Abstract
We evaluated the inducibility of nitrate-dependent Fe(II)-EDTA oxidation (NDFO) in non-growth, chloramphenicol-amended, resting-cell suspensions of Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Cells previously incubated with Fe(II)-EDTA oxidized ca. 6-fold more Fe(II)-EDTA than cells previously incubated with Fe(III)-EDTA. This is the first report of induction of NDFO by Fe(II).
TEXT
Although microbial nitrate-dependent Fe(II) oxidation (NDFO) was first reported more than a decade ago (1) and a number of bacterial species capable of NDFO have been reported (2, 3, 4, 5, 6, 7, 8, 9, 10), the biochemistry and enzymology of NDFO are still obscure. In contrast to our knowledge of respiratory electron transport during microbial iron reduction (11, 12, 13, 14, 15, 16) and electron transfer during acidophilic iron oxidation (11, 17), the mechanism of neutrophilic iron oxidation is largely unknown. Since no enzymatic machinery has been described for NDFO, questions remain whether Fe(II) oxidation is simply a combination of abiotic and enzymatic reactions occurring constitutively during NO3− reduction or an inducible, enzymatic process regulated by Fe(II). In a recent study, Chakraborty et al. demonstrated that Fe(II) oxidation provides an energetic benefit during mixotrophic growth of Acidovorax sp. strain 2AN using Fe(II) and acetate as an organic cosubstrate (2). Muehe et al. reported similar increased growth yields of another Acidovorax strain (18). Although these studies did not evaluate the inducibility of NDFO, reports of involvement of c-type cytochromes in Fe(II) oxidation by anoxic, phototrophic (19, 20), and microaerophilic (21) bacteria and a periplasmic, molybdopterin oxidoreductase in Fe(II) oxidation by a marine, neutrophilic bacterium (22) show that enzyme systems for Fe(II) oxidation at circumneutral pH exist and may be important for NDFO. In a recent review, Carlson et al. proposed a number of hypothetical Fe(II) oxidation mechanisms (23).
Although previous studies have provided evidence that NDFO is not an entirely abiotic reaction (2, 18), no studies have examined whether Fe(II) oxidation is catalyzed by enzymes that are inducible or are constitutively expressed during NO3− reduction. As a first step in attempting to understand biochemical mechanisms of NDFO, the induction experiments described here were designed to determine if previous incubation of two genera with Fe(II) resulted in altered Fe(II) oxidation ability. In addition, we wished to determine if enhanced activity was induced by Fe(II) or Fe(III).
Bacteria utilized.
The Acidovorax strain used in this study was isolated from an iron oxide-bearing sediment column inoculated with sediments from Dorn Creek, WI, and NDFO patterns by this isolate have been previously reported (2). Strain 2AN oxidized aqueous Fe2+, chelated Fe(II) [Fe(II)-EDTA], and solid-phase Fe(II) coupled to nitrate reduction mixotrophically in the presence of acetate in batch reactors, and enhanced growth concomitant with increased Fe(II) concentrations was demonstrated using a novel, continuous-flow system (2). Dechloromonas sp. strain UWNR4 was isolated as a nitrate reducer from nitrate-reducing enrichments obtained from Wisconsin River sediments, along with three other pure cultures, and was observed to be a robust Fe(II) oxidizer under mixotrophic conditions (24). Cultures used in experiments were recovered from frozen (−80°C) stock cultures or maintained under anoxic, NO3−-reducing conditions by using acetate as the growth substrate.
Utilization of Fe(II) as a nutrient or electron donor.
A batch growth experiment was designed to evaluate whether the Fe(II) oxidation-enhanced growth of Acidovorax sp. strain 2AN observed in previous work (2) could be explained by the presence of Fe as a nutrient as opposed to functioning as an electron donor. In this experiment and in the induction experiments described below, chelated Fe(II) was used instead of aqueous Fe2+ to avoid cell encrustation by Fe(III) oxyhydroxides and loss of metabolic activity (2, 25). NO2− often accumulates in NDFO experiments and can abiotically oxidize Fe2+ sorbed to cellular material or to biogenic Fe(III) oxyhydroxides that can serve as surface catalysts (26, 27, 28). Fe(II)-EDTA was specially chosen as the form of chelated Fe(II) to utilize in these experiments in order to minimize the potential for abiotic oxidation of chelated Fe(II) by NO3− or biogenic NO2− (28). Our previous work (2) showed that ca. 6 mM NO3− and ca. 5 mM Fe(II)-EDTA were nonreactive in 7-day incubations. Abiotic control experiments were done that also showed no reaction between ca. 2.5 mM NO2− and ca. 5 mM Fe(II)-EDTA after 6 days, whereas ca. 5 mM Fe(II) chelated by nitrilotriacetic acid (NTA) was rapidly oxidized by ca. 2 mM NO2− (see Fig. S1 in the supplemental material). Citrate-chelated Fe could not be used since Acidovorax sp. 2AN metabolizes citrate under NO3−-reducing conditions (data not shown).
Cells grown anaerobically on 10 mM acetate and 5 mM nitrate were anoxically washed twice by centrifugation (4°C for 20 min at 10,000 × g) using a basal medium without any electron donor and acceptor. The detailed composition of the basal medium has been described elsewhere (2). Similar numbers of cells, determined by an acridine orange direct counting method (29), were subsequently inoculated in triplicate batch reactors containing three different anoxic media, i.e., medium A (∼2 mM acetate), medium B [∼2 mM acetate and 5 mM Fe(II)-EDTA], and medium C [∼2 mM acetate and 5 mM Fe(III)-EDTA]. All media contained 5 mM NO3− as the sole electron acceptor and were buffered to pH 7 with 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]. Although the formation constant of Fe(III)-EDTA is higher than that of Fe(II)-EDTA, equilibrium Fe(II) speciation analysis of media B and C predicted that >99.8% of added Fe(II) or Fe(III) was chelated in both media (see Table S1 in the supplemental material). In addition, the free EDTA4− concentrations were calculated to be negligible (<10−9 mM) in both media. The reactors were incubated aphotically at 30°C and were sampled daily for growth measurement using the acridine orange method (29).
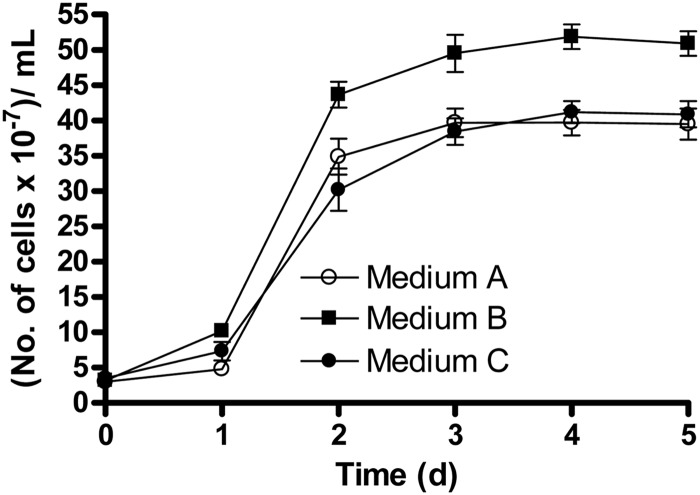
After 5 days of incubation, the number of cells (3.9 × 108 ± 0.3 × 108 cells/ml) in medium A was not noticeably different from the number of cells in medium C (4.1 × 108 ± 0.2 × 108 cells/ml) (Fig. 1), showing that the presence of 5 mM Fe(III)-EDTA had no significant effect on growth. However, the number of cells (5.1 × 108 ± 0.2 × 108 cells/ml) in medium B was ca. 25% higher than those in the other two media (Fig. 1). Our results demonstrate that the presence of Fe [as Fe(III)] as a nutrient was not sufficient to noticeably enhance the growth yield compared to when Fe(II) was present was an electron donor.
Fig 1.
Comparative growth of Acidovorax sp. 2AN in batch reactors using different media. All media contained 5 mM NO3−. Medium A contained 2 mM acetate, medium B contained 5 mM Fe(II)-EDTA and 2 mM acetate, and medium C contained 5 mM Fe(III)-EDTA and 2 mM acetate. Data are presented as means ± standard deviations (n = 3). When not shown, error bars are smaller than the symbols.
Induction experiments.
A batch growth experiment was conducted with Acidovorax sp. 2AN to evaluate the rates of Fe(II) oxidation by cells grown in medium A (acetate/nitrate) versus cells grown in medium B [Fe(II)-EDTA/acetate/nitrate]. Cells were harvested from 3-day-old cultures from both media by using anoxic washing as described above, followed by resuspension in basal medium. Two inocula with approximately equal cell densities were prepared to inoculate two sets of triplicate batch reactors containing medium B [with ca. 6.1 mM Fe(II)-EDTA]. The reactors were incubated aphotically at 30°C without shaking and were sampled daily to quantify Fe(II)-EDTA oxidation using o-phenanthroline as the colorimetric reagent (6).
After 3 days of incubation, ca. 2.5 mM Fe(II)-EDTA was oxidized by the cells pregrown on medium A [lacking Fe(II)-EDTA] with a mean oxidation rate of 0.83 mM/day (Fig. 2). Cells pregrown on medium B, however, oxidized Fe(II)-EDTA at a higher rate (ca. 1.7 mM/day) and oxidized ca. 5.1 mM Fe(II)-EDTA after 3 days. This experiment demonstrated that preincubation with Fe(II)-EDTA resulted in a more than 2-fold increase in the mean rate of Fe(II)-EDTA oxidation by strain 2AN and led us to hypothesize that exposure to Fe(II) induces higher rates of Fe(II) oxidation. Alternately, it is possible that cells in the medium B inoculum may have been acclimated to EDTA and high-Fe concentrations, resulting in subsequent higher Fe(II) oxidation rates. We therefore conducted additional experiments in which the different inocula were both grown in the presence of EDTA and high concentrations of Fe.
Fig 2.
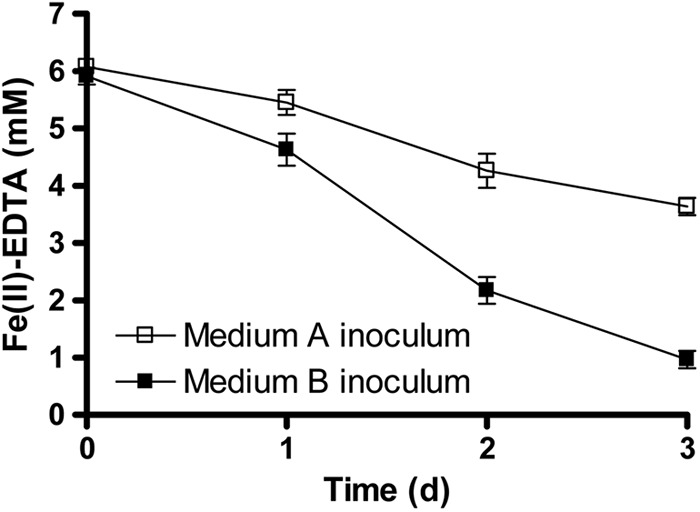
Comparison of Fe(II)-EDTA oxidation by Acidovorax sp. 2AN grown in medium A [without any Fe(II)] versus cells grown in medium B [with Fe(II)-EDTA]. The initial concentrations of cells were in the range of 11.4 × 107 to 13.3 × 107 cells/ml, and Fe(II)-EDTA was present at an initial concentration of ca. 6.1 mM. Data are presented as means ± standard deviations (n = 3). When not shown, error bars are smaller than the symbols.
Cell suspension experiments were done with both Acidovorax sp. 2AN and Dechloromonas sp. UWNR4 at high cell densities over short periods of time in the presence of chloramphenicol (CAP) to inhibit protein synthesis. Two different genera known to be capable of NDFO were utilized to ascertain whether results were specific only to Acidovorax species. Preliminary experiments were done to determine a suitable CAP concentration that would prevent protein synthesis but not interfere with respiratory electron transport. Utilizing 8 different CAP concentrations from 15 to 100 μM, we found that 30, 35, and 40 μM were the lowest concentrations that prevented growth, presumably by inhibition of protein synthesis (see Fig. S2a in the supplemental material). These three concentrations were subsequently tested for their effects on the rates of oxygen utilization by both Dechloromonas sp. UWNR4 and Acidovorax sp. 2AN. Of these three concentrations, 30 μM was observed to have the least effect (see Fig. S2b), and this concentration was selected as one that would inhibit protein synthesis but allow electron transport.
Cells were grown aerobically on nutrient broth to generate sufficient biomass and harvested by centrifugation. The cells were washed twice anoxically as described above and were added in approximately equal cell densities into medium B [5 mM Fe(II)-EDTA, 2 mM acetate, and 5 mM NO3−] and medium C [5 mM Fe(III)-EDTA, 10 mM acetate, and 5 mM NO3−]. Medium C [with Fe(III)-EDTA] was used to balance the possible toxic effects of EDTA, to maintain equivalent total Fe concentrations in both media, and to determine if enzymatic pathways of Fe(II) oxidation were specifically induced by ferrous iron. After 48 h of incubation at 30°C to allow induction of appropriate enzyme systems, the cells were washed with anoxic basal medium and cells from each of the growth conditions were separately used at a cell density of ca. 3.5 × 1010 to 4 × 1010 cells/ml in triplicate reactors (final volume of 5 ml) containing medium B [ca. 4 mM Fe(II)-EDTA and 5 mM NO3−] amended with 30 μM CAP. The reactors also contained 1.2 mM ethanol since ethanol was used as a solvent to prepare the CAP stock solution. To ensure non-growth conditions, acetate was not added to cultures in these short-term, resting cell assays. Two sets of control reactors lacking CAP were used with and without ethanol to test the effects of ethanol. The reactors were sampled anoxically every 30 min, and Fe(II)-EDTA oxidation was monitored.
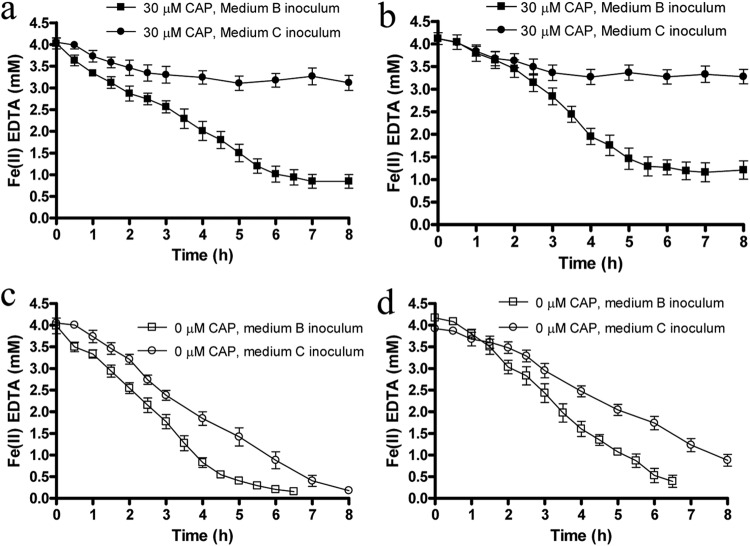
All control reactors lacking CAP showed almost complete Fe(II)-EDTA oxidation within 6 to 12 h of incubation (Fig. 3c and d). Controls without ethanol showed that 1.2 mM ethanol did not notably affect Fe(II)-EDTA oxidation rates (see Fig. S3 in the supplemental material). For CAP-inhibited suspensions of both Acidovorax sp. 2AN and Dechloromonas sp. UWNR4, cells that were previously incubated with Fe(II)-EDTA (medium B) oxidized ca. 75% (2.5 to 3 mM) of the initial Fe(II)-EDTA after 7 h of incubation (Fig. 3a and b). Fe(II)-EDTA oxidation rates were somewhat lower in CAP-containing suspensions than in controls lacking CAP (Fig. 3c and d; see Table S2 in the supplemental material), especially for strain 2AN, but continued oxidation took place for 5 to 7 h, showing that extensive oxidation was possible in cells preincubated with Fe(II), even if enzyme synthesis was inhibited.
Fig 3.
Fe(II)-EDTA oxidation in cell suspensions of resting cells of Acidovorax sp. 2AN (a and c) and Dechloromonas sp. UWNR4 (b and d) in the presence (a and b) and absence (c and d) of 30 μM CAP. The figure shows Fe(II) oxidation in reactors containing cells that were previously incubated with Fe(II)-EDTA (medium B inoculum) or with Fe(III)-EDTA (medium C inoculum). All cell suspensions initially contained ca. 4 mM Fe(II)-EDTA, 5 mM NO3−, and 1.2 mM ethanol. Data are presented as means ± standard deviations (n = 3). When not shown, error bars are smaller than the symbols.
The CAP-containing suspensions of cells previously incubated with Fe(III)-EDTA (medium C) showed a different oxidation pattern (Fig. 3a and b). Fe(II)-EDTA was initially oxidized (0.5 to 0.7 mM) for 1 to 1.5 h, after which time further oxidation slowed and eventually stopped. Further incubations of the reactors for 48 h did not display any more Fe(II)-EDTA oxidation (data not shown). This suggests that, although a finite Fe(II) oxidation capacity is present in cultures not previously incubated with Fe(II), continued oxidation in such cultures requires protein synthesis. In control cultures lacking CAP that were preincubated with Fe(III)-EDTA, Fe(II) oxidation continued (Fig. 3c and d), suggesting that cells were able to synthesize required enzymes after 1 to 1.5 h of incubation with Fe(II)-EDTA. However, Fe(II) oxidation rates were higher in similar control cultures preincubated with Fe(II)-EDTA (Fig. 3c and d; see Table S2 in the supplemental material).
Since the cells previously exposed to Fe(III)-EDTA did not exhibit continued oxidation of Fe(II)-EDTA, we inferred that NDFO in these strains is an inducible process and that induction is specific to Fe(II) as opposed to iron in general. Alternative explanations, such as coregulation of metal detoxification and antibiotic resistance (30, 31), can be argued, e.g., that previous Fe(II) exposure may have decreased CAP sensitivity. However, we observed somewhat greater average Fe(II) oxidation rates during the cell-suspension assay in controls lacking CAP (Fig. 3c and d) for cells preincubated with Fe(II)-EDTA, showing that Fe(II) exposure does not result in antibiotic resistance. In addition, Fe(II) oxidation has been suggested as a potential detoxification mechanism in the photoheterotrophic, Fe(II)-oxidizing strain Rhodobacter capsulatus SB1003 (32). However, upregulation of such a detoxification response seems unlikely in our studies as we have no evidence of either Fe(II)- or Fe(III)-EDTA toxicity (2). Contrarily, growth yield of strain 2AN was greater in the presence of Fe(II)-EDTA than in the presence of Fe(III)-EDTA or in the absence of either compound (Fig. 1). In addition, the toxic effects of Fe are typically attributed to oxidative stress caused by the generation of reactive oxygen species from Fenton-type reactions mediated by iron under aerobic conditions (33), which does not hold true in our case. Stress response by a reactive nitrogen species instead of Fe(II) is also highly speculative, since it would need to be a compound not formed during organotrophic NO3− reduction (our inocula were grown under such conditions) or one produced at higher concentrations during Fe(II) oxidation. Differences in free EDTA concentrations, if they existed, could explain differential toxicity responses, but free EDTA concentrations were negligible in the media with either Fe(II)- or Fe(III)-EDTA (see Table S1 in the supplemental material).
Since cells preincubated with Fe(III)-EDTA (medium C) were able to initially oxidize a finite amount of Fe(II) even when protein synthesis was inhibited, we formulated a hypothetical mechanism involving constitutive expression of a putative, outer-membrane-bound Fe(II) oxidoreductase under anoxic conditions, regardless of the presence or absence of Fe(II), and an Fe(II)-inducible, periplasmic electron carrier that is able to transfer electrons from the oxidoreductase to cytoplasmic membrane ETC components, e.g., the quinone pool. This mechanism presumes that the oxidoreductase can oxidize a finite amount of Fe(II) before it is completely reduced. In the absence of the inducible electron carrier, this results in an initial, but finite, oxidation capacity. The concept of a finite Fe(II) oxidation and electron storage capacity is somewhat similar to the “iron lung” or capacitance model proposed for Geobacter species by Lovley and coworkers (13, 34), who estimated a storage capacity of 1.6 × 10−17 mol electrons per cell. Interestingly, the Fe(II) oxidation capacity for cells of both genera used in our experiments is 1.5 × 10−17 to 1.7 × 10−17 mol Fe(II) per cell, based on a cell density of 3.5 × 1010 to 4.0 × 1010 cells/ml and oxidation of 0.6 mmol Fe(II) in CAP-inhibited cells prior to cessation of Fe(II) oxidation (Fig. 3a and b).
Due to the major differences in physiologies between Geobacter species and the genera used in our studies, the almost identical electron storage capacities may be fortuitous. Nevertheless, induction of Fe(II) oxidation in two physiologically similar bacteria capable of NDFO provides additional strong evidence that Fe(II) oxidation is enzymatic, particularly in a system using EDTA-chelated Fe in which abiotic reactions are minimized. The methodologies used in this work may be of use in designing appropriate biochemical, transcriptomic, or proteomic experiments that will further elucidate metabolic pathways of NDFO and evaluate alternative explanations to our hypotheses of induction solely by Fe(II).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the contributions of Bridget O'Connor for assistance with the induction experiments and Eric Roden for helpful discussions.
We acknowledge the National Science Foundation grant EAR-0525069 to F.P. for research support.
Footnotes
Published ahead of print 9 November 2012
Supplemental material for this article may be found at 10.1128/AEM.02709-12.
REFERENCES
- 1. Straub KL, Benz M, Schink B, Widdel F. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakraborty A, Roden EE, Schieber J, Picardal F. 2011. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl. Environ. Microbiol. 77:8548–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hafenbradl D, Keller M, Dirmeier R, Rachel R, Rossnagel P, Burggraf S, Huber H, Stetter KO. 1996. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308–314 [DOI] [PubMed] [Google Scholar]
- 4. Kappler A, Schink B, Newman DK. 2005. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 3:235–245 [Google Scholar]
- 5. Kappler A, Straub KL. 2005. Geomicrobiological cycling of iron. Rev. Mineral. Geochem. 59:85–108 [Google Scholar]
- 6. Kumaraswamy R, Sjollema K, Kuenen G, von Loosdrecht MCM, Muyzer G. 2006. Nitrate-dependent [Fe(II)EDTA]2− oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Syst. Appl. Microbiol. 29:276–286 [DOI] [PubMed] [Google Scholar]
- 7. Lack JG, Chaudhuri SK, Chakraborty R, Achenbach LA, Coates JD. 2002. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microb. Ecol. 43:424–431 [DOI] [PubMed] [Google Scholar]
- 8. Weber KA, Achenbach LA, Coates JD. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4:752–764 [DOI] [PubMed] [Google Scholar]
- 9. Weber KA, Hedrick DB, Peacock AD, Thrash JC, White DC, Achenbach LA, Coates JD. 2009. Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Appl. Microbiol. Biotechnol. 83:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber KA, Pollock J, Cole KA, O'Connor SM, Achenbach LA, Coates JD. 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 72:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bird LJ, Bonnefoy V, Newman DK. 2011. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 19:330–340 [DOI] [PubMed] [Google Scholar]
- 12. Croal LR, Gralnick JA, Malasarn D, Newman DK. 2004. The genetics of geochemistry. Annu. Rev. Genet. 38:175–202 [DOI] [PubMed] [Google Scholar]
- 13. Lovley DR. 2008. Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology 6:225–231 [DOI] [PubMed] [Google Scholar]
- 14. Lovley DR, Holmes DE, Nevin KP. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219–286 [DOI] [PubMed] [Google Scholar]
- 15. Richter K, Schicklberger M, Gescher J. 2012. Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl. Environ. Microbiol. 78:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi L, Richardson DJ, Wang Z, Kerisit SN, Rosso KM, Zachara JM, Fredrickson JK. 2009. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Environ. Microbiol. Rep. 1:220–227 [DOI] [PubMed] [Google Scholar]
- 17. Quatrini R, Appia-Ayme C, Denis Y, Jedlicki E, Holmes DS, Bonnefoy V. 2009. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genomics 10:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muehe EM, Gerhardt S, Schink B, Kappler A. 2009. Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. FEMS Microbiol. Ecol. 70:3–11 [DOI] [PubMed] [Google Scholar]
- 19. Croal LR, Jiao Y, Newman DK. 2007. The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe(II) oxidation in Rhodobacter capsulatus SB1003. J. Bacteriol. 189:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiao Y, Newman DK. 2007. The pio operon is essential for phototrophic Fe(II) oxidation in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 189:1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Wang Z, Belchik SM, Edwards MJ, Liu C, Kennedy DW, Merkley ED, Lipton MS, Butt JN, Richardson DJ, Zachara JM, Fredrickson JK, Rosso KM, Shi L. 2012. Identification and characterization of MtoA: a decaheme c-type cytochrome of the neutrophilic Fe(II)-oxidizing bacterium Sideroxydans lithotrophicus ES-1. Front. Microbiol. 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, Chan CS, Comolli LR, Ferriera S, Johnson J, Heidelberg JF, Edwards KJ. 2011. Mariprofundus ferooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS One 6:e25386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carlson HK, Clark IC, Melnyk RA, Coates JD. 2012. Towards a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: balancing electron uptake and detoxification. Front. Microbiol. 3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coby AJ, Picardal F, Shelobolina E, Xu H, Roden EE. 2011. Repeated anaerobic microbial redox cycling of iron. Appl. Environ. Microbiol. 77:6036–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schädler S, Burkhardt C, Hegler F, Straub KL, Miot J, Benzerara K, Kappler A. 2009. Formation of cell-iron-mineral aggregates by phototrophic and nitrate-reducing anaerobic Fe(II)-oxidizing bacteria. Geomicrobiol. J. 26:93–103 [Google Scholar]
- 26. Coby AJ, Picardal FW. 2005. Inhibition of NO3− and NO2− reduction by microbial Fe(III) reduction: evidence of a reaction between NO2− and cell surface-bound Fe2+. Appl. Environ. Microbiol. 71:5267–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper DC, Picardal FW, Schimmelmann A, Coby AJ. 2003. Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl. Environ. Microbiol. 69:3517–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Picardal F. 2012. Abiotic and microbial interactions during anaerobic transformations of Fe(II) and NOX−. Front. Microbiol. 3:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roden EE, Zachara JM. 1996. Microbial reduction of crystalline Fe(III) oxides: influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 30:1618–1628 [Google Scholar]
- 30. Stepanauskas R, Glenn TC, Jagoe CH, Tuckfield RC, Lindell AH, King CJ, McArthur JV. 2006. Coselection for microbial resistance to metals and antibiotics in freshwater microcosms. Environ. Microbiol. 8:1510–1514 [DOI] [PubMed] [Google Scholar]
- 31. Wright MS, Loefller Peltier G, Stepanauskas R, McArthur JV. 2006. Bacterial tolerances to metals and antibiotics in metal-contaminated and reference streams. FEMS Microbiol. Ecol. 58:293–302 [DOI] [PubMed] [Google Scholar]
- 32. Poulain AJ, Newman DK. 2009. Rhodobacter capsulatus catalyzes light-dependent Fe(II) oxidation under anaerobic conditions as a potential detoxification mechanism. Appl. Environ. Microbiol. 75:6639–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Touati D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1–6 [DOI] [PubMed] [Google Scholar]
- 34. Esteve-Núñez A, Sosnik J, Visconti P, Lovley DR. 2008. Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ. Microbiol. 10:497–505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.