Abstract
Introduction
Fibrosis in human diseases and animal models is associated with aberrant Wnt/β-catenin pathway activation. The regulation, activity, mechanism of action and significance of Wnt/β-catenin signaling in the context of systemic sclerosis (SSc) has not been characterized.
Methods
Expression of Wnt signaling pathway components in SSc skin biopsies was analyzed. The regulation of profibrotic responses by canonical Wnt/ß-catenin was examined in explanted human mesenchymal cells. Fibrotic responses were studied by proliferation, migration and gel contraction assays. The fate specification of subcutaneous preadipocytes by canonical Wnt signaling was evaluated.
Results
Analysis of published genome-wide expression datasets revealed elevated expression of the Wnt receptor Fzd2 and the Wnt target Lef1, and decreased expression of Wnt antagonists Dkk2 and Wif1 in skin biopsies from subsets of dcSSc patients. Immunohistochemistry showed increased nuclear β-catenin expression in these biopsies. In vitro, Wnt3a induced ß-catenin activation, stimulated fibroblast proliferation, migration, gel contraction and myofibroblast differentiation, and profibrotic gene expression. Genetic and pharmacological approaches were used to demonstrate that these profibrotic responses involved autocrine TGF-β signaling via Smads. In contrast, in explanted subcutaneous preadipocytes Wnt3a repressed adipogenesis and promoted myofibroblast differentiation.
Conclusions
Canonical Wnt signaling was hyperactivated in SSc skin biopsies, and in explanted mesenchymal cells Wnt3a stimulated fibrogenic responses while suppressing adipogenesis. Together, these results indicate that Wnts have potent profibrotic effects and canonical Wnt signaling plays an important role in the pathogenesis of fibrosis and lipoatrophy in SSc.
Introduction
Systemic sclerosis (SSc) is a chronic autoimmune disease of unknown etiology associated with vascular injury, inflammatory responses and tissue fibrosis (1). Fibrosis, the distinguishing pathological hallmark of SSc, is characterized by overproduction of collagen and other extracellular matrix (ECM) components by fibroblasts and myofibroblasts accompanied by progressive loss of subcutaneous adipose tissue. The source of ECM-producing activated fibroblasts within the lesional tissue in SSc is controversial (2). In situ transition of fibroblasts into α-smooth muscle actin (SMA)-positive myofibroblasts, tissue accumulation of bone marrow-derived progenitor cells trafficking from the circulation, and transdifferentiation of epithelial cells, vascular endothelial cells and pericytes are some of the putative mechanisms underlying the expansion of the pool of biosynthetically activated mesenchymal cells. Transforming growth factor-β (TGF-β) is the master regulator of fibroblast activation and myofibroblast differentiation. Ligand binding to the type I TGF-ß receptor causes phosphorylation of cytoplasmic Smad2 and Smad3, promoting Smad heterocomplex formation and nuclear accumulation. The Smad complex selectively binds to Smad-binding elements, recruits the histone acetyltransferase p300 and other coactivators, and activates or represses target gene transcription (3). In addition to TGF-β, multiple cytokines and growth factors capable of inducing fibroblast activation and differentiation have been implicated in the pathogenesis of fibrosis (4).
Wnts comprise a multigene family of secreted glycoproteins that provide essential developmental signals during embryogenesis (5). ß-catenin is a central mediator in canonical Wnt signaling (6). Binding of Wnt ligands to cell surface receptor Frizzed 2 (FZD2) and co-receptors low-density lipoprotein receptor-related protein 5 (LRP5) and LRP6 inhibits the activation of glycogen synthetase kinase (GSK)-3ß, which blocks ß-catenin phosphorylation, ubiquitination and degradation. Active unphosphorylated β-catenin consequently accumulates in the cytoplasm, and translocates into nucleus, where it serves as transcriptional co-activator for the DNA-binding factors lymphocyte enhancer factor (LEF) and T-cell factor (TCF). Although β-catenin/TCF-LEF mediated transcription occurs ubiquitously in all tissues, the genes targeted are cell-type and context-dependent (7). Secreted frizzled-related proteins (sFRPs), Wnt inhibitory factors (WIFs) and Dickkopf-homolog proteins (DKKs) can interact with extracellular Wnt proteins or Wnt receptors to block Wnt/β-catenin signaling and negatively modulate Wnt responses (8). In addition, a protein called inhibitor of β-catenin and T cell factor 4 (ICAT), identified by yeast two-hybridization, functions as an intracellular inhibitor of Wnt/β-catenin signaling that competes for the β-catenin/TCF binding interface (9).
Whereas the importance of deregulated Wnt/β-catenin signaling in a variety of benign and malignant human diseases has been long appreciated, its significance in the context fibrogenesis has only recently begun to be investigated (10). Aging-associated fibrosis of muscle has been attributed to up-regulation of canonical Wnt signaling, which results in skewed progenitor cell differentiation toward a fibrogenic rather than myogenic phenotype (11). In transgenic mice, activation of canonical Wnt signaling resulted in exuberant cutaneous wound healing and increased local collagen synthesis (12). Multiple genes involved in tissue repair and fibrosis are known to be transcriptional targets of Wnt/ß-catenin, although the mechanism of their regulation is generally not well-defined (13-16). Genome-wide transcriptional profiling of lungs from patients with idiopathic pulmonary fibrosis (IPF) revealed elevated expression of genes coding for Wnt ligands, receptors, regulators, and targets such as osteopontin and WISP1 (17, 18). Moreover, lung fibroblasts explanted from patients with IPF maintain activated ß-catenin signaling ex vivo even in the absence of on-going Wnt stimulus (15). Other studies showed evidence of increased Wnt expression and activity in skin and serum from patients with SSc (19, 20). Moreover, nuclear β-catenin, a marker for active canonical Wnt signaling, was found to be strongly up-regulated in lungs from patients with SSc-associated pulmonary fibrosis (21). Together, these observations highlight a consistent association between aberrant Wnt/β-catenin signaling and pathological fibrosis in multiple organs and species.
Because little is known about Wnt/β-catenin signaling in SSc and its relevance for disease pathogenesis, we undertook an investigation of the expression and activity of the Wnt/β-catenin axis in SSc skin biopsies and in explanted human skin fibroblasts and subcutaneous progenitor cells. The results demonstrate impaired Wnt antagonism with consequent hyperactivation of canonical Wnt signaling in the lesional skin. Induction of Wnt signal transduction in explanted normal fibroblasts stimulated migration, proliferation, collagen gel contraction and myofibroblast differentiation, and enhanced the expression of fibrosis-related genes. In contrast, in subcutaneous adipocytes, Wnt3a inhibited adipogenesis, at least in part, by repressing the adipogenic master regulator peroxisome proliferator-activated receptor-gamma (PPARγ), resulting in fibroblastic differentiation with induction of Type I collagen and α-SMA expression in these cells. Taken together, the present findings support a key role for aberrant Wnt/β-catenin signaling in the development and progression of fibrosis in SSc, and elucidate the underlying mechanism of action. Taken together with recent reports in mouse models of fibrosis and various fibrosing disorders, these results provide the rationale for exploring therapies targeting aberrant Wnt/β-catenin signaling in the treatment of SSc.
Materials and Methods
Fibroblast and subcutaneous preadipocyte cultures
Primary cultures of neonatal fibroblasts were established by explantation from foreskins as described (22). Skin biopsies of healthy adult volunteers and patients with SSc were performed upon informed consent and in compliance with Northwestern University Institutional Review Board for Human Studies (the clinical characteristics of the patients are available from the corresponding author). Embryonic fibroblasts from Smad3−/− and wildtype mice were a gift from W.H. Schnaper (Northwestern University). Unless otherwise indicated, fibroblasts were maintained at 37°C in an atmosphere of 5% CO2 in Eagle’s Minimum Essential Medium (EMEM) or Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% vitamins, 1% penicillin/streptomycin, and 2 mM L-glutamine (from BioWhittaker, Walkersville, MD). For all experiments, fibroblasts were studied between passages 4 and 8. Human subcutaneous preadipocytes from non-obese adults (Zen-Bio, Research Triangle Park, NC) were maintained in preadipocyte maintenance medium (PM-1, Zen-Bio). In selected experiments, recombinant mouse Wnt3a (R&D Systems, Minneapolis, MN) or LiCl (Sigma, St. Louis, MO) was added to the cultures at indicated concentrations. Levels of active TGF-β1 in culture supernatants were determined by enzyme-linked immunosorbent assays (ELISA) (R&D). Cell viability was determined by Trypan blue dye exclusion assays.
Adipogenic differentiation
Preadipocytes were induced to undergo adipogenic differentiation in vitro as previously described (22). Briefly, confluent cultures were incubated in DM-2 adipogenic differentiation medium for four days, followed by incubation with AM-1 adipogenic maintenance medium (from Zen-Bio) for up to a further 7 days prior to harvesting. To assess intracellular lipid accumulation, cells were washed with PBS, fixed in 10% formalin for 60 min and washed with 60% isopropanol. After drying, cells were stained with Oil Red O (0.5% Oil Red O dye in 60% isopropanol, Sigma) for 30 min, followed by gentle rinse before microscopic visualization (23). Lipid-containing cells were counted in four random microscopic fields (400x) for each experimental condition, and experiments were repeated at least three times with consistent results.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Total RNA was isolated from confluent fibroblasts or adipocytes using TRIzol Reagent (Invitrogen, Carlsbad, CA). Reverse transcription for real-time quantitative PCR (qPCR) was performed using SuperScript First-strand synthesis system (Invitrogen) according to manufacturer’s protocol. Real-time qPCR reactions were performed on ABI-Prism 7300 sequence detection PCR machine (Applied Biosystem, Forster City, CA) according to the manufacturer’s protocol (22). Relative mRNA expression levels were normalized with Gapdh levels in each sample and determined by calculating ΔΔCt, as detailed in the manufacturer’s guidelines (Applied Biosystem).
Adenovirus infection, transient transfection and RNA interference
Fibroblasts or preadipocytes at early confluence were infected with replication-incompetent adenoviral vectors expressing human Wnt3a (Ad-Wnt3a) (from T-C. He, University of Chicago), constitutively active ß-catenin (Ad-β-cateninca)(from J. Kitajewski, Columbia University) or GFP (Ad-GFP). In other experiments, fibroblasts were transiently transfected with expression vectors for Fzd2 (Origene, Rockville, MD), or with short interfering RNAs coding for Wif1, Dkk2 or β-catenin (Ctnnb1), or scrambled control siRNAs (Dharmacon, Lafayette, CO). Following incubation for indicated periods, cultures were harvested and whole cell lysates were subjected to Western analysis, or total RNA was isolated and subjected to real-time qPCR. In selected experiments, adenovirus-infected cells were transiently transfected with TOPFlash reporter constructs (from R. Moon, University of Washington), which contain eight copies of the TCF binding site upstream of luciferase, using Superfect Reagent (Qiagen, Valencia, CA). All experiments were performed in triplicates.
Cadherin-free β-catenin binding assays
To evaluate activation of canonical Wnt signaling, intracellular levels of cadherin-free β-catenin were determined by pull-down assays using GST-ICAT as described (9). Briefly, at the end of the experiments, cells were harvested, solubilized in a nonionic detergent buffer (1% Nonidet P-40, 50 mM Tris, pH 7.5, 150 mM NaCl, and 2 mM EDTA including protease inhibitors), and centrifuged at 14,000 g. Cellular β-catenin was affinity-precipitated using GST-ICAT immobilized to glutathione-coupled Sepharose (Sigma), washed with buffer A (buffer A: 10 mM Tris, pH 8.0, 140 mM NaCl, 1 mM EDTA, 0.1% NP-40, and 10 μg/ml leupeptin and aprotinin), and subjected to SDS-PAGE and Western blot analysis using β-catenin antibody (BD Biosciences, San Jose, CA).
Proliferation, migration and gel contraction assays
Foreskin fibroblasts infected with Ad-Wnt3a or Ad-GFP (30 MOI) were seeded (1000 cells/well) in 96-well plates. Proliferation rates were determined using CellTiter 96® Non-Radioactive Cell Proliferation Assay Kits (Promega, Madison, WI). The modulation of cell migration was evaluated by in vitro wound-healing assays, as described (23). Briefly, monolayers of Ad-GFP or Ad-Wnt3a-infected fibroblasts were incubated in serum-free medium for 12 h, and scratch wounds were induced using standard p1000 pipette tips. Wounds were monitored for up to 48 h by phase contrast microscopy, and wound gap length was determined at three different sites in each sample at indicated intervals. For collagen gel contraction assay, fibroblasts infected with Ad-GFP or Ad-Wnt3a were seeded in Type I collagen gels (BD Biosciences), and following incubation in DMEM in the presence of 10% FBS for the indicated periods, gel diameters were determined (21). The results are expressed as percentage of gel area compared to initial gel area.
Western analysis
At the end of the incubation periods, cultures were harvested, and equal amounts of whole cell lysates proteins were subjected to electrophoresis in 4–15% Tris–glycine gradient gels (22). Proteins were then transferred to PVDF membranes, blocked with 10% fat-free milk in TBST buffer, and incubated with the following primary antibodies: β-catenin, Fatty acid binding protein 4 (FABP4)(1: 1000, BD Biosciences), active β-catenin (1:1000, Millipore, Jaffrey, NH), ICAT (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH (1:3000, Invitrogen), α-SMA (1:2000; Sigma) or Type I collagen (1:400; Southern Biotechnology, Birmingham, AL). Membranes were washed three times, incubated with appropriate secondary antibodies for 45 min, and antigen–antibody complexes were visualized by chemiluminescence (Pierce Biotechnology, Rockford, IL).
Immunofluorescence
Preadipocytes were incubated in DM2 medium for 2 days to induce adipogenic differentiation, infected with Ad-Wnt3a (30 MOI), and incubated in AM medium for a further 96 h. Skin fibroblasts were incubated in media with Wnt3a for 4 h, or infected with Ad-Wnt3a (30 MOI) for 5 days. At the end of the incubation, cells were fixed in 4% paraformaldehyde, washed in PBS, incubated with primary antibodies against β-catenin or α-SMA (1:1000) for 120 min, followed by incubation with Alexa® 488- or Alexa® 594 conjugated chicken-anti-mouse antibodies (Invitrogen) for 60 min. Nuclei were identified by 4’-6-diamidino-2-phenylindole (DAPI) staining. Non-immune IgG was used as a negative control in each experiment. Following stringent washing, slides were examined under a Zeiss UV Meta 510 confocal microscope (Carl Zeiss, Jena, Germany). Each experiment was repeated at least three times with consistent results.
Immunohistochemistry
Immunohistochemistry of forearm skin biopsies from SSc patients or healthy adults was performed on formalin fixed paraffin sections as previously described, using antibody against β-catenin (BD Biosciences) at 1:100 dilution (24). Substitution of the primary antibody with isotype-matched irrelevant IgG served as negative controls. Nuclear β-catenin-positive and total fibroblast-like cells were counted in 4-5 random fields from one section/biopsy under 400 × magnifications.
Analysis of cDNA microarray datasets
Data were analyzed from a microarray dataset of genome-wide expression using skin biopsies from patients with SSc and from healthy controls (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9285). The dataset has been previously described in extensive detail (25). Genes known from the literature to be involved in Wnt signaling were extracted from the microarray dataset, and those that showed expression with statistically significant difference between SSc and healthy control biopsies were further analyzed. The expression level of each gene was centered on its mean value across all arrays.
Statistical analysis
One-way Anova was used to analyze the microarray data. Tukey post-hoc test was used to examine the difference between intrinsic subsets. In other experiments, the data are presented as means ± SD. The significance of differences between experimental and control groups was determined by Student’s t-test and the value of p<0.05 was considered statistically significant. Student t-tests were performed using GraphPad t-test calculator.
Results
Aberrant Wnt/β-catenin activation in SSc
To gain insight into the potential significance of Wnt/β-catenin axis in SSc, we first examined the expression of Wnt pathway components in genome-wide expression datasets that were generated using skin biopsies from a cohort of SSc patients and healthy controls (25). This cohort comprises 17 well-characterized patients with diffuse cutaneous SSc (dcSSc), and 7 patients with limited cutaneous SSc (lSSc), along with 3 patients with localized scleroderma and 6 healthy controls. As shown previously, unbiased hierarchical clustering using a list of genes that showed the most consistent expression between the forearm-back pairs for each patient, but the most diversity across patients, yielded five intrinsic subsets distinguished by unique gene expression signatures (25). Analysis of a replication cohort that includes longitudinal analysis of skin biopsies has demonstrated that within an intrinsic subset, these gene expression signatures are stable (Pendergrass, Lemaire, Layatis and Whitfield M. Ms. submitted). There was no statistical difference between levels of the Wnt ligands (Wnt1-11) among the five intrinsic subsets. In contrast, expression of the Wnt receptor Fzd2 was significantly increased (p<0.01) in the diffuse-proliferative SSc subsets (Fig. 1A). Importantly, the expression of the Wnt inhibitors Dkk2 and Wif1 was significantly decreased in the biopsies clustering in the same intrinsic subsets comprised entirely of patients with dcSSc (p<0.01), whereas they showed increased expression in biopsies clustering in the inflammatory intrinsic subset (p<0.01) comprising dcSSc, lSSc and localized scleroderma biopsies.
Figure 1. Wnt signaling is increased in systemic sclerosis.
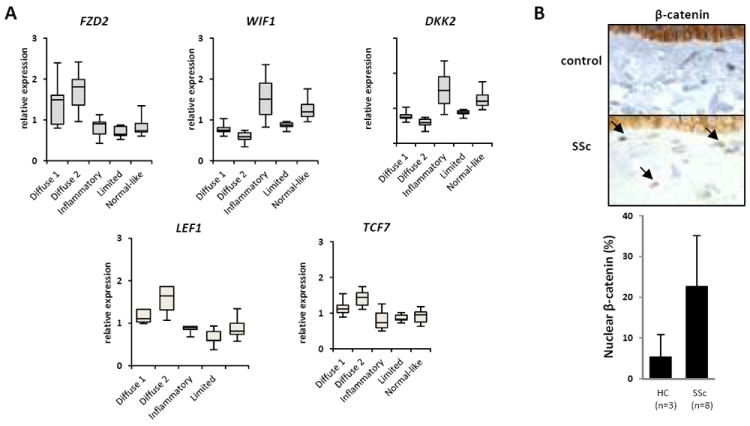
A. Analysis of Wnt pathway gene expression in skin biopsy-based microarray datasets. The generation of the microarray datasets based on skin biopsies from patients with dSSc (n=17), lSSc (n=7), localized scleroderma (n=3) and healthy controls (n=6), and identification of five intrinsic subsets was described previously (25). Expression levels for each gene are shown as plot boxes representing the middle half of the distribution of the data points from the 25th (“lower hinge”) to the 75th percentile (“upper hinge”). The line across the box represents the median. The lines above and below the box indicate the maximum and minimum data values respectively. B. Immunohistochemistry of β-catenin. Skin biopsies from SSc patients (n=8) and healthy controls (n=3) were examined. Upper panels, representative images. Arrows indicate nuclear β-catenin-positive fibroblast-like cells in the dermis. Original magnification × 400. Lower panel, nuclear β-catenin positive cells in the dermis quantified as described under Materials and Methods are shown as means ± SD.
The combination of increased Wnt receptor expression coupled with decreased expression of Wnt inhibitors in these skin biopsies might be expected to give rise to hyperactivation of Wnt/β-catenin signaling. Indeed, expression of the Wnt target gene Lef1 showed significant increase in biopsies clustering in the diffuse-proliferation intrinsic molecular subsets. Moreover, Tcf7, a transcription factor mediating Wnt/β-catenin responses, showed similarly increased expression. As would be expected in the presence of hyperactived canonical Wnt signaling, dermal fibroblast-like cells showed elevated nuclear β-catenin accumulation in SSc skin biopsies (Fig. 1B). While the proportion of nuclear β-catenin-positive cells in the lesional dermis was increased in biopsies from SSc patients with both early (<2 yrs) and late-stage disease, no significant correlation between β-catenin expression and modified Rodnan skin score was found (data not shown).
In explanted fibroblasts, Lef1 mRNA expression was up-regulated by both Wnt3a and infection with Ad-β-cateninca, confirming that Lef1 was a target of canonical Wnt signaling (Fig. 2A). To confirm directly that elevated expression of the Wnt receptor Fzd2, or reduced expression of the Wnt antagonists Dkk2 or Wif1 resulted in hyperactivated Wnt/ß-catenin signaling in fibroblasts, a series of gain-of-function and loss-of-function experiments were undertaken. These experiments revealed that ectopic expression of Fzd2 or RNAi-mediated knockdown of either Wif1 or Dkk2 in fibroblasts enhanced their sensitivity to exogenous Wnt ligand (Fig. 2B).
Figure 2. Wnt3a stimulates fibroblasts proliferation, migration and gel contraction.
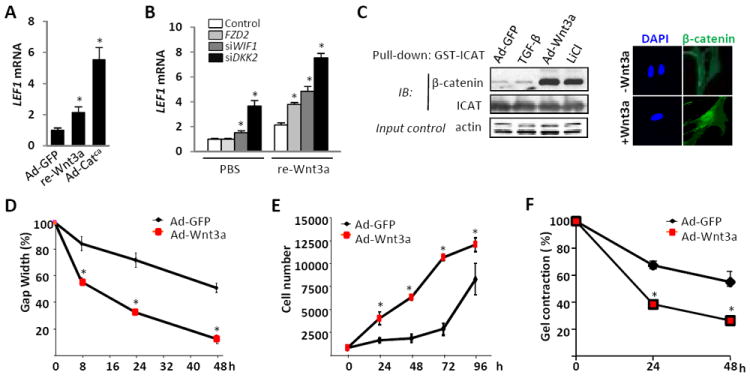
Confluent foreskin fibroblasts were infected with indicated expression vectors (Ad-GFP, Ad-Catca or Ad-Wnt3a, 30 MOI) or transiently transfected with Fzd2, or siRNA specific for Wif1 or DKK2 or corresponding scrambled control siRNAs, followed by incubation for 24-48 h in media with Wnt3a (100 ng/ml) or LiCl (30 mM). A, B. RNA was isolated and examined by real-time qPCR. Results, normalized with Gapdh, are shown as means ± SD of triplicates from three independent experiments. * p < 0.05. C. Left panels, whole cell lysates were subjected to GST-ICAT pull-down assays (see Material and Methods). Active β-catenin binding to ICAT was probed with a pan-catenin antibody. Representative images. Right panels, fibroblasts were incubated with Wnt3a for 4 h, fixed and probed with DAPI (blue) and β-catenin antibody (green), and examined by immunofluorescence microscopy. Representative images, original magnification × 400. D. Fibroblast migration was monitored by measuring scratch width at three different sites in each sample. Results are shown as means ± SD of triplicates from an experiment representative of three. * p < 0.05. E. Proliferation assays. * p< 0.05. F. Gel contraction assays. Results expressed as the percentage of gel area compared to time 0 are shown as mean ± SD of a triplicates from representative experiment of three independent experiments. * p <0.05.
Wnt3a stimulates profibrotic responses in normal fibroblasts
In view of the observed hyperactivation of Wnt/β-catenin signaling in SSc, we focused on the well-characterized canonical Wnt ligand Wnt3a. The effect of Wnt3a on canonical β-catenin signaling was evaluated by a combination of ICAT pull-down assays, immunocytochemistry and transient transfection assays. Levels of N-terminal unphosphorylated (active) β-catenin were significantly increased in Ad-Wnt3a-infected as well as LiCl-treated fibroblasts (Fig. 2C, left panels). Immunocytochemistry demonstrated that Wnt3a induced rapid nuclear accumulation of β-catenin in these fibroblasts (Fig. 2C, right panels). Transient transfection assays showed significant stimulation of the canonical Wnt reporter TOPFlash by Wnt3a infection, as well as by recombinant Wnt3a (data not shown).
Fibroblast migration, proliferation and collagen are critical aspects of both wound healing and pathological fibrogenesis. Modulation of wound healing by Wnt3a was determined by in vitro scratch assays. A pipette tip was used to make a liner scratch in the confluent monolayers of fibroblasts infected with Ad-Wnt3a, and fibroblast migration was monitored for up to 48 h. As shown in Figure 2D, ectopic Wnt3a signaling accelerated fibroblast migration. Moreover, inducing β-catenin activity by either recombinant Wnt3a treatment, or by Ad-Wnt3a infection, significantly increased fibroblast proliferation (Fig. 2E, and data not shown). Fibroblast contractility, determined using collagen lattice gel contraction assays, was similarly enhanced by Ad-Wnt3a, as well as by recombinant Wnt3a (Fig. 2F, and data not shown).
To evaluate the effect of Wnt3a on the expression of genes involved in fibrogenesis, foreskin fibroblasts were infected with Ad-Wnt3a. Real-time qPCR showed a significant dose-dependent stimulation of Col1A2 and α-SMA mRNA expression (Figs. 3A, B). The levels of both active β-catenin and total β-catenin were increased in the Ad-Wnt3a infected fibroblasts, as expected. Parallel experiments showed that Ad-Wnt3a induced the formation α-SMA-positive stress fibers characteristic of myofibroblasts (Fig. 3C). Consistent results were seen in multiple independent experiments with fibroblasts explanted from different donors.
Figure 3. Wnt3a stimulates profibrotic gene expression and myofibroblast differentiation.

Confluent foreskin fibroblasts were infected with Ad-GFP or Ad-Wnt3a (30 MOI or as indicated) for up to 6 d. A. RNA was isolated at indicated intervals and subjected to real-time qPCR. Results, normalized with Gapdh, are shown as means ± SD of triplicates from three independent experiments. * p < 0.05. B. Whole cell lysates were subjected to Western blot analysis. Representative immunoblots. C. Fibroblasts were stained with DAPI (blue) or immunostained with antibodies against α-SMA (green). Original magnification × 400.
Canonical TGF-β signaling mediates Wnt3-induced profibrotic responses
Because TGF-β, a pivotal trigger for profibrotic responses, has been implicated in Wnt-induced myofibroblast differentiation (26), we sought to delineate its role in mediating the profibrotic effects of Wnt3a. The results of real-time qPCR analysis showed a time- and dose-dependent increase in Tgfb1 mRNA expression in fibroblasts stimulated by recombinant Wnt3a that was prevented by siRNA-mediated knockdown of β-catenin (Fig. 4A and data not shown). Moreover, incubation with recombinant Wnt3a, as well as with LiCl, resulted in increased Smad2 and Smad3 phosphorylation, which was prevented by pre-incubation of cultures with a neutralizing anti-TGF-β1 antibody, indicating the involvement of autocrine TGF-ß signaling. Persistent Wnt3a expression by adenovirus infection in fibroblasts similarly induced increased levels of Tgf-β1 mRNA and secretion of active TGF-β1 (Fig. 4C and data not shown).
Figure 4. Smad-dependent TGF-β signaling mediates profibrotic Wnt3a responses.

Confluent fibroblasts were transfected with siRNA against β-catenin (siCtnnb1) or scrambled control siRNA (A), and then incubated in media with or without Wnt3a (100 ng/ml) for up to 24 h (A, B), or infected with Ad-Wnt3a for 6 d (C, D). A. RNA was isolated at indicated intervals (left panel) or following Wnt3a incubation for 120 min, and subjected to real-time qPCR. Results, normalized with 18S RNA, represent the means ± SD of triplicate determinations in representative experiment. * p < 0.05. B. Whole cell lysates were examined by Western analysis. Representative blots. C. Levels of active TGF-β1 in culture supernatants were determined by ELISA. The results represent the means ± SD of duplicate determinations from three independent experiments. * p < 0.05. D. Cultures were incubated with or without SB431542 (10 μM) for a further 24 h, and RNA was examined by real-time qPCR. Results, normalized with Gapdh, represent the means ± SD of triplicate determinations. * p < 0.05. E. Confluent cultures of Smad3-null and wildtype mouse embryonic fibroblasts were infected with Ad-GFP or Ad-Wnt3a. Following six days of incubation, RNA was subjected to real-time qPCR. The results, normalized with 36b4, represent the means ± SD of triplicate determinations. * p <0.05.
Complementary genetic and pharmacological approaches were taken to establish the requirement for autocrine TGF-β/Smad signaling in mediating the profibrotic effects of Wnt3a. Pretreatment of fibroblasts with SB431542, a specific ALK5 inhibitor markedly attenuated the Wnt3a-induced stimulation of multiple profibrotic genes (Fig. 4D). Similarly, blocking TGF-β signaling with a neutralizing antibody to TGF-ß abrogated the stimulation of collagen synthesis induced by Wnt3a (data not shown). Furthermore, in Smad3-null MEFs Wnt3a failed to stimulate collagen gene expression, while stimulation of axin2 was unaffected in these cells, indicating that Smad2/3 dependence of Wnt3a signaling was selective for fibrogenic responses (Fig. 4E). Together, the results from experiments using gene deletion and pharmacological inhibition of TGF-ß and Smad signaling implicate Smad2/3-mediated autocrine TGF-β signaling in the profibrotic responses induced by Wnt3a.
Wnt3a inhibits preadipocyte-to-adipocyte differentiation
The best characterized biological function of the canonical Wnt/β-catenin signaling is cell fate specification during embryogenesis (5). In fibrosis, a potential source of excessive myofibroblasts might be mesenchymal progenitor cells (27). Recent studies indicate that multipotent preadipocytes can trans-differentiate into fibroblast-like cells, suggesting that these progenitors might be another potential source of myofibroblasts (28). We have previously shown in vivo that genetic disruption of the development of adipose tissue in transgenic mice overexpressing Wnt10b resulted in dermal fibrosis, with dense collagen deposition displacing the subcutaneous adipose layer (20). In order to explore the possibility that Wnt modulation of adipogenic differentiation by might play a role in fibrogenesis, explanted subcutaneous preadipocytes were first induced to differentiate into adipocytes by incubation in DM-2 medium, followed by recombinant Wnt3a treatment or Ad-Wnt3a infection. The results with both approaches showed that Wnt3a abrogated the accumulation of cytoplasmic lipid droplets induced by DM-2 (Fig. 5A and data not shown). Moreover, Ad-Wnt3a changed the preadipocyte morphology from round to spindle-shaped with smaller nuclei (Fig. 5A). The anti-adipogenic effects of Wnt3a were more pronounced in differentiating preadipocytes than in fully differentiated mature adipocytes (data not shown). Real-time qPCR showed that induction of Pparγ1 and Pparγ2, which are the key molecular regulators of adipogenesis, as well as Fabp4, a well-known marker of adipocyte differentiation, were significantly suppressed in the presence of Wnt3a (Fig. 5B and data not shown). In contrast to the profibrotic responses induced by Wnt3a in fibroblast, the inhibitory effects of Wnt3a on adipogenic gene expression were was Smad2/3-independent (Fig. 5C).
Figure 5. Wnt3a suppresses preadipocyte differentiation.
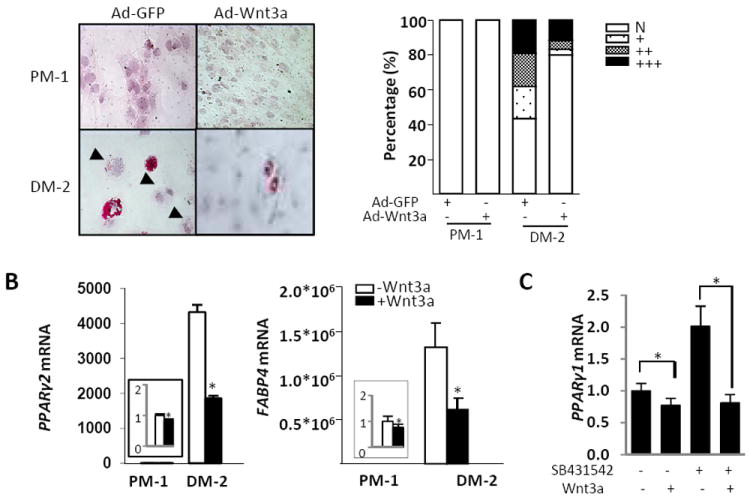
A. Human subcutaneous preadipocytes were incubated in PM-1 or DM-2 medium for 2 d, and infected with Ad-GFP or Ad-Wnt3a (30 MOI). Following a further 5 d, cells were fixed and stained with Oil Red O. Left panels, cytochemistry (original magnification 400×). Arrows indicate weak red staining in differentiated adipocytes. Right panel, quantitation of Oil Red O staining. 120 cells for each condition in five different fields (magnification 400×) were scored (N, none; +, small droplets, weak staining; ++, moderate staining; +++, large droplets). B. Preadipocytes were incubated in media with or without Wnt3a (100 ng/ml) for 7 days. RNA was isolated and subjected to real-time qPCR. The results, normalized with Gapdh, represent the means ± SD of triplicate determinations. * p<0.05. C. Preadipocytes were induced to adipogenic differentiation for 7 d. Cultures were then pretreated with SB431542 for 15 min and incubated with recombinant Wnt3a (100 ng/ml) for 24 h. RNA was isolated and subjected to real-time qPCR analysis. The results, normalized with Gapdh, represent the means ± SD of triplicate determinations. * p<0.05.
Wnt3a stimulates preadipocyte differentiation into myofibroblasts
Multipotent mesenchymal progenitor cells can be induced to differentiate into myofibroblasts (28). In order to investigate the effects of Wnt3a on preadipocyte differentiation toward other mesenchymal fates, human subcutaneous preadipocytes were infected with Ad-Wnt3a for up to 3 days. In these experiments, Wnt signaling increased the expression of α-SMA protein and mRNA in preadipocytes (Fig. 6A and data not shown). Similar results were seen with differentiated adipocytes (Fig. 6A, lanes 3 and 4). Furthermore, Wnt3a stimulated Type I collagen synthesis and the formation of α-SMA-positive stress fibers in these cells, while simultaneously suppressing the induction of FABP4 (Fig. 6B). The induction of Type I collagen by Wnt3a was attenuated by SB431542 pretreatment, indicating a role for TGF-β/Smad signaling this process. These results therefore demonstrate that in multipotent mesenchymal progenitor cells, Wnt3a not only suppressed adipogenesis via negative regulation of PPAR-γ via autocrine TGF-β signaling, but simultaneously induced Smad2/3-dependent myofibroblast differentiation. The cumulative result of these Wnt/β-catenin-induced profibrotic and anti-adipogenic activities is potent stimulation of fibroblast activation and fibrogenesis.
Figure 6. Wnt3a stimulates preadipocytes to myofibroblast differentiation.
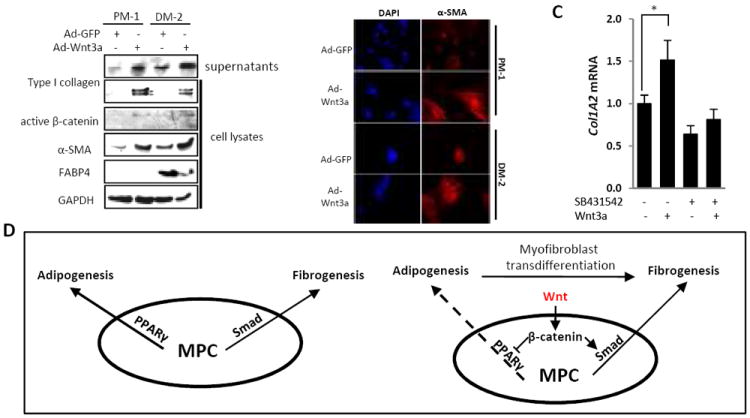
Human subcutaneous preadipocytes were incubated in PM-1 or DM-2 medium for 4 d, and infected with Ad-GFP or Ad-Wnt3a (30 MOI) for 3 d. A. Whole cell lysates and supernatants were subjected to Western analysis. Representative Western blots. B. Cells were stained with DAPI (blue) or immunostained antibody against α-SMA (red). Original magnification × 400. C. Preadipocytes incubated in DM-2 medium for 7 d were incubated with recombinant Wnt3a (100 ng/ml) for 24 h in the presence or absence of SB431542. RNA was isolated and subjected to real-time qPCR analysis. The results, normalized with Gapdh, represent the means ± SD of triplicate determinations. * p<0.05. D. Mesenchymal progenitor cells (MPC) undergo PPARγ – dependent adipogenesis or TGF-β/Smad-dependent fibrogenesis (left panel). In the presence of Wnt3a (right panel), PPARγ is suppressed, while TGF-β is enhanced, resulting in repression of adipogenesis, myofibroblast transdifferentiation and enhanced fibrogenesis.
Discussion
The Wnts have essential roles in embryonic morphogenesis, stem cell homeostasis and cell fate determination, and genetic or acquired abnormalities of Wnt expression or signaling are associated with various diseases (29). Here, we showed that the Wnt/β-catenin pathway is hyperactivated in skin biopsies from a subset of patients with dcSSc. Wnt3a promoted myofibroblast differentiation via Smad-dependent autocrine TGF-β signaling, while suppressing adipogenic differentiation of preadipocytes and inducing their differentiation into myofibroblasts. The net effect of these combined stimulatory and inhibitory activities is to promote fibrogenesis. Wnt signaling is normally tightly regulated by the balance of Wnt ligands and their inhibitors, and functional loss of Wnt antagonists result in hyperactivation of Wnt signaling (30-32). In cancers, hypermethylation of Wnt antagonists reduces their expression, with resultant increase in Wnt activity (33). While deregulation of Wnt signaling is implicated in oncogenesis and metastasis (34, 35), the potential relevance of Wnt signaling and its deregulation in fibrogenesis has only recently begun to be appreciated, and the underlying mechanisms remain to be elucidated (17, 36-38).
In the present studies we found that Wnt3a directly induced TGF-β1 expression and activity in normal fibroblasts. Wnts and TGF-β are known to regulate each other’s production and activity in a reciprocal manner (39). Wnt3a has been previously shown to induce expression of TGF-β and its receptors in multiple cell types in addition to fibroblasts (13, 26, 40). In silico analysis using the UCSC Genome Browser revealed enhanced transcription factor accumulation on the TGF-ß1 gene promoter in ß-catenin-expressing cells, suggesting direct transcriptional stimulation of TGF-ß1 induced via the canonical Wnt signaling pathway (data not shown). Moreover, canonical Wnt signaling can also directly modulate the intensity of TGF-β/Smad signaling (41). On the other hand, TGF-β impacts on Wnt signaling at multiple levels, including stimulation of both Wnt production and β-catenin activity (42, 43). These observations highlight the intimate reciprocal cross-regulation and extensive intracellular cross-talk that exists between the Wnt/β-catenin and TGF-β signaling pathways, and is likely to have significant impact on fibrogenesis.
A striking pathological finding in SSc skin is the progressive loss of subcutaneous adipose tissue and its replacement by scar, resulting in characteristic skin tethering (24). Adipogenesis is under complex regulation by the nuclear hormone receptor PPARγ, the master regulator in adipogenic lineage specification (43). The Wnt/β-catenin pathway is implicated in mesenchymal stem cell fate decisions in part via suppression of PPARγ. The down-regulation of PPARγ by Wnts is thought to be mediated via COUP-TFII (44), microRNA (45) and epigenetic modification (46). The present results demonstrate that Wnt3a promoted preadipocyte-to-myofibroblast differentiation while inhibiting adipogenesis in a PPARγ-dependent manner. A comparable paradigm for Wnt-regulated mesenchymal cell fate switching involving PPARγ is reported in adipogenic-osteoblastogenic differentiation (47).
Since pathological fibrosis in multiple human diseases and various animal models is consistently associated with aberrant Wnt/β-catenin signaling, drugs that target the Wnt cascade have enormous potential as novel therapeutics. Several Wnt inhibitors are in preclinical or phase I clinical trial in cancers (48). In animal models of fibrosis, inhibition of Wnt signaling by blockade of the β-catenin/TCF-mediated transcription exerted potent anti-fibrotic effects (49, 50). Blockade of Wnt signaling with paricalcitol or the peptide-mimetic ICG-001 resulted in attenuation of renal fibrosis in a mouse model (51, 52). These observations provide further support for the pivotal role of aberrant Wnt/β-catenin signaling in various forms of fibrosis, and indicate the feasibility of targeting Wnts to prevent or reverse the process.
In summary, the demonstration that impaired Wnt antagonism is associated with Wnt/β-catenin pathway hyperactivation in skin biopsies from a subsets of SSc patients expands on our similar findings in SSc-associated lung fibrosis (21). Canonical Wnt/β-catenin signaling in fibroblasts stimulated their proliferation, migration, gel contraction and myofibroblasts differentiation. These potent profibrotic Wnt responses involved Smad-dependent autocrine TGF-β signaling. At the same time, Wnt3a also inhibited adipogenesis in progenitor cells and switched their differentiation towards the myofibroblasts lineage. Taken together with emerging findings, the results implicate Wnt/β-catenin signaling in fibrogenesis by concomitant inhibition of adipogenesis and promotion of myofibroblast activation and differentiation. Therefore, Wnt/β-catenin pathway is a promising target for anti-fibrotic therapeutic approaches.
Acknowledgments
We are grateful for helpful discussions with Warren Tourtellotte and members of the Varga Lab.
This work was supported by grants from the National Institute of Health (AR-42309) and from the Scleroderma research Foundation.
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga J, Whitfield ML. Transforming growth factor-beta in systemic sclerosis (scleroderma) Front Biosci (Schol Ed) 2009;1:226–35. doi: 10.2741/s22. [DOI] [PubMed] [Google Scholar]
- 4.Wei J, Bhattacharyya S, Tourtellotte WG, Varga J. Fibrosis in systemic sclerosis: Emerging concepts and implications for targeted therapy. Autoimmun Rev. 2011;10(5):267–75. doi: 10.1016/j.autrev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka SS, Kojima Y, Yamaguchi YL, Nishinakamura R, Tam PP. Impact of WNT signaling on tissue lineage differentiation in the early mouse embryo. Dev Growth Differ. 2011 doi: 10.1111/j.1440-169X.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 6.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 7.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2(9):e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129(7):1614–27. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottardi CJ, Gumbiner BM. Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions. Am J Physiol Cell Physiol. 2004;286(4):C747–56. doi: 10.1152/ajpcell.00433.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bowley E, O’Gorman DB, Gan BS. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138(1):141–50. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 12.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, et al. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99(10):6973–8. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal. 2007;1(3-4):175–83. doi: 10.1007/s12079-007-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 2010;285(11):8196–206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W, Tan R, Dai C, Li Y, Wang D, Hao S, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem. 2010;285(32):24665–75. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19(8):5576–87. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaire R, Farina G, Bayle J, Dimarzio M, Pendergrass SA, Milano A, et al. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J Invest Dermatol. 2010;130(6):1514–23. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: A novel mouse model for scleroderma? Arthritis Rheum. 2011 doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, et al. Nuclear {beta}-catenin is Increased in SSc Pulmonary Fibrosis and Promotes Lung Fibroblast Migration and Proliferation. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, et al. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS ONE. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M, Melichian DS, Chang E, Warner-Blankenship M, Ghosh AK, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174(2):519–33. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtola J, Myers J, Akhtar-Zaidi B, Zuzindlak D, Sandesara P, Yeh K, et al. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135(13):2321–9. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3(7):e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS ONE. 6(5):e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya S, Wei J, Varga J. The mechanistic basis of fibrosis in systemic sclerosis. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(31):22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 29.Lam AP, Gottardi CJ. beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23(6):562–7. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133(11):2149–54. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- 32.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119(4):837–51. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying Y, Tao Q. Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics. 2009;4(5):307–12. doi: 10.4161/epi.4.5.9371. [DOI] [PubMed] [Google Scholar]
- 34.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1(7):563–77. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coluzzi F, Di Bussolo E, Mandatori I, Mattia C. Bone metastatic disease: taking aim at new therapeutic targets. Current medicinal chemistry. 2011;18(20):3093–115. doi: 10.2174/092986711796391660. [DOI] [PubMed] [Google Scholar]
- 36.Gardner H, Shearstone JR, Bandaru R, Crowell T, Lynes M, Trojanowska M, et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 2006;54(6):1961–73. doi: 10.1002/art.21894. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann C, Akhmetshina A, Dees C, Palumbo K, Zerr P, Beyer C, et al. Inhibition of glycogen synthase kinase 3beta induces dermal fibrosis by activation of the canonical Wnt pathway. Annals of the rheumatic diseases. 2011;70(12):2191–8. doi: 10.1136/ard.2010.147140. [DOI] [PubMed] [Google Scholar]
- 38.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS medicine. 2008;5(3):e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Esteban C, Capdevila J, Kawakami Y, Izpisua Belmonte JC. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development. 2001;128(16):3189–95. doi: 10.1242/dev.128.16.3189. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy TL, Centrella M. Novel links among Wnt and TGF-beta signaling and Runx2. Mol Endocrinol. 24(3):587–97. doi: 10.1210/me.2009-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22(1):106–20. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JH, Chen WL, Sider KL, Yip CY, Simmons CA. beta-catenin mediates mechanically regulated, transforming growth factor-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2011;31(3):590–7. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011 doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009;106(14):5819–24. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, et al. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics. 11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukamoto H, Zhu NL, Asahina K, Mann DA, Mann J. Epigenetic cell fate regulation of hepatic stellate cells. Hepatol Res. 2011;41(7):675–82. doi: 10.1111/j.1872-034X.2011.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2011 doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 16(12):3153–62. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 49.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107(32):14309–14. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(49):21110–5. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, et al. Targeted Inhibition of {beta}-Catenin/CBP Signaling Ameliorates Renal Interstitial Fibrosis. J Am Soc Nephrol. 2011 doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22(1):90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]


