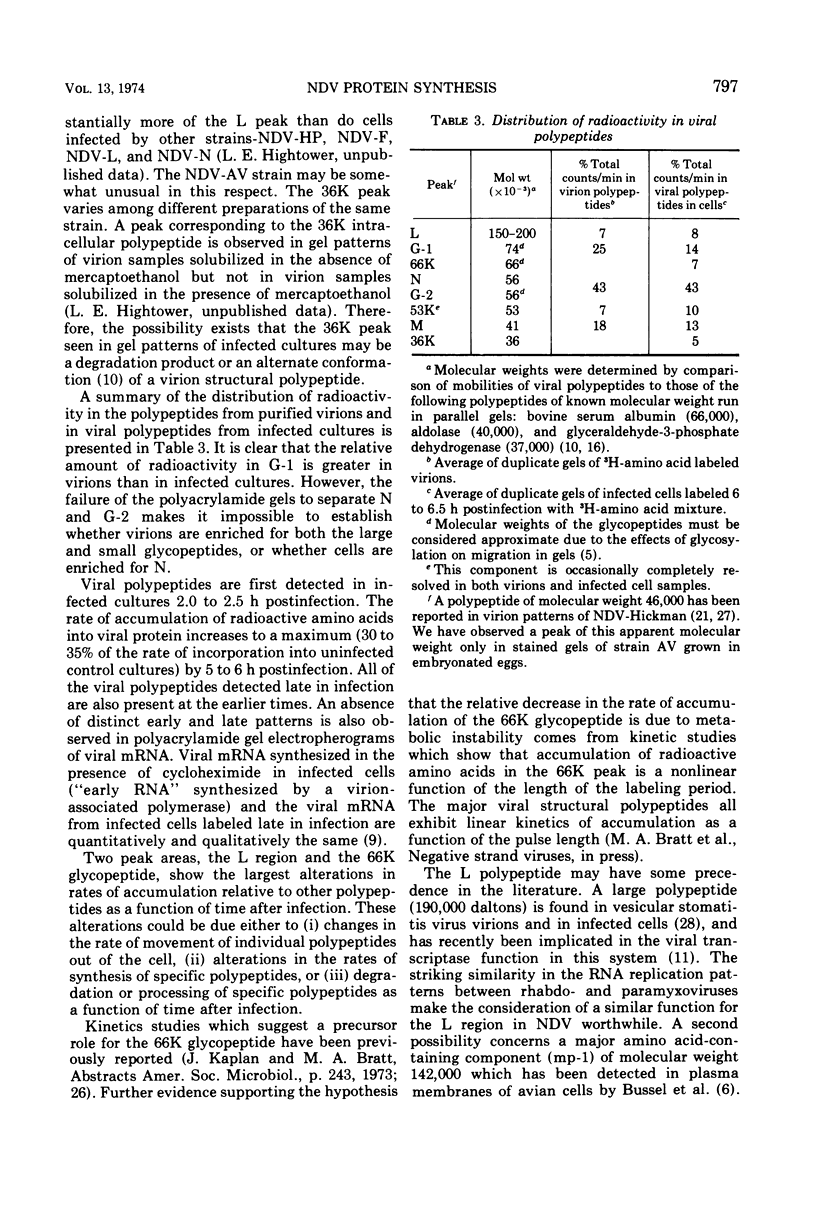
A double-isotopic label difference analysis of polyacrylamide gels has been used to distinguish between cellular and viral protein accumulation in infected cells and to quantify the kinetics of accumulation of viral polypeptides. This technique, coupled with the determination of total radioactive amino acid incorporation in infected cultures, has revealed the following kinetic patterns. Viral polypeptides are first detected in infected cultures 2.0 to 2.5 h postinfection. The rate of accumulation of radioactive amino acids in viral polypeptides increases to a maximum (30 to 35% of the rate of accumulation in uninfected control cultures), whereas the rate of accumulation of radioactive amino acids in host-cell protein decreases to a minimum (20% of the rate of accumulation in uninfected control cultures) by 5 to 6 h postinfection. All of the viral polypeptides detected late in infection are also present at the earlier times, and the major virion structural polypeptides are present in approximately the same (N/G-2, 53K) or slightly increasing (L, G-1, M) relative amounts. One peak area containing a nonstructural glycopeptide with an apparent molecular weight of 66,000 shows significant alterations in rates of accumulation during infection. Inhibition in the rate of radioactive amino acid incorporation into both trichloroacetic acid-soluble and acid-precipitable material during infection has been demonstrated. However, these two inhibition phenomena can be uncoupled temporally by incubating infected cultures at 36 C instead of the usual 40 C, suggesting that they may not be directly related.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. J., Reeve P. The proteins of Newcastle disease virus. 2. Virus-induced proteins. Microbios. 1972;5(20):247–257. [PubMed] [Google Scholar]
- Bikel I., Duesberg P. H. Proteins of Newcastle disease virus and of the viral nucleocapsid. J Virol. 1969 Oct;4(4):388–393. doi: 10.1128/jvi.4.4.388-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Robinson W. S. Membrane proteins of uninfected and Rous sarcoma virus- transformed avian cells. J Virol. 1973 Aug;12(2):320–327. doi: 10.1128/jvi.12.2.320-327.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Relationship between the ribonucleic acid synthesizing capacity of ultraviolet-irradiated Newcastle disease virus and its ability to induce interferon. J Virol. 1971 Oct;8(4):500–508. doi: 10.1128/jvi.8.4.500-508.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. S., Bratt M. A. Separation of the messenger RNAs of Newcastle disease virus by gel electrophoresis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2544–2548. doi: 10.1073/pnas.70.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J. M., Rogers A. W., Miller R. G., Jr The identification of labelled structures on autoradiographs. Nature. 1973 Apr 27;242(5400):612–613. doi: 10.1038/242612a0. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kingsbury D. W. Separation of Newcastle disease virus proteins by polyacrylamide gel electrophoresis. Virology. 1969 Apr;37(4):597–604. doi: 10.1016/0042-6822(69)90277-3. [DOI] [PubMed] [Google Scholar]
- Haslam E. A., Cheyne I. M., White D. O. The structural proteins of Newcastle disease virus. Virology. 1969 Sep;39(1):118–129. doi: 10.1016/0042-6822(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Huo W. H., Wilson D. E. Degradation of cellular ribonucleic acid in Newcastle disease virus infected cells. J Gen Virol. 1969 Mar;4(2):245–251. doi: 10.1099/0022-1317-4-2-245. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Darnall D. W. Protein subunits: a table (second edition). Science. 1969 Oct 3;166(3901):126–128. doi: 10.1126/science.166.3901.126. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Meager A., Burke D. C. Virus RNA and protein synthesis in cells infected with different strains of Newcastle disease virus. J Gen Virol. 1971 Oct;13(1):111–120. doi: 10.1099/0022-1317-13-1-111. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Choppin P. W. Proteins and glycoproteins of paramyxoviruses: a comparison of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1971 Jan;7(1):47–52. doi: 10.1128/jvi.7.1.47-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- Reeve P., Alexander D. J., Pope G., Poste G. Studies on the cytopathic effects of Newcastle disease virus: metabolic requirements. J Gen Virol. 1971 Apr;11(1):25–34. doi: 10.1099/0022-1317-11-1-25. [DOI] [PubMed] [Google Scholar]
- Samson A. C., Fox C. F. Precursor protein for Newcastle disease virus. J Virol. 1973 Sep;12(3):579–587. doi: 10.1128/jvi.12.3.579-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELOCK E. F., TAMM I. Biochemical basis for alterations in structure and function of HeLa cells infected with Newcastle disease virus. J Exp Med. 1961 Nov 1;114:617–632. doi: 10.1084/jem.114.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. E. Inhibition of host-cell protein and ribonucleic acid synthesis by Newcastle disease virus. J Virol. 1968 Jan;2(1):1–6. doi: 10.1128/jvi.2.1.1-6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]



