Abstract
Intracellular free iron of Escherichia coli was determined by whole-cell electron paramagnetic resonance spectrometry. Ultrahigh pressure (UHP) increased both intracellular free iron and cell lethality in a pressure-dose-dependent manner. The iron chelator 2,2′-dipyridyl protected cells against UHP treatments. A mutation that produced iron overload conditions sensitized E. coli to UHP treatment.
TEXT
Many pathogenic bacteria are killed by brief exposure to ultrahigh pressure (UHP) treatment at ≥300 MPa (1). Food processors use lethal pressures (300 to 800 MPa) at ambient temperatures to pasteurize foods with minimal loss of nutritional and sensory qualities (2). However, the mechanism of UHP-induced microbial inactivation is yet to be fully understood.
Transcriptional analysis of pressure-treated Escherichia coli, using a DNA microarray technique, revealed significant alterations in the expression of genes related to the assembly of iron-sulfur ([Fe-S]) clusters (3). The cysteine desulfurase of the SUF system, sufS, was significantly downregulated (by 1.9-fold), and the entire suf operon appeared to be downregulated. The SUF system is primarily in charge of [Fe-S] synthesis under iron starvation conditions. Repression of the suf operon is likely due to increased level of Fe-Fur, the iron-bound ferric uptake regulator, when intracellular free iron is replete (4). The authors hypothesized that UHP treatment disrupts the iron homeostasis, leading to at least a transient increase in intracellular free iron, thus contributing to lethality via production of damaging by-products (i.e., hydroxyl radical produced via the Fenton reaction). The objective of this study was to investigate the relationship between intracellular free iron and UHP-induced lethality of E. coli.
UHP treatments increase intracellular free iron of E. coli.
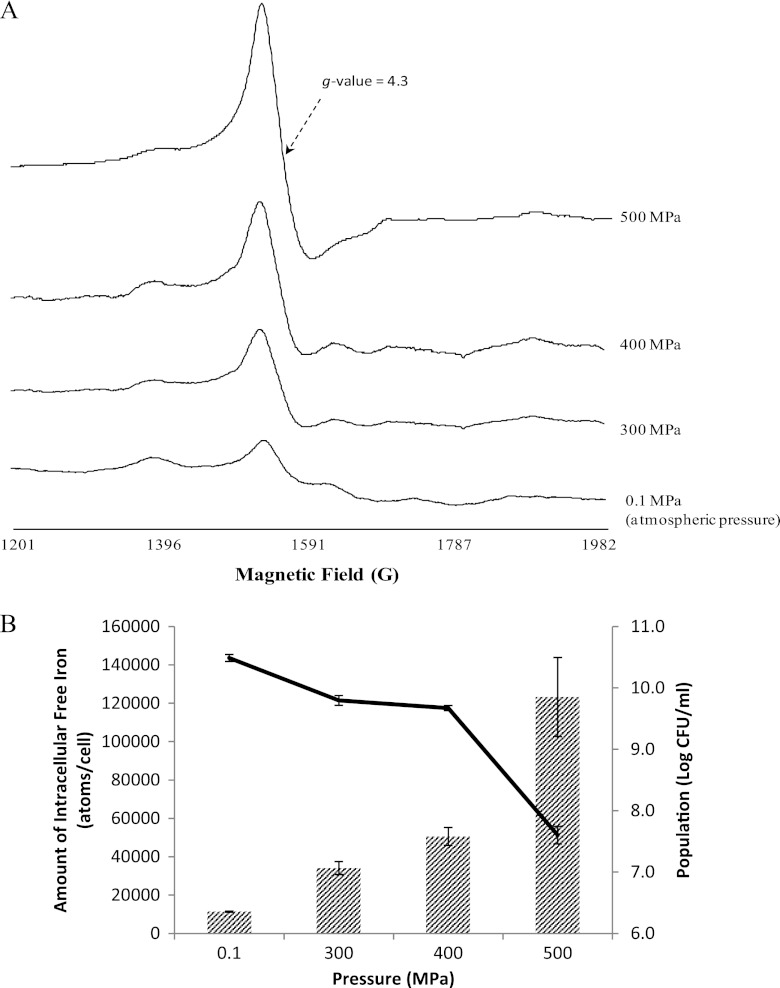
The amounts of intracellular free iron of E. coli K-12 before and after 1-min treatments with 300 to 500 MPa (Quintus QFP6; Flow Pressure Systems, Kent, WA) were determined using whole-cell electron paramagnetic resonance (EPR) spectroscopy (5). Briefly, stationary-phase cells were harvested and suspended in Tris buffer, pressure treated or not, incubated briefly in Luria-Bertani (LB) broth containing 20 mM deferoxamine mesylate (DF) (Calbiochem, La Jolla, CA), and resuspended in Tris buffer containing glycerol, and the resulting concentrated cell suspension was subjected to EPR analysis (Bruker ESP X-band spectrometer; Bruker, Billerica, MA). The reagent DF chelates the intracellular free iron, and the resulting complex produces a sharp EPR signal with a g-value of 4.3 (5). The amplitude of the first derivative of the signal was directly proportional to the concentration of iron in standard solutions (data not shown). Treatment of E. coli with UHP increased the concentration of intracellular free iron in a pressure-dose-dependent fashion. The higher the pressure that was applied, the greater the amplitude of the iron signal that was observed (Fig. 1A). Iron measurements were normalized by cell population; therefore, the number of iron atoms per cell (Fe/cell) was calculated. As depicted in Fig. 1B, the intracellular free iron content significantly increased from 1.1 × 104 Fe/cell for untreated cells to 3.4 × 104, 5.1 × 104, and 1.2 × 105 Fe/cell after 300-, 400-, and 500-MPa UHP treatments, respectively (P < 0.05). UHP-induced lethality followed a similar trend, with milder UHP treatments (300 and 400 MPa) resulting in less than a 1.0-log-CFU/ml reduction and the 500-MPa treatment inactivating 2.9 log CFU/ml (Fig. 1B). Pressure dose dependence of both lethality and free iron concentration implies that intracellular free iron is correlated to high-pressure-induced lethality of E. coli. Previous studies suggested that accumulation of O2−, a by-product of aerobic metabolism in most bacteria, could leach iron from the oxidatively labile [Fe-S] clusters by attacking the weak iron-sulfide ligands (6, 7). Aertsen et al. (8) proposed an endogenous oxidative burst as the cause of cell damage by UHP. It is possible that lethal pressure treatments (in excess of 300 MPa) induce the release of iron from [Fe-S] via enhanced oxidative reactions, and the accumulation of free iron finally leads to DNA damage and cell death.
Fig 1.
Changes in intracellular free iron and corresponding changes in viability of Escherichia coli K-12 subjected to various ultrahigh-pressure (UHP) treatments (0.1 to 500 MPa for 1 min at 25 ± 2°C). (A) Iron electron paramagnetic resonance signals from a whole-cell preparation of E. coli subjected to various UHP treatments. (B) E. coli survivors (line) and amounts of free intracellular iron (hatched bars) after different pressure treatments. Error bars represent standard errors from three independent experiments.
Contribution of intracellular iron status to the barotolerance of E. coli.
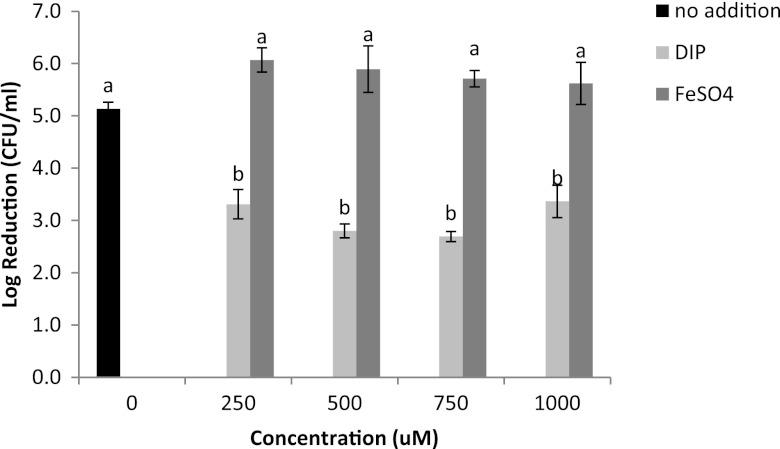
Due to the increase of intracellular free iron in UHP-treated E. coli, we speculated that cells may vary in barotolerance, depending on the level of intracellular iron. Iron overload and iron deprivation conditions were achieved by adding FeSO4 and the iron chelator 2,2′-dipyridyl (DIP; Sigma-Aldrich, St. Louis, MO), respectively, at levels of 250 to 1,000 μM. The basal condition refers to cells grown in LB broth without the addition of iron or iron chelators; this medium contains ∼5.8 μM iron. E. coli cells grown under different iron availability conditions were collected, resuspended in Tris buffer, and subjected to UHP treatments (500 MPa, 1 min). Under the basal condition, UHP decreased the E. coli population by 5.1 log CFU/ml, whereas addition of DIP significantly (P < 0.05) increased the barotolerance of E. coli, regardless of its concentration, with an average population decrease of 3.1 log CFU/ml (Fig. 2). DIP is a highly membrane-permeable iron chelator that has been used to dequench fluorescent metal sensors intracellularly (9). It is possible that DIP chelates the free intracellular iron released by UHP treatment, forming a redox-inactive chelate, thus protecting cells against the damage associated with excess free iron. According to a prior study, treatment with iron chelators protected E. coli cells against the lethal effect of H2O2, probably by capturing intracellular iron and blocking the Fenton reaction (10). Supplementation of the growth medium with extra FeSO4 did not significantly (P > 0.05) affect the barotolerance of E. coli (Fig. 2). Iron acquisition is tightly regulated by Fur to prevent potential cellular toxicity (4); therefore, a high level of intracellular iron is hard to achieve by increasing the availability of extracellular iron.
Fig 2.
Inactivation of Escherichia coli K-12 cultured under basal, iron deprivation, or iron overload conditions when treated with UHP treatments (500 MPa, 1 min, 25 ± 2°C). Error bars represent standard errors (n = 3). Different letter designations indicate significant differences in inactivation (P < 0.05).
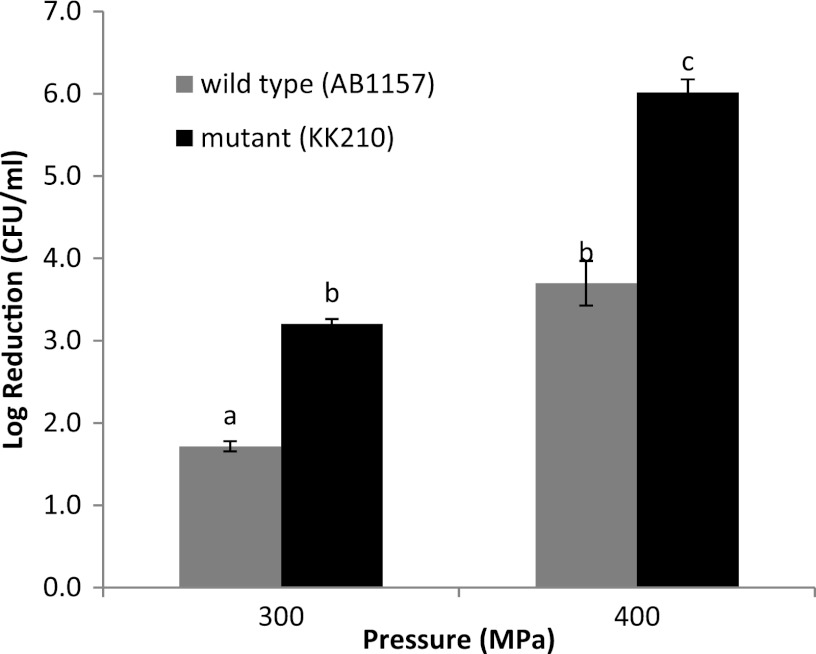
It has been reported that Fur deficiency overloads E. coli cells with intracellular free iron (6). Therefore, the Fur mutant (E. coli KK210) and its wild-type strain (E. coli AB1157), grown in LB to the stationary phase, were collected and treated with UHP (300 to 500 MPa, 1 min). The E. coli mutant was significantly (P < 0.05) more sensitive to UHP than was the wild type (Fig. 3), indicating that elevated intracellular free iron contributes to UHP-induced lethality. The 500-MPa treatments decreased the E. coli KK210 population below the detection limit of the enumeration procedure (i.e., >7-log-CFU/ml reduction; data not shown).
Fig 3.
Reductions in populations of Escherichia coli AB1157 (wild type) and KK210 (Fur mutant) when subjected to UHP treatments (300 to 400 MPa, 1 min, 25 ± 2°C). Error bars represent standard errors (n = 3). Different letter designations indicate significant differences in inactivation (P < 0.05).
In conclusion, UHP increases intracellular free iron of E. coli in a pressure-dose-dependent manner. The iron chelator protects E. coli against UHP, probably by chelating excess intracellular free iron released during the treatment. E. coli cells with intracellular free iron overload are highly susceptible to UHP treatment. These data provide strong evidence that bacterial lethality by UHP is influenced by the level of intracellular free iron.
Footnotes
Published ahead of print 2 November 2012
REFERENCES
- 1. Mackey BM, Mañas P. 2008. Inactivation of Escherichia coli by high pressure, p 53–85 In Michiels CW, Bartlett DH, Aertsen A. (ed), High-pressure microbiology. ASM Press, Washington, DC [Google Scholar]
- 2. Thakur BR, Nelson PE. 1998. High-pressure processing and preservation of foods. Food Rev. Int. 14:427–447 [Google Scholar]
- 3. Malone AS, Chung YK, Yousef AE. 2006. Genes of Escherichia coli O157:H7 that are involved in high-pressure resistance. Appl. Environ. Microbiol. 72:2661–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Semsey S, Andersson AMC, Krishna S, Jensen MH, Massé E, Sneppen K. 2006. Genetic regulation of fluxes: iron homeostasis of Escherichia coli. Nucleic Acids Res. 34:4960–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woodmansee AN, Imlay JA. 2002. Quantitation of intracellular free iron by electron paramagnetic resonance spectroscopy. Methods Enzymol. 349:3–9 [DOI] [PubMed] [Google Scholar]
- 6. Keyer K, Imlay JA. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U. S. A. 93:13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liochev SI, Fridovich I. 1994. The role of O2− in the production of HO·: in vitro and in vivo. Free Radic. Biol. Med. 16:29–33 [DOI] [PubMed] [Google Scholar]
- 8. Aertsen A, Spiegeleer PD, Vanoirbeek K, Lavilla M, Michiels CW. 2005. Induction of oxidative stress by high hydrostatic pressure in Escherichia coli. Appl. Environ. Microbiol. 71:2226–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kakhlon O, Cabantchik Z. 2002. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 33:1037–1046 [DOI] [PubMed] [Google Scholar]
- 10. Asad NR, Leitáo AC. 1991. Effects of metal ion chelators on DNA strand breaks and inactivation produced by hydrogen peroxide in Escherichia coli: detection of iron-independent lesions. J. Bacteriol. 173:2652–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]